Abstract
The aim of this study was to compare inflammatory response and synovial metaplasia in implant-based immediate breast reconstruction with a biological mesh (Veritas®) with that of a synthetic mesh (TIGR® Matrix Surgical Mesh). We hypothesize that the inflammatory response and formation of synovial metaplasia might be different and the rate of capsular contracture therefore different. The patients were recruited from the Gothenburg TIGR®/Veritas® Study (ClinicalTrials.Gov identifier NCT02985073). All referrals for bilateral immediate breast reconstruction were assessed for inclusions. During the operation, the patients were randomized to which sides the biological and the synthetic mesh were going to be applied. During the implant exchange biopsies were taken. Biopsies were taken from 30 breasts in 15 patients. There seem to be more myofibroblast and neovascularization in the biological meshes than in the synthetic and the collagen fibers seem to be aligned in an irregular pattern with both parallel and vertical fibers. In the synthetic meshes, there were more giant cells and foreign body reaction and the collagen fibers were loosely and well aligned, oriented parallel to the surface of the implant. Synovial metaplasia was seen in the majority of both the biological and the synthetic meshes. The histological patterns in early capsules from biological and synthetic meshes vary considerably. Nonetheless, it is unknown what role different cell types have in capsular formation in the long run and there was no difference in clinical capsular contracture at the clinical follow-up in this study.
Introduction
Meshes and matrices were introduced in breast surgery in 2001 [Citation1]. They quickly became popular and are now used in the majority of implant based immediate breast reconstructions [Citation2]. The first meshes were biological and called acellular dermal matrices (ADMs) [Citation2,Citation3]. More recently, synthetic meshes have been introduced on the market [Citation3]. Theoretically, the mesh work as a scaffold that integrates when the patient’s own fibroblasts, myofibroblasts, lymphocytes, macrophages, granulocytes, and mast cells grow into it [Citation4]. Thus, it gives mechanical support and facilitates the wound healing by influencing cell infiltration and migration. The mechanism of integration is not fully understood [Citation5] and seems to vary between different mesh types and preparations [Citation4,Citation5]. There are no human histological studies specifically comparing integration of biological and synthetic meshes and few studies comparing the long-term clinical results [Citation3,Citation6].
The most common complication in implant-based breast reconstruction and augmentation is capsular contracture [Citation7]. It is still unclear what causes it but a multifactorial process, with an excessive inflammation and foreign body reaction, is thought to have an important role. Inflammation can lead to activation of macrophages, lymphocytes, fibroblasts, myofibroblasts, and mast cells, and the formation of fibrosis through the production of collagen and consequent shrinking of the implant capsule and capsular contracture [Citation7–9]. One of the stated advantages of meshes is that they might impede capsule formation around implants and thereby diminish the risk of capsular contracture [Citation10]. The theory is that meshes might decrease the inflammatory response [Citation11–14]. Evidence of a decreased inflammation has been seen in histological studies were inflammatory markers in biopsies from the integrated mesh and the native subpectoral pocket have been compared [Citation11–13]. The diminished inflammatory response could be explained by that the retained extracellular matrix proteins in biological meshes facilitate wound healing [Citation15], which implies that the process might be different in biological and synthetic meshes.
The development of a fibrous capsule around an implant is a dynamic process that progresses from a loosely organized to a well-organized cellular structure and finally into a fibrous sheet [Citation7,Citation16]. When an implant or tissue expander (TE) is introduced, a chronic proliferative inflammatory process is initiated around it. It starts with the formation of a synovial-like metaplastic membrane (‘synovial metaplasia’) [Citation4]. Synovial metaplasia is histologically very similar to a fibrous form of the synovial membrane found around joints, but can be up to 10 times thicker. It is thought that the implant causes friction and fibroblast inhibition resulting in a tissue response producing synovial metaplasia [Citation4]. Synovial metaplasia may have a protective effect against capsular contracture [Citation17] as it could delay the progression toward a fibrous capsule [Citation16]. Synovial metaplasia has never been studied specifically in the context of different meshes.
