Abstract
Introduction
Infection by Mycobacterium ulcerans constitutes a neglected tropical disease whose prevalence seems to have overrun those of cutaneous tuberculosis and leprosy. Its aggressivity depends on a mycolactone toxin. Lesions may involve skin, tendon and bone with a large spectrum of manifestations: non-ulcerative (papules, nodules, plaques), ulcerative and oedematous presentations as well as osteomyelitis with muscular contraction and ankylosis. Upper limbs account for more than two thirds of the infection sites. Surgical treatment may involve tendon transpositions, partial and total skin grafts. Amputation is relegated to extreme cases.
Material and methods
Selected iconography from patients during the last 15 years is presented. At least 1500 cases had partial skin grafts (anterior thigh). Total skin grafts (inguinal region) were used in about 200 cases. Complex lesions involved 9 ilioinguinal flaps (5 boys, 4 girls, mean age 11.2 years, range 2–16 years), 5 tendon transfers (4 boys, one girl, mean age 15.4 years, range 12–19 years) and 3 resections of the first carpal row (2 girls, 1 boy, mean age 8 years, range 4–15 years).
Results and discussion
Out of 9 ilioinguinal flaps mild, marginal necrosis was the only complication in 2 patients without flap loss. Mean hospital stay was 26.44 days (range, 18–41 days), with return to full weight-bearing after a mean of 12 weeks (range 9–25 weeks) after discharge. Functional thumb opposition to allow pencil prehension was achieved in all three cases of resection of first carpal row resection without postoperative complications.
Introduction
Buruli ulcer (BU), is a necrotizing skin condition caused by Mycobacterium ulcerans.It is the third most common mycobacterial infection worldwide, though in some areas its prevalence seems to have overrun those of tuberculosis and leprosy [Citation1–3].
The name of the disease comes from the district in Uganda where a particular epidemic was observed in the 1960s. Some skin ulcers described in 1897 in Kampala may be those of the condition. It was also found in Bairnsdale (Australia) in 1948 and the pathogenous bacteria was isolated in that year [Citation4].
BU is a neglected tropical disease that appears in more than 2000 people each year. There is considerable variation among years with a peak of 5954 registered cases [Citation5] in 2004. West African countries remain the hotspot for the disease, but there are endemic areas and sporadic cases in Asia, Oceania and the Americas [Citation6,Citation7]. Flooded wetlands, marshes and rice pads are usually pointed as suitable biotopes for the disease. M. ulcerans or its DNA may be isolated on aquatic flora and fauna.
Environmental factors may be at the origin of a current re-emergence of the disease: extensive irrigation schemes, deforestation, overpopulation and global climate change have been cited as having a role in it. Unlike other Mycobacteriae, M. ulcerans is a non-communicable disease. The virulence of M. ulcerans is mainly based on its ability to synthesise mycolactone, a necrotising exotoxin with immunosuppressive properties. Several studies aim to find a therapeutic use of mycolactone anesthesic properties or to produce specific antibodies against it. Mycolactone-producing mycobacteriae have radiated from a specialized strain of M. marinum as common ancestor. Five different mycolactone structures (A/B, C, D, E, F) have been identified. Mycolactone A/B is found in M. ulcerans strains from Africa, Japon and Southeast Asia. It is by far the most aggressive type. C strains are typical of Australian M. ulcerans while D strains are present in China. Mycolactone E is produced by M. liflandii. Mycolactone F is produced by M. pseudoshotsii. Another peculiar attribute of M. ulcerans colonies is their ability to surround their outer boundaries by glycolipidic extracellular matrix. This matrix hampers the action of antibiotic therapy [Citation8–14].
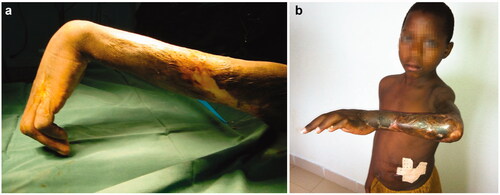
BU can be seen in a broad spectrum of evolutive lesions: non-ulcerative (papules, nodules, plaques), ulcerative and oedematous () as well as osteomyelitis (). Children under 15 years of age account for more than a half of new cases and even infants may be hosts to the disease [Citation15]. One third of them represent upper limb localisations. Typically, papules and nodules are non-painful. Most lesions are detected in the ulcerative stage () when subdermal necrosis has expanded beyond the visible margins of the ulcer.
Figure 1. Buruli ulcer shows a broad spectrum of skin lesions: Non-ulcerative (papules, nodules, plaques) (a), ulcerative (b) and edematous (c).
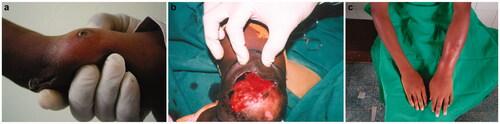
Figure 2. Osteomyelitis as an insidious, destructive condition. Radiographic features (b) may overpass surface clinical appreciation (a).
