Abstract
This post hoc analysis from a multicenter study (NCT01674634) was designed to evaluate the efficacy of collagenase Clostridium histolyticum (CCH) treatment in patients with different stages of Dupuytren contracture. Previously untreated patients who received two concurrent injections of CCH in two affected joints in the same finger were assessed by disease severity (Tubiana stage). The mean (SD) improvement in total fixed flexion contraction (FFC) 31 days post-CCH treatment in 181 patients was: 71.1 (36.5)% for Tubiana I, 77.0 (21.0)% for Tubiana II, 72.0 (20.4)% for Tubiana III and 66.4 (22.2)% for Tubiana IV. Treatment of metacarpophalangeal and proximal interphalangeal joints in the same finger resulted in a mean (SD) improvement of 82.5 (24.8)% and 66.4 (27.9)%, respectively. In conclusion, CCH is an effective treatment alternative for all stages of Dupuytren contracture and it provides a less invasive treatment alternative to surgery with similar short-term efficacy in patients with more severe disease.
Introduction
Dupuytren disease (OMIM 126900) is a benign fibroproliferative progressive disorder, affecting the palmar fascia of the hands and fingers, and is characterized by flexion contracture within the joints of the fingers. The prevalence varies between populations and age groups and the disease is more common among men and in people of Northern European descent [Citation1–3]. The disease is heterogeneous involving both genetic and environmental factors [Citation4,Citation5]. The onset and progression varies and occasionally other fibroproliferative conditions, such as Peyronie disease (OMIM 171000) and plantar fibromatosis (or Ledderhose disease), are associated with Dupuytren contracture.
Although there is no curative treatment for Dupuytren disease, there are several treatment options of which limited fasciectomy (LF) is the most frequently used and considered the gold standard for treatment [Citation5–7]. Disadvantages of this procedure are the risk of surgical complications [Citation8] and the long postoperative recovery period [Citation9]. Percutaneous needle fasciotomy (PNF) is less invasive and uses a needle to puncture the cord until it can be disrupted by mechanical force. This is completed during one visit and has relatively low complication rates [Citation10]. One drawback with this technique is a tendency for early recurrence [Citation6,Citation11,Citation12]. The affected cords can also be disrupted enzymatically by collagenase Clostridium histolyticum (CCH), the first-in-class biologic treatment of Dupuytren disease, which is injected into the cord followed by a finger extension procedure 1 to 3 days postinjection to break the cord [Citation13]. Both PNF and CCH provide an outpatient treatment option with shorter recovery compared to LF [Citation14].
Factors, such as patient’s preference, age, disease severity, financial considerations and the physician’s personal preference and experience with the available techniques, are determinants for the treatment option chosen for an individual patient. The fact that there are no randomized controlled studies comparing all three available treatment modalities complicates decision making, and results from separate studies are used as guidance to provide the best treatment option. However, the comparison of such study results is hampered by the different patient populations, the complexity of the disease, possible co-morbidities, the number of joints and fingers involved and the lack of uniform definitions of efficacy and the way recurrence is determined [Citation15]. For example PNF treatment appears less effective than LF in more severe cases with disease classified as Tubiana III or IV [Citation16], emphasizing the need to take disease stage into account when comparing different study results. Treatment outcome is also affected by the type of joints studied, for example, metacarpophalangeal (MP) joints typically have better treatment outcome than proximal interphalangeal (PIP) joints [Citation9], which may further complicate study comparisons.
This paper describes a post hoc analysis from a large open-label phase 3 b study on CCH that evaluated the efficacy and safety of two concurrent injections of CCH in two joints [Citation13]. This analysis evaluates CCH treatment efficacy by disease severity (Tubiana stage) on short-term treatment outcomes. The CCH results are compared descriptively with results from the only randomized clinical trial with LF and PNF. Therefore, the patient population had to match with regards of baseline characteristics, hence only previously untreated patients with both MP and PIP joints affected in the same finger were selected for this post hoc analysis [Citation16].
Methods
Study design
This is a post hoc analysis of a previously published multicenter, open-label, phase 3 b study that investigated the safety and efficacy of two concurrent CCH injections (0.58 mg/injection) for two joint contractures (MP and/or PIP) caused by palpable cords in the same hand (www.clinicaltrials.gov identifier: NCT01674634) [Citation13]. The objective of this post hoc analysis was to assess the improvement of the total fixed flexion contractures (FFC), 31 days after CCH treatment, by Tubiana stage.
The original study was conducted according to ICH GCP guidelines and the Declaration of Helsinki and it was approved by the relevant Ethics Committees [Citation13].