We hypothesize that the inflammatory response and formation of synovial metaplasia might be different when biological and synthetic meshes are used in immediate breast reconstruction and the rate of capsular contracture therefore different. The aim of this study was to compare inflammatory response and synovial metaplasia in implant-based immediate breast reconstruction with a biological mesh (Veritas®) with that of a synthetic mesh (TIGR® Matrix Surgical Mesh).
Patients and methods
Study design and sample size
The patients were recruited from the Gothenburg TIGR®/Veritas® Study (ClinicalTrials.Gov identifier NCT02985073). The Regional Ethical Committee of Gothenburg reviewed and approved the study (189-16). Procedures followed were in accordance with the Helsinki Declaration of 1964, as revised, and the Good Clinical Practice (GCP) guidelines. Personal data were treated in accordance with the General Data Protection Regulation (GDPR).
The primary outcome for the Gothenburg TIGR®/Veritas® Study is complication frequency and the sample size was calculated based on that. Histological studies similar to the present study have had a sample size of about 15–20 patients [Citation11,Citation12,Citation18].
Participants
All referrals for bilateral immediate breast reconstruction, to our department, were assessed for inclusions. The department is located in one of seven university hospitals in Sweden. At the first consultation a final assessment for eligibility was performed. All patients who met the inclusion criteria were asked for participation. Inclusion criteria were 18 years of age or older and indication for a bilateral prophylactic mastectomy and an immediate breast reconstruction. Exclusion criteria were inability to give informed consent, previous breast surgery, smoking, and BMI > 30 kilograms/meters2. Indication and surgical technique were discussed at a multi-disciplinary team (MDT) conference in all cases. All patients gave their written informed consent to participate in the study.
Surgical technique, meshes and randomization
The surgical technique has been described previously [Citation19,Citation20] and was identical in the two breasts, with the exception of the mesh used. All reconstructions were performed by the same plastic surgeons (HH or EH). Traditionally, a two-stage approach has been used for immediate breast reconstruction in our department and to avoid introducing one-stage reconstruction and mesh-based reconstruction at the same time, a two-stage approach was used in all cases. Different general surgeons performed the mastectomies. Both meshes used are degradable. Biological Veritas® Collagen Matrix (Synovis Surgical Innovations, St. Paul, MN, USA) is a xenograft made of bovine pericardium. It is composed of non-cross-linked propylene oxide-treated acellular collagen matrix [Citation21–23]. Synthetic TIGR® Matrix Surgical Mesh (Novus Scientific, Uppsala, Sweden) is knitted from two types of fibers: a fast degrading copolymer between glycolide and trimethylene carbonate and a slow-degrading copolymer between lactic and trimethylene carbonate. The fast degrading part gives extra strength during the healing phase (4 month) and gradually becomes softer and more flexible. The slow-degrading part is completely resorbed after about three years [Citation24]. In all cases an anatomical tissue expander (TE) (CPX®, Mentor Worldwide LLC, CA, USA) was used. It was exchanged for a permanent implant about three months after the initial operation. Two suction drains were used for each breast, one subpectoral and one subcutaneous. The drains were kept in place until the output was less than 40 ml per 24 h. Prophylactic perioperative and postoperative antibiotics (cloxacillin or clindamycin, in case of allergy) were given until the drains were removed.
During the operation, the patients were randomized to which sides the biological and the synthetic mesh were going to be applied. The design was parallel and the intended allocation ratio in the groups was 1:1. The allocation sequence was concealed and a sealed envelope process and a simple randomization approach were used. The patients were blinded to on which side they had which mesh.
Clinical data collection
Collected demographic data included age at surgery, complications, time between the first operation and the implant exchange (i.e. time after implantation the biopsies were taken), and if the mesh was clinically integrated at the second operation. The clinical presence of capsular contracture was evaluated by two surgeons (HH and EH) and classified according to Baker [Citation25].
