Abstract
Degloving injuries of the fingers represent a reconstructive challenge. Even if poorly described in literature, the proximal ulnar perforator flap (PUPF), based on perforator of the anterior ulnar recurrent artery or directly on a perforator branch of the ulnar artery, meets the requested criteria for the ideal coverage. We performed a cadaveric study in order to clarify the anatomical basis and vascularization of the PUPF flap. Eight injected upper limb specimens were dissected for this study: perforators were followed down to their origin and classified in terms of number, length, diameters and distances between their emergence and specific pre-determined landmarks as the medial humeral epicondyle. At least one ulnar perforator in the proximal third of the forearm was identified in all the specimens. In 50% of the upper limbs, the perforator branch came directly from the ulnar artery, while in the 87.5% a perforator branch came from the anterior recurrent ulnar artery; in 3 out of 8 cases both perforator branches were described. Mean lengths of the perforator branch were 57.9 mm and 44.3 mm, respectively and the mean diameters measured at their origin were 0.99 mm and 1.17 mm respectively. Our data illustrate the consistency of at least one perforator branch from the proximal third of the ulnar artery, most commonly coming from the anterior recurrent ulnar artery. Considering our results, the PUPF could be a good alternative to the classical free flaps for the resurfacing of the finger defects.
Introduction
Circumferential defect associated with degloving of digit after hand trauma or oncological resection represents a challenge in terms of reconstruction. Soft tissue disruption is often associated with the exposure and possible infection of tendons, joints and bones. Local or regional flaps can only occasionally satisfy the requirements for coverage of degloved fingers and free tissue transfer should often be considered in complex cases. The ideal coverage should be pliable, sensate and aesthetically pleasing with low donor-site morbidity [Citation1,Citation2].
Despite the lesser operative time and surgical skills required, traditional local and loco-regional flaps used in finger reconstruction can be burdened by insufficient coverage firstly due to the limited flap advancement [Citation2] and can commonly lead to unaesthetic outcomes.
To date, also considering the constant microsurgical improvement, several free flap have been used in finger defects coverage [Citation3,Citation4].
While the radial aspect of the forearm has been extensively studied, there is a lack of reports regarding the ulnar aspect [Citation5]. In this regard, only few reports in the past literature described the proximal ulnar perforator flap (PUPF) as an option for the reconstruction of middle fingers or thumb [Citation6,Citation7].
The dorsal ulnar artery perforator flap was firstly proposed by Becker and Gilbert for hand coverage in order to avoid the sacrifice of an axial vessel [Citation8]. On the other hand, a reverse flow ulnar perforator flap can be harvested to cover defects of the dorsum or palm of the hand, the first interdigital space and the palmar surface of the wrist [Citation9–11].
Besides that, the PUPF, based on a perforator of the anterior ulnar recurrent artery or directly on a perforator branch of the ulnar artery, can be considered an option in proximal defect (e.g. coverage around the elbow), as pedicled flap, and a further option in fingers reconstruction, after eventually defatting procedure to adapt it to the defect size, when thin and pliable skin reconstruction is needed.
Against this background the aim was to clarify through a careful anatomic study, the anatomical basis and vascularization of the PUPF flap, in order to evaluate its feasibility for clinical use in hand digital reconstruction.
Material and methods
Between October and November 2019, eight fresh injected cadaveric upper limbs (4 left, 4 right of different specimens) were dissected for this study. The specific age, gender and nationality of the specimens were not reported. Red latex was injected into the axillary arteries 30 min before the dissection at ICLO Teaching and Research Center in Arezzo (Italy). The markings were placed as follow: a line connecting the pisiform and the medial humeral epicondyle on the volar forearm was used to divide the forearms into three equal segments (proximal, middle and distal) (). In the proximal segment, parallel of the longitudinal axis, an explorative incision around 8–10 cm length on the volar forearm was performed () and a supra fascial dissection in radio-ulnar direction under 4.0× loupes magnification was conducted to visualize the perforators. Each perforator was tracked from the skin to the origin of the ulnar artery. The number of perforators, length, diameter (at the origin and perforating point) and distances between the origin or cutaneous end point and the medial humeral epicondyle were recorded in the proximal third (). Measurements were realized on the photographic material with ImageJ Fiji software [Citation12], while the statistical analysis was performed with GraphPad Prism 8 (GraphPad Software, SanDiego, CA, USA.