Patient population
The original study enrolled 715 patients who had at least two FFC of 20 degrees or greater on the same hand in MP and/or PIP joints (not thumbs) that were caused by palpable cords and who had a positive tabletop test [Citation13]. This post hoc analysis comprises a subpopulation of previously untreated patients enrolled in the original study who received two injections and had at least one follow-up efficacy assessment. We present the analysis of two populations, one population including previously untreated patients with two injections in the same finger (181 patients: 181 MP joints and 181 PIP joints) that was used for the analysis of FFC by Tubiana and as well for joint specific analyses, and one population including all previously untreated patients (346 patients: 461 MP joints and 231 PIP joints) that was used for the joint specific analysis only. Previously untreated patients were included to better match patients in previous studies on LF and PNF [Citation16] to allow for indirect comparisons.
Efficacy assessment
The primary outcome measure of this post hoc analysis was the change in total FFC 31 days after CCH treatment. The total FFC was calculated as the sum of the passive extension deficit (measured in degrees) of affected MP and PIP joints located in the same finger. Assessment of distal interphalangeal (DIP) joints was not included since such data were not collected in the original study. The total FFC, including MP and PIP joints in the same finger, was used as an approximation for total passive extension deficit (TPED) in the whole finger. Total FFC is presented according to Tubiana stage [Citation17], which represents a generally accepted classification measuring total flexion deformity for a single affected digit; Stage I; TPED of 1–45°, stage II; TPED 46–90°, stage III; TPED of 91–135°, stage IV; TPED >135° [Citation17,Citation18].
Statistical analysis
Raw data for the statistical analyses were available from the trial ‘Safety and Efficacy of Two Concurrent Injections of AA4500 in Adult Subjects With Multiple Dupuytren’s Contractures’ (NCT01674634, [Citation13]) by kind permission of Endo Pharmaceuticals. The change in the total FFC after treatment was calculated and presented for previously untreated patients. Results are presented for patients with two treated joints in the same finger by Tubiana stage (Tubiana I to IV) and by joint type (MP, PIP). An ANOVA was performed to test for any significant differences in treatment outcome in the different Tubiana stages.
Results
Demographics
Previously untreated patients who received two concurrent injections of CCH in cords affecting MP and PIP joints in the same finger were subject of this post hoc analysis (n = 181). The demographics and baseline characteristics were similar to that of all previously untreated patients and to the total population, which has been published previously [Citation13]. Most subjects were male (85%), white (98%) and the mean (SD) age was 64.1 (9.9) years ().
Table 1. Demographics and baseline characteristics of previously untreated patients with Dupuytren disease.
Improvement in total FFC by Tubiana stage after CCH treatment
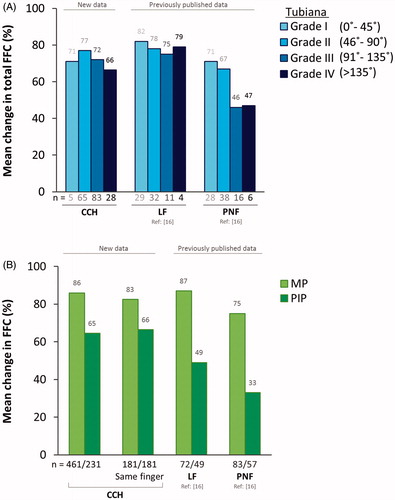
To investigate if CCH treatment efficacy is similar independent of disease advancement, previously untreated patients who received two concurrent injections in two affected joints in the same finger (one injection per joint, 1MP:1PIP) were assessed by Tubiana stage (). The mean (SD) improvement in FFC, 31 days after treatment, was in the same range for all Tubiana stages: 71.1 (36.5)% for Tubiana I, 77.0 (21.0)% for Tubiana II, 72.0 (20.4)% for Tubiana III and 66.4 (22.2)% for Tubiana IV, indicating similar treatment outcome independent on disease severity. An ANOVA analysis showed no statistically significant differences in the treatment outcome between the different Tubiana groups (p = .17). The overall mean (SD) improvement in FFC independent of Tubiana stage was 72.9 (21.5)% (), which is in the same range as the improvement among previously treated patients, 70.6 (23.1)% (n = 169) (Supplementary Table 1). It is also in the same range as the total population in the original publication who had two treated joints in the same finger, 72 (22)% (n = 350), and included both previously treated and untreated patients [Citation13].