Biopsies and histological analyses
During the implant exchange, one or two 4 mm full-thickness intra-operative punch biopsies were taken by the same surgeons (HH or EH). They were taken from the periprosthetic capsule, at the interface between the integrated mesh and the native subpectoral pocket, in each breast. The biopsies were fixed immediately in 10% neutral buffered formalin, paraffin-embedded, sectioned in 5 μm and stained with hematoxylin/eosin and trichrome –Masson to visualize connective tissue. The sections were examined by Nikon light microscope. The same pathologist (PB) performed all evaluations and was blinded to where the tissue sections had been taken.
The morphological evaluation was based on the presence of polymorphonuclear cells (both neutrophils and eosynophils), lymphocytes, plasma cells, macrophages, giant cells, neovascularisation, and foreign body reaction. Collagen fibers were evaluated for thickness and orientation of collagen fibers [Citation17]. Synovial metaplasia (SM) was evaluated morphologically as ‘early appearing’, ‘mature looking’ and ‘developed, hyalinized form’ [Citation16,Citation17].
Immuno-histochemical evaluation was performed with monoclonal antibody for α-smooth muscle actin (α- SMA), a marker of myofibroblast (Clone 1A4, DAKO, Glostrup, Denmark). Immunohistochemistry was performed using Ventana Benchmark Nova Ultra system and assessed with semi-quantitative scoring system based on a 4-point graded scale, where 0 = no staining, 1 = minimal/mild staining, 2 = moderate staining, and 3 = severe staining [Citation11].
Statistics
Descriptively, frequencies were given for continuous variables. The morphological evaluations were considered interval scales. Differences between samples from synthetic and biological meshes were analyzed using non-parametric Wilcoxon signed-rank test for related samples as the two samples came from the same patient. All tests were two-tailed and a p-value of .05 or less was considered statistically significant. All analyses were performed with IBM SPSS Version 25 for Mac (SPSS Inc Chicago, IL, USA).
Results
Biopsies were taken from 30 breasts in 15 patients. All of the cases were prophylactic cases and none of the patients had received radiotherapy. Each patient had one breast operated on with synthetic mesh and one with biological mesh and at least one biopsy was taken from each side. Median age at the first operation was 35.6 years (min 24.7 and max 58.4 years). Two patients were operated on with Wise-pattern mastectomies and the rest via a sub-mammary incision. The nipple areolar complex was preserved in all cases. Median mastectomy weight was 267.5 grams (min 70 and max 544 grams). Time between mesh insertion and biopsy was a median of 111 days (min 70 and max 145 days). Between the insertion and the biopsy one patient needed a seroma puncture on the side with synthetic mesh. During the operations all meshes were well integrated macroscopically and there was no evidence of clinical infection in any case. In all patients, the seroma formation was more pronounced on the biological side. The clinical follow-up after the implant exchange was a median of 520 days (range 188 to 679 days). All breasts were evaluated as Baker class I or II.
Morphology
Histological investigation revealed a range of histological changes seen between specimens and broadly three patterns of tissue reaction, labeled type A-C, can be described: Type A: Most prominently characterized by a thin capsule with a network of loosely and well aligned collagen fibers, oriented parallel to the surface of the implant. Immunohistochemistry demonstrated either absence of myofibroblasts or a discrete network of parallel, loosely arranged actin positive cells. On the implant surface synovial metaplasia was seen in practically all cases but in different stages of development, mostly ‘mature’ with a clearly visible pseudo-synovial cell layer. Also, distinct and well-organized groups of foreign material (mesh) was surrounded by heavy infiltrates of macrophages and giant cells of foreign body type. Lymphocytes, and notably infiltrates of eosinophils, were often seen. Usually no neutrophils occurred. The inflammatory infiltrates were well defined and closely attached to and around granulomas containing mesh fibers. Type B: Primarily characterized by deeper fibrosis than type A and with collagen fibers aligned in irregular pattern with both parallel and vertical fibers. Actin-positive myofibroblast were prominent, arranged in thick bundles, following the arrangement of the collagen fibers. Foreign body granulomas were either absent or loosely organized but mostly without foreign material. Inflammatory infiltrates were of variable intensity, not as well organized as in type A. Composition of inflammatory cells was similar. Even here eosinophils were often present with no neutrophils. Type C: An intermediate pattern with morphological features of both type A and B, impossible to define as either one. Typically, the fibrosis was arranged both with areas of regular collagen fibers, but also other areas of irregular collagen fiber. Foreign bodies (mesh fibers) were often seen. The most common reaction in biopsies from biological mesh breasts was type B, which was seen in 71% of the cases, and from synthetic meshes type A, which was seen in 64% of the cases (, ) (p = .022). None of the patients had the same type of reaction in both breasts. The immune-histochemical actin staining revealed that there were more myofibroblast in the biological meshes than in the synthetic (p = .211) (). Two of the biological biopsies were not possible to evaluate as there was only hyaline material in them.