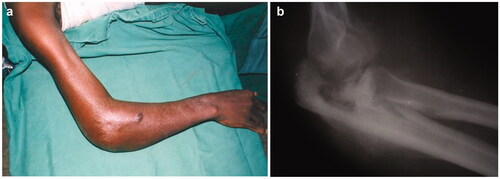
Figure 3. Ulcerative forms (a) and their secondary scarring outcomes in the forearm and wrist (b, c).
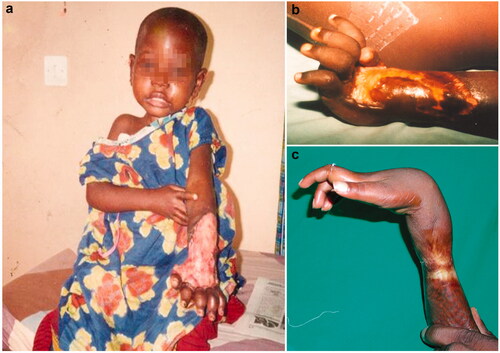
Poverty, isolation and lack of transportation in rural areas are root to the late referral of the condition. However, in well-informed populations from endemic areas those first, evolutive lesions are recognised as suspect. Social ostracism and shame may also have a negative impact on early communication of symptoms [Citation16–18]. Osteomyelitis may occur after contiguous spread from adjacent soft tissue and haematogenous seeding, as well as a postoperative complication. Differential diagnoses depend on the clinical stage of the lesions. Non-ulcerative manifestations may evocate mycosis, psoriasis, leprosy, repetitive trauma, onchocerciasis, filariasis or insect bites. Ulcerative lesions may be similar to those of leishmaniosis, cutaneous tuberculosis, yaws or necrotising fasciitis. Due to the slow growing nature of M. ulcerans and specific incubation [Citation19] laboratory diagnosis has been extremely difficult until the advent of PCR [Citation20].
Hystopathological analysis remains a diagnosis tool in selected centres and there are some reports on biopsies and fine needle aspiration as main diagnostic tool [Citation21]. Until 2004 surgical excision of lesions embodied the main treatment. Nowadays, WHO recommendations emphasise antibiotherapy by 8 weeks of intramuscular streptomycin (15 mg/kg) plus oral rifampicin (10 mg/kg). An alternative treatment consists of 4 weeks of rifampicin plus streptomycin followed by 4 weeks of rifampicin plus oral clarithromycin. Streptomycin is contraindicated during pregnancy and its side effects include hearing impairment, vertigo and nystagmus. Rarer rifampicin side effects involve jaundice, hepatitis and renal failure [Citation22]. Current studies are looking for easier oral modalities of treatment. A series from Australia presents favourable outcomes after surgery and a rifampicin-ciprofloxacin association [Citation23]. Amikacine has been suggested as possible adjuvant treatment in complicated osteomyelitis [Citation24]. Coinfection by HIV entails a particular challenge [Citation25]. Currently, there are research lines on possible therapeutic roles for avermectine or bacteriophages. Efforts in public campaigning for early detection in endemic areas as well as recent advances towards a vaccination may change the future trends of the disease.
All surgical perspectives emphasise the role of patient stabilisation before surgery. Following WHO guidelines infection is ‘stabilised’ when PCR is negative. Pre-operative status is a very important step in management. It must include screening for HIV, drepanocytosis, malnutrition and intestinal parasites. There is little gain in performing a complicated reconstruction on severely malnourished patients. Surgical reasoning must be the same as for deep burns or severe chronic scars [Citation26,Citation27]. Once the patient is stable and the antibiotic treatment has begun, the timing for surgical treatment is a matter of controversy. Unfortunately, national health coverage is incomplete in many rural, remote areas and the ideal status before coping with surgery may take several months. On the other hand, impaired access to health facilities may even determine the onset of surgery before having completed antibiotic treatment. Though the overall treatment has considerably changed since the days in which antibiotic therapy was empirical, coverage of wide ulcerative areas may still require several operatory procedures [Citation28]. Physical therapy is required before and after surgery to prevent ankyloses [Citation29]. A recent, single report proposes the use of negative pressure in the healing process [Citation30]. Relapses would seem less frequent when antibiotherapy is continued after excision but there are scarce studies until now [Citation31–33].
The exposed areas of face and limbs are the most frequent infection sites. Lesions may involve skin, tendon and bone. In what refers to hand, the dorsal surface seems more prone to infection than the palmar one. Advanced stages of the disease may appear in variable combinations of flexion of the elbow, hyperextension of metacarpophalangeal joints and wrist involvement. Surgical treatment may include a variety of strategies: simple excision, partial skin graft, total skin grafts, flaps, tendon transpositions, arthrodesis and amputation in a minority of cases. Most patients would need more than one procedure [Citation34–36]. Arthrodesis by pins (Kirschner wires) in a small number of children may be necessary. Arthrodesis in growing patients is complicated; materials are not so easily available in every country and follow-up constraints are important. Nerve grafting is not a routine but interposition of grafts (sural nerve, medial antebrachial cutaneous nerve) may be required in a handful of patients. Our first aim should be to maximise function (hand grip, elbow flexion), then skin or flap coverage, and finally in third place to ease social interaction/aesthetics.