Table 2. Change in total FFC following two concurrent injections of collagenase Clostridium histolyticum (one injection per joint; 1MP:1PIP) in the same finger in previously untreated patients with Dupuytren disease divided by Tubiana stage.
Treatment efficacy in different joints
MP joints generally have greater treatment success than PIP joints, therefore individual joint analyses were performed [Citation9]. The mean (SD) improvement in total FFC, 31 days after CCH treatment of MP and PIP joints in the same finger, was 82.5 (24.8)% for MP joints (n = 181) and 66.4 (27.9)% for PIP joints (n = 181). A similar level of improvement was observed when including all MP and PIP joints in the analysis, independent on if MP and PIP were located in the same finger or not (N = 346, MP [n = 461], PIP [n = 231]) ().
Table 3. Change in total FFC following collagenase Clostridium histolyticum treatment of MP and PIP joints in previously untreated patients with Dupuytren disease.
Discussion
In this post hoc analysis, we present data indicating that CCH is effective in all severities of Dupuytren disease. Due to the absence of any clinical trial comparing the different treatment options for this disease, we have selected subgroups to closely match that of a previously published randomized prospective study on LF and PNF, using similar definitions, in order to evaluate the short-term treatment outcome of available treatments.
In the PNF and LF study that included previously untreated patients with Dupuytren disease, the majority of the fingers (PNF: 75% and LF: 80%) were in Tubiana I or II, while the previously untreated population in the CCH study included patients with more severe disease stages (only 39% in Tubiana I or II). To take this difference in disease stage into consideration, we analyzed the total FFC by Tubiana stage. The average improvement in total FFC after CCH treatment in the population of fingers with two treated joints ranged from 66% to 77% between Tubiana stages. This is similar, or slightly lower, than the average improvement previously reported after LF treatment that ranged from 75% to 82% (). This new information reveals that some patients traditionally considered suitable for surgery might also be well suited for CCH treatment.
Figure 1. Comparison of CCH, LF and PNF treatment efficacy. (A) Mean change in total FFC (%) 31 days after CCH treatment (previously untreated patients, 1MP:1PIP in the same finger) or 6 weeks after LF and PNF treatment (previously untreated patients, overall joint distribution of 3MP:2PIP), by Tubiana grade. LF and PNF measures include DIP joint assessment and therefore represent TPED values. (B) Mean change in FFC (%) in MP and PIP joints 31 days after CCH treatment or 6 weeks after LF and PNF treatment. CCH: collagenase Clostridium histolyticum; FFC: fixed flexion contracture; LF: limited fasciectomy; MP: metacarpophalangeal; PIP: proximal interphalangeal; PNF: percutaneous needle fasciotomy; TPED: total passive extension deficit.
Our post hoc analysis strived to be as similar as possible to the randomized controlled trial of LF and PNF is one way of reanalyzing data to optimize the use of available patient level data. However, there are other means of indirectly comparing treatment options where patient level data cannot be accessed. The conclusion of this post hoc analysis is confirmed by the results from a meta-analysis of 10 studies comparing the same three treatment options concluding an equivalent clinical efficacy in the short and medium term for CCH and fasciectomy [Citation19].
PNF is generally more favorable for fingers in Tubiana I and Tubiana II () [Citation16]. The comparable outcome of CCH and PNF treatment in lower Tubiana stages is in line with other studies focusing on less severe cases of Dupuytren disease [Citation14,Citation20,Citation21]. A limitation of the post hoc analysis presented here is the lack of data for DIP joints; instead the total FFC for MP and PIP was used as an approximation of TPED. A consequence of this approach of approximating TPED, where the population was selected for having both MP and PIP joints treated in the same finger, is the relatively high disease severity in such group. The milder forms of the Dupuytren disease typically involve only one joint, explaining the low number of patients in Tubiana 1 (n = 5) in this post hoc analysis, which possibly makes the data in this group less representative of the actual patient population. However, the successful treatment of CCH in a population with mild disease severity and with a representative joint distribution has been confirmed in earlier studies [Citation22,Citation23].
Joint distribution can be a factor to consider when interpreting results from different studies. Tubiana I usually involves only one joint, and since MP joints typically have better treatment outcomes than PIP joints, it is important to consider the study population in detail when comparing data from different studies. The more severe stages of Dupuytren disease typically span several adjacent joints with varying degree of flexion extension deficit. To take this in consideration a joint-specific comparison of the available treatment options was performed. The improvement of MP joints was similar between the different treatment options, ranging from 75% to 87%, while the improvement of PIP joints was lower and varied more, ranging from 33% to 66% ().