Figure 1. Examples of biopsies. Masson trichrome staining, original magnification 40x (a,d); 100x (b,e); α-smooth muscle actin, original magnification 40x (c,f). (A–C) illustrates a type A reaction and d-f a type B reaction. Small arrow: synovial metaplasia. Arrow head: mesh fibers. Asterix: collagen fibers. Thick arrow: actine positive myofibroblasts.
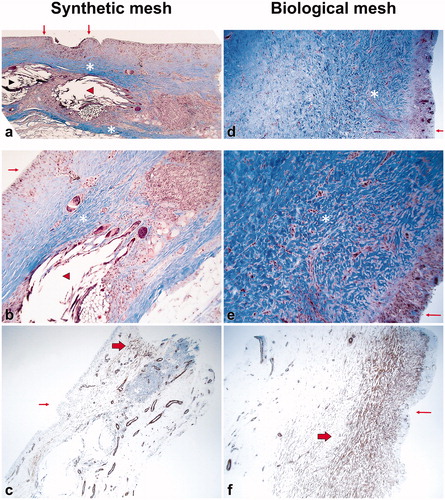
Table 1. Morphological evaluation of the biopsies.
Table 2. Result of actin immunohistostaining.
Discussion
This study compares inflammatory response and synovial metaplasia in implant-based immediate breast reconstruction with a biological mesh with that of a synthetic mesh. In brief, there seem to be more myofibroblast and neovascularization () in the biological mesh than in the synthetic and the collagen fibers seem to be aligned in an irregular pattern with both parallel and vertical fibers. In the synthetic meshes, there were more giant cells and foreign body reaction () and the collagen fibers were loosely and well aligned, oriented parallel to the surface of the implant. Synovial metaplasia was seen in the majority of both the biological and the synthetic meshes.
One of the stated advantages of meshes is a decreased risk for capsular contracture [Citation2]. It has been speculated that biological meshes give a better long-term result as they are more similar to naturally occurring extracellular matrices [Citation26] and might send signals that mimic the natural tissue environment [Citation4]. Nonetheless, such theories have not been confirmed in clinical studies, where similar capsular contraction rates have been seen [Citation27].
Animal studies [Citation10,Citation28] have suggested biological meshes give lower capsular contracture rates as they do not induce a chronic inflammation and a foreign body response and that a thinner fibrous capsule is formed. Previous human histological studies comparing biopsies from integrated meshes and native subpectoral capsule in the same patients have demonstrated that the capsule with mesh have significantly lower levels of vessel proliferation, inflammatory markers, fibrosis, myofibroblast activity, and collagen I deposition than the native capsule [Citation11–13]. One study has shown a decreased level of giant cells and foreign body granuloma in mesh capsules [Citation13], whereas another study failed to confirm this [Citation12]. Studies on different implants [Citation17,Citation29] have indicated the foreign body reaction could be lower when polyurethane-coated implants are used than when textured implant are used, which contradicts that an early strong foreign body reaction is the determining factor in long-term capsular contracture rates [Citation30]. In the present study, more foreign body reaction and giant cells (), could be seen in the synthetic meshes. The findings indicate that the tissue reaction might differ considerably or have a different time course depending on whether a biological or a synthetic mesh is used. However, it is difficult to interpret the implications for long-term capsular contracture, due to the short time between insertion of the mesh and the biopsy (median 111 days).