Figure 1. (A) Schematic view of the forearm three segments division. The circles in red highlights the proximal third which is the object of investigation in our anatomical study. P: pisiform bone; ME: medial epicondyle. (B) A volar side, skin incision, parallel of the longitudinal axis of the forearm, was performed in the proximal third. A supra fascial dissection in radio-ulnar direction under 4.0× loupes magnification was conducted to visualize the perforators. FRC: flexor carpi radialis muscle; FUC: flexor carpi ulnaris muscle; ME: medial epicondyle; *: perforator branch. Scale bar (1 cm).
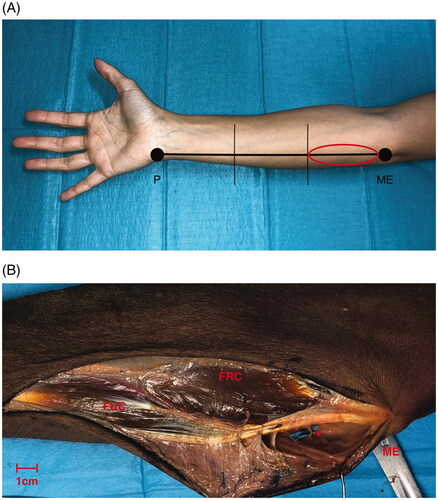
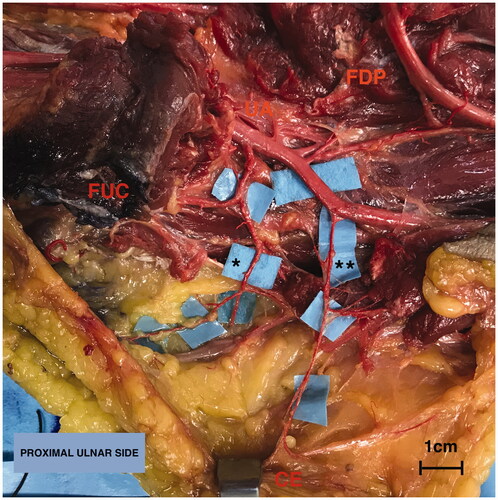
Figure 2. Anatomy of the upper limb in the proximal third (volar side) focusing on the ulnar artery (UA). Overturning proximally the insertion of the flexor carpi ulnaris muscle (FUC), the first tract of the UA is exposed running on the flexor digitorum profundus muscle (FDP): it gives firstly the recurrent branches on the ulnar edge, following by the common ulnar interosseous division on the radial side. Secondly, between its the proximal and middle third the UA gives a further branch on the ulnar side (**). Legend: (*) Anterior recurrent ulnar perforator artery, (**) direct ulnar perforator artery, cutaneous end point (CE), flexor carpi ulnaris muscle (FUC) and flexor digitorum profundus muscle (FDP). Scale bar (1 cm).
Results
In all specimens, at least one ulnar perforator was identified. In almost all the cases it was an intramuscular perforator through the flexor carpi ulnaris (50%) or flexor digitorum superficialis muscles (37.5%). Only in one specimen, septocutaneous course of one perforator was reported.
In 4 out of 8 upper limbs (50%) the perforator branch came directly from the ulnar artery. In one case (12.5%) the perforator originated directly from the brachial artery just above the ulnar fold (). In 7 out of 8 cases (87.5%) a perforator branch came from the anterior ulnar recurrent artery, while in 3 out of 8 specimens both of the perforator branches were described ().
Figure 3. Single case (one out eight specimens) in which the perforator (*) originated directly from the brachial artery above the ulnar fold. The red string indicates the brachial artery (BA). Flexor carpi ulnaris muscle (FUC). Scale bar (1 cm).