This post hoc analysis is limited to an indirect comparison of short-term outcome. The long-term outcome is also of interest. However, this is even more complicated to compare. The study on LF and PNF included evaluation of long-term efficacy showing 21% overall recurrence rate for LF and 85% for PNF within five years. The retreatment rates were 10% for LF, 63% for PNF and 33% of the PNF patients needed a third medical intervention during the five years [Citation12,Citation24]. The recurrence rate for CCH is studied in 644 patients and the rate of recurrence was 47% within five years in previously successfully treated joints and the retreatment rate was 16% for successfully treated joints and 18% for all treated joints (196 of 1081). There are currently two randomized clinical trials published with one year follow-up data and one with two year follow-up data for PNF versus CCH, showing similar outcome between the groups. However, due to relatively small populations, low disease severity and mainly MP joints [Citation14,Citation20,Citation25], data may not be representative for the normal distribution of patients with Dupuytren disease. The definitions of recurrence may also not be equal between studies. Some study-specific joints, others study whole fingers and some studies do not take retreatments in consideration [Citation16].
Local treatment strategies, previous training, treatment safety profile, funding situation and the experience of the treating physician are factors influencing the choice of treatment in Dupuytren disease. Patient-related considerations include age, disease severity, the presence of diathesis [Citation26], concomitant diseases and the risk of recurrence and wound infection [Citation5,Citation27,Citation28], time of recovery and ultimately the patient’s preference. Elderly patients, who have slow progression of contracture and concomitant diseases, might benefit from one of the less invasive treatment options. In addition, the underlying status of the affected joints, skin condition, cord location, cord size, cord thickness, potential nerve involvement and scarring from previous treatments all influence the decision.
All of these factors, and the potential risks and complications of each procedure, are important to consider when choosing the optimal treatment for an individual patient. However, the lack of randomized controlled clinical trials evaluating the relative effectiveness of the different treatments in different disease stages makes it difficult to evaluate therapy alternatives. While the results of our analysis are restricted to the limits of the methodology, this is a post hoc analysis of a large, prospective open-label trial, controlled to support the label expansion of two instead of only one injection of CCH in the same hand [Citation13]. Raw data were stratified into relevant populations allowing for comparison to the study on LF and PNF [Citation16]. Our data suggest that CCH is a valid treatment option for all Tubiana stages, including patients with more advanced disease.
Ethical approval
Informed consent was provided by the patients in the original study. Since this is a post hoc analysis, any further details on this is not provided here.
Disclosures statement
GM Pess: Advisory Board for Sobi, Speaker Bureau for Endo Pharmaceuticals and royalties from Biomet, Inc.
L Dahlin: investigator in the trial of the post hoc analysis presented in this manuscript and in a trial sponsored by Polyganics, Netherlands.
M Sundbom, K Wilson and D Lindqvist: employees of Sobi and holders of equity interest in Sobi.
Supp_Table_1.docx
Download MS Word (31.7 KB)Acknowledgements
We thank Tine Weis (Gentofte Hospital, Hellerup, Denmark), Frederik Verstreken (Ortopedisch Centrum Antwerpen, Belgium) and Zsolt Szabo (BAZ University County Teaching Hospital, Miskolc, Hungary) for valuable discussions and participation in an advisory board meeting regarding treatment of Dupuytren disease. We also thank Hans Olivecrona (Sobi, Sweden) for continuous intellectual contribution and support during this project and Kristina Lindsten (Sobi, Sweden) for medical writing support in accordance with good publication practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Additional information
Funding
References
- Gudmundsson KG, Arngrı́msson R, Sigfússon N. Epidemiology of Dupuytren's disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;153:291–296.
- Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren's contracture in the Erlangen University Hospital–a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60. PubMed PMID: 17610744; PubMed Central PMCID: PMCPMC1950875.
- Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren's contracture in men and women. J Hand Surg Br. 1999;24:456–459.
- Larsen S, Krogsgaard DG, Aagaard Larsen L, et al. Genetic and environmental influences in Dupuytren's disease: a study of 30,330 Danish twin pairs. J Hand Surg Eur Vol. 2015;40:171–176.
- Eckerdal D, Nivestam A, Dahlin LB. Surgical treatment of Dupuytren's disease - outcome and health economy in relation to smoking and diabetes. BMC Musculoskelet Disord. 2014;15:117.
- Huisstede BM, Hoogvliet P, Coert JH, et al. Dupuytren disease: European hand surgeons, hand therapists. And Physical Medicine and Rehabilitation Physicians Agree on a Multidisciplinary Treatment Guideline: results from the HANDGUIDE Study. Plast Reconstr Surg 2013;32:964e–976e.