In previous research, myofibroblast have been found in the majority of Baker grade 3-4 contractures [Citation31–34]. It has been proposed that this is a sign of an active phase of wound contracture and the myofibroblasts diminish when the scar is mature [Citation31,Citation32]. The stronger myofibroblast reaction and scar formation seen in the biological meshes could merely indicate that it is integrated faster than the synthetic mesh. On the other hand, a study [Citation35] comparing textured and smooth expanders, where biopsies were taken during the exchange to a permanent implant, suggested that an early difference in occurrence of myofibroblast could explain later capsular contracture. In that study [Citation35], significantly more fibroblasts were seen around smooth expanders than around textured expanders. The expanders were replaced by the same textured permanent silicone implants in both groups and at a clinical two-year follow-up there were more capsular contracture in the group initially operated on with smooth expanders [Citation35]. Therefore, the initial tissue reaction, and amount of myofibroblasts, could be significant for the later capsular contracture rates. In our study, this would imply that the stronger myofibroblast reaction in the biological mesh () could be unfavorable capsule contracture wise. On the other hand, animal studies [Citation29,Citation36] have revealed a much stronger myofibroblast reaction around polyurethane-coated implants than textured implants. Polyurethane-coated implants generally have lower capsular contracture rates than textured implants [Citation30], which could suggest that an early strong myofibroblast reaction is favorable for long-term capsular contracture formation. In brief, myofibroblasts are part of an active phase of wound contracture and diminish when the scar is mature [Citation31,Citation32]. Even though abundant myofibroblasts can be found in Baker 3-4 capsules [Citation31–34], the role of a strong myofibroblast reaction in the early phases of implantation in the formation of capsular contractures remains unclear.
It has also been hypothesized that the collagen orientation plays a key role in capsular formation [Citation34]. The study [Citation35], described above, comparing textured and smooth expanders, showed that collagen fiber orientation was irregular in the capsule surrounding the smooth expanders and regularly arranged and with a more even thickness in the textured implants [Citation35]. On the other hand, highly aligned collagen fibers could lead to a greater force of contracture and capsular formation [Citation31,Citation34], whereas uncontracted capsule have more loosely arranged, multidirectional collagen fibers [Citation34]. In the present study, the collagen orientation was generally more regular in the synthetic meshes than in the biological meshes (), which could indicate that biological meshes are more favorable.
Synovial metaplasia has been proposed as a protective factor against capsular contracture [Citation17]. In the long term it occurs mostly in Baker I/II capsules [Citation34,Citation37]. However, the role of synovial metaplasia in the early tissue reaction phase remains to be fully elucidated, as it seems to be present in most early capsules [Citation29]. Our findings are in accordance with this, as synovial metaplasia could be seen in most capsules in both groups ().
A weakness of the present study is that biopsies were taken on only one occasion and therefore the course could not be studied. Therefore, it is difficult to evaluate whether the meshes give different responses or the time course merely is different. The two meshes are integrated and absorbed with different speed [Citation21–24] which affects the healing process and scar formation. The stronger myofibroblast reaction and scar formation seen in the biological meshes could indicate that it is integrated faster than the synthetic mesh.
Other weaknesses include that the patients were followed clinically for only a short period of time. The patients need to be followed longer to established the true clinical capsular contraction rate. In addition, the Baker classification for capsular contracture has never been validated and carry a subjective component. Moreover, the sample size is small.
A strength of this study is that it compares synthetic and biological meshes in the same patients and patient related factors affecting healing and scar formation should be of little importance when the results are evaluated. In addition, the same type of implants were used in all breasts, which is a strength as implant texture seems to influence the tissue reaction [Citation35]. Moreover, both histological and clinical evaluations have been performed.