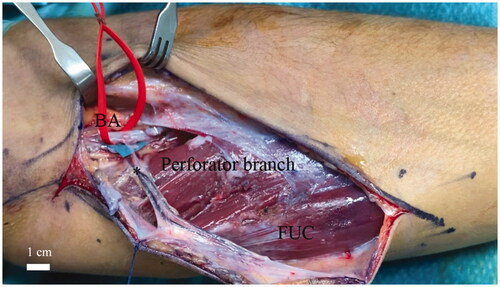
Table 1. Number of perforators, diameters at the origin and at the perforator point of the ulnar perforator or anterior recurrent ulnar perforator artery in the eight injected specimens dissected.
The mean lengths of the perforator branch coming from ulnar artery and anterior ulnar recurrent artery were 57.9 mm and 44.3 mm, respectively. The average distances between the medial humeral epicondyle (ME) and the origin of the perforators (O) from the main vessel were reported as 94.6 mm and 62.4 mm in the direct ulnar artery pattern and/or anterior recurrent artery pattern, respectively. The mean distances between medial humeral epicondyle and the cutaneous end point (CE) were 108.4 mm and 74.6 mm in the direct ulnar artery pattern and/or anterior recurrent artery pattern, respectively ().
Table 2. Distances between the origin and medial humeral epicondyle or distances between the cutaneous end point and medial humeral epicondyle and the pedicle length for each type of perforator (direct ulnar perforator or anterior recurrent ulnar perforator) in the eight injected specimens dissected.
The mean diameters measured at the origin (OP) of the perforator were 0.99 mm and 1.17 mm in the direct ulnar artery pattern and/or anterior recurrent artery pattern, respectively, while the average diameters measured at the perforating point (PP) were 0.70 mm and 0.84 mm respectively ().
No significant differences between both perforators were found for the diameter at the perforating point and the pedicle length (Mann-Whitney test p value > 0.05), while significant differences were mentioned for the vessel diameters at the origin point and distances from the medial epicondyle (Mann-Whitney test, p value < 0.05).
Detailed anatomical measurements are provided in and .
Discussion
The reconstruction of soft tissue defects of the hand continues to be a challenge for plastic surgeons. The ideal reconstruction should be functional, minimizing the donor-site morbidity, avoiding the sacrifice of main vascular pedicles and ideally reducing the hospitalization and recovery time [Citation1].
A variety of local and free flaps, including both muscular and fascial, have been proposed in hand lesions reconstruction. Moreover, the forearm is an ideal source of perforator flaps which have the advantages of being thin, pliable skin for hand and finger coverage and avoiding the sacrifice of the main vessels due to peripheral dissection [Citation13].
Focusing on the ulnar aspect of the forearm, according to Sun et al. two main clusters of perforators along the entire ulnar artery, one in the proximal third and the other one in the distal fourth of the forearm, were identified.
These two groups of perforators give cutaneous branches through the flexor digitorum superficialis and the flexor carpi ulnaris muscle space, allocated at 45.7 mm proximally to the pisiform bone and at 77.3 mm distally to the medial epicondyle, with an average diameter of 0.75 mm and a mean pedicle length of 14–6 mm [Citation14].
In the majority of the cases the ulnar artery along its length gives musculocutaneous (69%) and septocutaneous (31%) perforators [Citation6]: however, our data, limited to eight specimens, underlined the predominant intramuscular course [Citation15].
Similarly, to our findings, Wei et al. stated that on average, two perforators emerged in the proximal third from the ulnar anterior recurrent artery with an average vessel diameter of 0.8 mm. In 85% of the cases (in a total of 35 cases) another perforator (mean diameter 0.7 mm) coming from the ulnar artery was visualized at the junction of the proximal and middle third of the distance between the pisiform and medial epicondyle [Citation6].