- Dias J, Bainbridge C, Leclercq C, et al. Surgical management of Dupuytren's contracture in Europe: regional analysis of a surgeon survey and patient chart review. Int J Clin Pract. 2013;67:271–281.
- Denkler K. Surgical complications associated with fasciectomy for dupuytren's disease: a 20-year review of the English literature. Eplasty 2010;10:e15.
- Crean SM, Gerber RA, Le Graverand MP, et al. The efficacy and safety of fasciectomy and fasciotomy for Dupuytren's contracture in European patients: a structured review of published studies. J Hand Surg Eur Vol. 2011;36:396–407.
- Pess GM, Pess RM, Pess RA. Results of needle aponeurotomy for Dupuytren contracture in over 1,000 fingers. J Hand Surg Am. 2012;37:651–656.
- McMillan C, Binhammer P. Steroid injection and needle aponeurotomy for Dupuytren disease: long-term follow-up of a randomized controlled trial. J Hand Surg Am. 2014;39:1942–1947.
- van Rijssen AL, Werker PM. Percutaneous needle fasciotomy for recurrent Dupuytren disease. J Hand Surg Am. 2012;37:1820–1823.
- Gaston RG, Larsen SE, Pess GM, et al. The efficacy and safety of concurrent collagenase Clostridium Histolyticum injections for 2 dupuytren contractures in the same hand: a prospective, multicenter study. J Hand Surg Am. 2015;40:1963–1971.
- Scherman P, Jenmalm P, Dahlin LB. One-year results of needle fasciotomy and collagenase injection in treatment of Dupuytren's contracture: a two-centre prospective randomized clinical trial. J Hand Surg Eur Vol. 2016;41:577–582.
- Werker PM, Pess GM, van Rijssen AL, et al. Correction of contracture and recurrence rates of Dupuytren contracture following invasive treatment: the importance of clear definitions. J Hand Surg Am. 2012;37:2095–2105.
- van Rijssen AL, Gerbrandy FS, Ter Linden H, et al. A comparison of the direct outcomes of percutaneous needle fasciotomy and limited fasciectomy for Dupuytren's disease: a 6-week follow-up study. J Hand Surg Am. 2006;31:717–725.
- Tubiana R, LC, Hurst LC, Badalamente MA, M, E. Dupuytren’s disease. Martin Dunitz, London. 2000;ISBN 1-85317-475-0.
- Bainbridge C, Dahlin LB, Szczypa PP, et al. Current trends in the surgical management of Dupuytren's disease in Europe: an analysis of patient charts. Eur Orthop Traumatol. 2012;3:31–41.
- Sanjuan-Cervero R, Carrera-Hueso FJ, Vazquez-Ferreiro P, et al. Efficacy and adverse effects of collagenase use in the treatment of Dupuytren’s disease: a meta-analysis. Bone Joint J. 2018;100-B:73–80.
- Strömberg J, Ibsen-Sörensen A, Fridén J. Comparison of treatment outcome after collagenase and needle fasciotomy for dupuytren contracture: a randomized, single-blinded, clinical trial with a 1-year follow-up. J Hand Surg Am. 2016;41:873–880.
- Zhou C, Hovius SER, Pieters AJ, et al. Comparative effectiveness of needle aponeurotomy and collagenase injection for dupuytren's contracture: a multicenter study. Plast Reconstr Surg Glob Open. 2017;5:e1425.
- Gilpin D, Coleman S, Hall S, et al. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren's disease. J Hand Surg Am. 2010;35:2027–2038.
- Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren's contracture. N Engl J Med. 2009;361:968–979.
- van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren's disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469–477.
- Skov ST, Bisgaard T, Sondergaard P, et al. Injectable collagenase versus percutaneous needle fasciotomy for dupuytren contracture in proximal interphalangeal joints: a randomized controlled trial. J Hand Surg Am. 2017;42:321–328.
- Degreef I, De Smet L. Risk factors in Dupuytren’s diathesis: is recurrence after surgery predictable? Acta Orthop Belg. 2011;77:27–32.
- Atroshi I, Nordenskjold J, Lauritzson A, et al. Collagenase treatment of Dupuytren's contracture using a modified injection method: a prospective cohort study of skin tears in 164 hands, including short-term outcome. Acta Orthop. 2015;86:310–315.
- Riley KN, Herman IM. Collagenase promotes the cellular responses to injury and wound healing in vivo. J Burns Wounds 2005;74:e8.