In conclusion, the histological patterns in early capsules from biological and synthetic meshes vary considerably. There seem to be more myofibroblast and neovascularization in the biological mesh than in the synthetic and the collagen fibers seem to be aligned in an irregular pattern with both parallel and vertical fibers. In the synthetic meshes, there were more giant cells, inflammation, and foreign body reaction and the collagen fibers were loosely and well aligned, oriented parallel to the surface of the implant. Synovial metaplasia was seen in the majority of both the biological and the synthetic meshes. Nonetheless, it is unknown what role different cell types have in capsular formation in the long run [Citation11,Citation38] and there was no difference in clinical capsular contracture at the clinical follow-up in this study. The significance of our findings on the long-term capsular contracture frequency needs to be studied further.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Duncan DI. Correction of implant rippling using allograft dermis. Aesthetic Surg J. 2001;21(1):81–84.
- JoAnna Nguyen T, Carey JN, Wong AK. Use of human acellular dermal matrix in implant- based breast reconstruction: evaluating the evidence. J Plastic Reconstruct Aesthetic Surg. 2011;64(12):1553–1561.
- Logan Ellis H, Asaolu O, Nebo V, et al. Biological and synthetic mesh use in breast reconstructive surgery: a literature review. World J Surg Onc. 2016;14(1):121.
- Boháč M, Danišovič Ľ, Danihel Ľ, et al. Histological and immunohistochemical characteristics of capsular synovial metaplasias that form around silicone breast implants. Biologia. 2018;73(1):107–112.
- Capito AE, Tholpady SS, Agrawal H, et al. Evaluation of host tissue integration, revascularization, and cellular infiltration within various dermal substrates. Ann Plast Surg. 2012;68(5):495–500.
- Dieterich M, Stubert J, Gerber B, et al. Biocompatibility, cell growth and clinical relevance of synthetic meshes and biological matrixes for internal support in implant-based breast reconstruction. Arch Gynecol Obstet. 2015;291(6):1371–1379.
- Headon H, Kasem A, Mokbel K. Capsular contracture after breast augmentation: an update for clinical practice. Arch Plast Surg. 2015;42(5):532–543.
- Siggelkow W, Faridi A, Spiritus K, et al. Histological analysis of silicone breast implant capsules and correlation with capsular contracture. Biomaterials. 2003;24(6):1101–1109.
- Poeppl N, Schreml S, Lichtenegger F, et al. Does the surface structure of implants have an impact on the formation of a capsular contracture? Aesth Plast Surg. 2007;31(2):133–139.
- Stump A, Holton LH, 3rd, Connor J, et al. The use of acellular dermal matrix to prevent capsule formation around implants in a primate model. Plastic Reconstruct Surg. 2009;124(1):82–91.
- Leong M, Basu CB, Hicks MJ. Further evidence that human acellular dermal matrix decreases inflammatory markers of capsule formation in implant-based breast reconstruction. Aesthetic Surg J. 2015;35(1):40–47.
- Yu D, Hanna KR, LeGallo RD, et al. Comparison of histological characteristics of acellular dermal matrix capsules to surrounding breast capsules in acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg. 2016;76(5):485–488.
- Basu CB, Jeffers L. The role of acellular dermal matrices in capsular contracture: a review of the evidence. Plastic Reconstruct Surg. 2012;130(5 Suppl 2):118S–124S.
- Hille-Betz U, Kniebusch N, Wojcinski S, et al. Breast reconstruction and revision surgery for implant-associated breast deformities using porcine acellular dermal matrix: a multicenter study of 156 cases. Ann Surg Oncol. 2015;22(4):1146–1152.
- Wong AK, Schönmeyer BH, Singh P, et al. Histologic analysis of angiogenesis and lymphangiogenesis in acellular human dermis. Plastic Reconstruct Surg. 2008;121(4):1144–1152. Epub 2008/03/20.
- Ko CY, Ahn CY, Ko J, et al. Capsular synovial metaplasia as a common response to both textured and smooth implants. Plastic Reconstruct Surg. 1996;97(7):1427–1433.
- Bassetto F, Scarpa C, Caccialanza E, et al. Histological features of periprosthetic mammary capsules: silicone vs. polyurethane. Aesth Plast Surg. 2010;34(4):481–485.
- Basu CB, Leong M, Hicks MJ. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plastic Reconstruct Surg. 2010;126(6):1842–1847.