Altogether, the same authors found on average seven perforators coming from the entire course of the ulnar artery between the medial epicondyle and the pisiform with a mean pedicle length of 27 mm and each diameter greater than 0.5 mm [Citation6].
In our experience we identified one perforator arising directly from the ulnar artery in the passage between the proximal and middle third in 50% of the cases and one perforator coming from the anterior ulnar recurrent artery (87.5% of the cases). In 3 out of 8 specimens (37.5%%) both of these perforators were present. Moreover, we observed that the proximal skin of the forearm was perfused by a perforator arising from the brachial artery in one case (12.5%). The diameter of the direct perforator at the origin point from the proximal third of the ulnar artery was in mean 1 mm (range 0.92−1.18 mm) with an average length of 57.9 mm (range 46.7−64.6 mm). The external diameter of the perforator of anterior ulnar recurrent artery at the origin point resulted in mean 1.17 mm (range 0.95−1.29 mm) with an average length of 44.3 mm (range 23.6−83.3 mm).
Conversely, the average external diameter at the perforating point was reported as 0.70 mm (range 0.52−0.85 mm) and 0.84 mm (range 0.63−0.98 mm) respectively.
The distances between the origin and the medial humeral epicondyle were 94.6 mm (range 73.1−125.8 mm) and 62.4 mm (range 34.6−94.1 mm) respectively for the direct ulnar and the ulnar recurrent artery perforators.
In this work we evaluated a comparison between the two main ulnar artery perforators of the proximal third, specifically characterizing the by number, diameters at the origin and perforating point, pedicle length and distances of the vessel from the origin or cutaneous termination to the medial humeral epicondyle. No significant differences between both perforators were found for the diameter at the perforating point and the pedicle length (Mann-Whitney test, p value > 0.05). On the contrary, significant differences between the diameter at the origin and distances previously mentioned, were evidenced (Mann-Whitney test, p value < 0.05).
The main advantages of the PUPF are that it can be harvested under locoregional anesthesia and it preserves the main vascular supply (ulnar artery). The PUPF has ideal texture, thinness and hairlessness for finger soft-tissue reconstruction and it could be raised as sensate flap by including the medial cutaneous nerve [Citation16]. A superficial vein should always be included in the flap as venous drainage, due to frequent mismatch between the size of the comitantes veins perforator and the receiving dorsal veins of the finger. The superficial vein of the forearm, which has a more suitable diameter, could be easily dissected and include in the flap [Citation11,Citation17].
Primary closure of the donor site is generally possible with defects of less than 4 cm in width. Bigger skin paddles will need skin grafting but will still have a cosmetically and functionally better outcome than the radial forearm or distal ulnar perforator flap in which skin grafting relies on the tenosynovium tissue [Citation6,Citation18,Citation19]. On the other hand, the PUPF flap requires advanced microsurgical skill technique, both for the intramuscular or suprafascial dissection as well as for vascular anastomoses. The small dimensions and the short length of the vascular pedicle represent the major limits of the application of this free flap in non-hand reconstruction scenarios. Specifically, the diameters of the dominant digital artery are comparable with the perforator diameters of our findings and a further distally dissection of the ulnar or radial dominant digital artery can overcome the shorter perforator pedicle length.
Preoperatively, we recommend a proper imaging investigation as a Color Doppler ultrasonography to identify the perforating vessels (existence, position, diameter and blood flow velocity).
In case of dimensions mismatch with the recipient vessels or damage of the vessel during dissection and harvesting, a convention ulnar flap, more proximal or distal can always be a backup plan, however it will sacrifice one of the main vessels in forearm.
The proximal ulnar artery perforator flap is a thin, pliable and non-hairy skin paddle with minimal donor-site morbidity and constant blood supply that can offer an ideal resurfacing for digital defect in both volar and dorsal sides [Citation1,Citation14]. The present anatomical results may be helpful in safely planning the harvesting and design of the proximal ulnar perforator flap as it can be considered a good surgical alternative to the classical free flaps described in the current literature for the resurfacing of the finger defects.