- Hallberg H, Lewin R, Bhatti Söfteland M, et al. Complications, long-term outcome and quality of life following Surgisis and muscle covered implants in immediate breast reconstruction: a case-control study with a six year follow-up. Eur J Plast Surg. 2018;42:33.
- Hallberg H, Lewin R, Elander A, et al. TIGR((R)) matrix surgical mesh – a two-year follow-up study and complication analysis in 65 immediate breast reconstructions. J Plastic Surg Hand Surg. 2018;52(4):253–258.
- Luo X, Kulig KM, Finkelstein EB, Nicholson MF, et al. In vitro evaluation of decellularized ECM-derived surgical scaffold biomaterials. J Biomed Mater Res. 2017;105(3):585–593.
- Mofid MM, Meininger MS, Lacey MS. Veritas(R) bovine pericardium for immediate breast reconstruction: a xenograft alternative to acellular dermal matrix products. Eur J Plast Surg. 2012;35(10):717–722.
- Gaertner WB, Bonsack ME, Delaney JP. Experimental evaluation of four biologic prostheses for ventral hernia repair. J Gastrointest Surg. 2007;11(10):1275–1285.
- Hjort H, Mathisen T, Alves A, et al. Three-year results from a preclinical implantation study of a long-term resorbable surgical mesh with time-dependent mechanical characteristics. Hernia. 2012;16(2):191–197.
- Spear SL, Baker JL Jr. Classification of capsular contracture after prosthetic breast reconstruction. Plastic Reconstruct Surg. 1995;96(5):1119–1123.
- Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv. 2014;32(2):462–484.
- Hallberg H, Elander A, Kölby L, et al. A biological or a synthetic mesh in immediate breast reconstruction? A cohort-study of long-term Health related Quality of Life (HrQoL). Eur J Surg Oncol. 2019;45(10):1812–1816.
- Komorowska-Timek E, Oberg KC, Timek TA, et al. The effect of AlloDerm envelopes on periprosthetic capsule formation with and without radiation. Plastic Reconstruct Surg. 2009;123(3):807–816.
- Silva EN, Ribas-Filho JM, Czeczko NG, et al. Histological evaluation of capsules formed by silicon implants coated with polyurethane foam and with a textured surface in rats. Acta Cir Bras. 2016;31(12):774–782.
- Duxbury PJ, Harvey JR. Systematic review of the effectiveness of polyurethane-coated compared with textured silicone implants in breast surgery. J Plastic Reconstruct Aesthetic Surg. 2016;69(4):452–460.
- Hwang K, Sim HB, Huan F, et al. Myofibroblasts and capsular tissue tension in breast capsular contracture. Aesth Plast Surg. 2010;34(6):716–721.
- Rudolph R, Abraham J, Vecchione T, et al. Myofibroblasts and free silicon around breast implants. Plastic Reconstruct Surg. 1978;62(2):185–196.
- Baker JL, Jr., Chandler ML, LeVier RR. Occurrence and activity of myofibroblasts in human capsular tissue surrounding mammary implants. Plastic Reconstruct Surg. 1981;68(6):905–912.
- Bui JM, Perry T, Ren CD, et al. Histological characterization of human breast implant capsules. Aesth Plast Surg. 2015;39(3):306–315.
- Kuriyama E, Ochiai H, Inoue Y, et al. Characterization of the capsule surrounding smooth and textured tissue expanders and correlation with contracture. Plastic Reconstruct Surg Global Open. 2017;5(7):e1403.
- Silva EN, Ribas-Filho JM, Tabushi FI, et al. Smooth muscle alpha actin immunoexpression (alpha-Sma) and CD-117 antibody (C-Kit) in capsules formed by polyurethane foam-coated silicone implants and with textured surface: a study on rats. Aesth Plast Surg. 2019;43(1):233–242.
- Prantl L, Schreml S, Fichtner-Feigl S, et al. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plastic Reconstruct Surg. 2007;120(1):275–284.
- Kamel M, Protzner K, Fornasier V, et al. The peri-implant breast capsule: an immunophenotypic study of capsules taken at explantation surgery. J Biomed Mater Res. 2001;58(1):88–96.