Conclusion
In this study we illustrate the consistent anatomy of at least one perforator branch from the proximal third of the ulnar artery, commonly coming from the anterior recurrent artery, a collateral of the ulnar artery.
The proximal ulnar artery perforator flap could represent a valid alternative to the classical free flaps described in the current literature for the soft tissue finger defects.
Institutional review approval
This study was designed and registered within the internal database held in the Microsurgery and Hand Surgery unit, ASST ‘Sette Laghi’, University of Insubria, Varese, Italy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cherubino M, Corno M, Valdatta L, et al. Thumb reconstruction with thin proximal ulnar perforator free flap. J Hand Surg Am. 2017;42(2):e133–e138.
- Tang JB, Elliot D, Adani R, et al. Repair and reconstruction of thumb and finger tip injuries: a global view. Clin Plast Surg. 2014;41(3):325–359.
- Arnež ZM, Ramella V, Papa G, et al. Is the LICOX® PtO2 system reliable for monitoring of free flaps? Comparison between two cohorts of patients. Microsurgery. 2019;39(5):423–427.
- Horta R, Silva P, Costa-Ferreira A, et al. Microsurgical soft-tissue hand reconstruction: an algorithm for selection of the best procedure. J Hand Microsurg. 2011;3(2):73–77.
- Guimberteau JC, Goin JL, Panconi B, et al. The reverse ulnar artery forearm island flap in hand surgery: 54 cases. Plast Reconstr Surg. 1988;81(6):925–932.
- Wei Y, Shi X, Yu Y, et al. Vascular anatomy and clinical application of the free proximal ulnar artery perforator flaps. Plast Reconstr Surg Glob Open. 2014;2(7):e179.
- Xiao C, Bao Q, Wang T, et al. Clinical application and outcome of the free ulnar artery perforator flap for soft-tissue reconstruction of fingers in five patients. Plast Reconstr Surg. 2013;131(1):132e–133e.
- Becker C, Gilbert A. [The ulnar flap]. Handchir Mikrochir Plast Chir. 1988;20:180–183.
- Unal C, Ozdemir J, Hasdemir M. Clinical application of distal ulnar artery perforator flap in hand trauma. J Reconstr Microsurg. 2011;27(9):559–565.
- Vergara-Amador E. The retrograde ulnar dorsal flap: surgical technique and experience as island flap in coverage of hand defects. Tech Hand up Extrem Surg. 2015;19(3):90–94.
- Liu J, Zheng H. Free distal ulnar artery perforator flaps for the reconstruction of a volar defect in fingers. J Plast Reconstr Aesthet Surg. 2014;67(11):1557–1563.
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675.
- Chen SH, Xu DC, Tang ML, et al. Measurement and analysis of the perforator arteries in upper extremity for the flap design. Surg Radiol Anat. 2009;31(9):687–693.
- Sun C, Hou ZD, Wang B, et al. An anatomical study on the characteristics of cutaneous branches-chain perforator flap with ulnar artery pedicle. Plast Reconstr Surg. 2013;131:329–336.
- Yu P, Chang EI, Selber JC, et al. Perforator patterns of the ulnar artery perforator flap. Plast Reconstr Surg. 2012;129(1):213–220.
- Jawad AS, Harrison DH. The island sensate ulnar artery flap for reconstruction around the elbow. Br J Plast Surg. 1990;43(6):695–698.
- Cherubino M, Bolletta A, Baroni T, et al. Anatomical study and clinical application of ulnar artery proximal perforator flaps. J Reconstr Microsurg. 2020.DOI: https://doi.org/10.1055/s-0040-1716321
- Mathy JA, Moaveni Z, Tan ST. Perforator anatomy of the ulnar forearm fasciocutaneous flap. J Plast Reconstr Aesthet Surg. 2012;65(8):1076–1082.
- Hakim SG, Trenkle T, Sieg P, et al. Ulnar artery-based free forearm flap: review of specific anatomic features in 322 cases and related literature. Head Neck. 2014;36(8):1224–1229.