Material and methods
Ethics statement
The study was approved by the Ethical Committee of the ICR. Written informed consent for all procedures was obtained from the legal representatives of all patients. The subjects of the study are made anonymous by the assignment of numbers. Facial features that could lead to identification were pixelated in photographs.
In our series, we have incomplete records for minor forms although no less than 1500 partial skin grafts (mostly from anterior thigh) were performed in the last 15 years at our institution and small rural facilities. Some procedures took place in a mobile operating room that serves as temporary facility for innermost districts of the country.
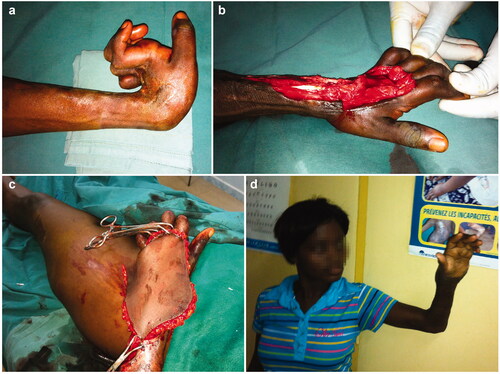
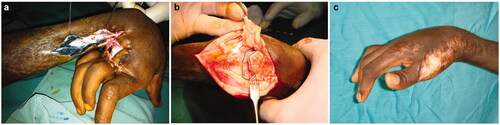
In addition to partial skin grafts, total skin grafts (mostly from the inguinal region as donor site) account for nearly 200 procedures (). Nine ilioinguinal flaps (5 boys and 4 girls with a mean age of 11.2 years, range 2–16 years) have been chosen as therapeutic option in severe defects of the dorsal aspect of the hand (). 5 tendon transfers (4 boys and one girl with a mean age of 15.4 years, range 12–19 years) were performed in a very heterogeneous array of techniques to draw any conclusions and some controversy persists about the most appropriate moment for intervention. We favour lengthening in sliding/bayonetted fashion but we have used grafting after harvesting material from tensor fascia lata, plantaris and palmaris longus muscles. These techniques were inspired by previous experience of the senior authors with leprosy cases. 3 resections of the first carpal row (2 girls and 1 boy with a mean age of 8 years, range 4–15 years, all right-handed) in extreme cases of wrist ankylosis have been an unusual means to tackle with particular, severe cases ().
Results
Ilioinguinal flaps were performed in 9 patients. Mild postoperative complications (very limited, superficial skin necrosis of some margins) were seen in 2 patients (22.22%), with no flap loss. No donor-site complications were observed. The mean postoperative length of hospital stay was 26.44 days (range, 18–41 days), with patients returning to full weight-bearing after a mean of 12 weeks (range 9–25 weeks) after discharge.
All 3 patients that underwent resections of the first carpal rows required physiotherapy to some extent with variable compliance. However, functional thumb opposition to allow pencil prehension was achieved in all three cases. No postoperative complications were seen in those 3 patients. Though in surgeon's eyes the aesthetic result was poor in one of the cases, as a rule, the patients themselves were satisfied with the functional and cosmetic improvements.
Discussion
Surgery still keeps an adjuvant role in the handling of Buruli ulcer. Unfortunately, long pre-operative antibiotherapy as well as transportation problems continue to be common riddles that cause too many patients to be missing for follow-up in developing countries. This lack of follow-up impedes the formulation of comprehensive clinical series of surgical outcomes. In the long run, early diagnosis and widespread accessibility to antibiotics should decrease the number of patients who need an operative treatment. On the other hand, antibiotic resistance and environmental changes may play an important role on future trends.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- WHO Updated February 2017 Media Centre Fact Sheets: Buruli ulcer (Mycobacterium ulcerans infection); [cited 2017 Oct 30]. Available from: http://www.who.int/mediacentre/factsheets/fs199/en/.
- Amofah G, Bonsu F, Tetteh C, et al. Buruli ulcer in Ghana: results of a national case search. Emerg Infect Dis. 2002;8(2):167–170.
- Debacker M, Aguiar J, Steunou C, et al. Mycobacterium ulcerans disease (Buruli ulcer) in rural hospital, Southern Benin, 1997–2001. Emerg Infect Dis. 2004;10(8):1391–1398.
- MacCallum P, Tolhurst JC, Buckle G, et al. A new mycobacterial infection in man. J Pathol. 1948;60(1):93–122.
- WHO. 2017. Neglected diseases. Buruli ulcer. Number of new cases of Buruli ulcer reported (2002–2016).; [cited 2017 Oct 30]. Available from: http://apps.who.int/neglected_diseases/ntddata/buruli/buruli.html.
- WHO. 2012. Treatment of Mycobacterium ulcerans infection (Buruli ulcer). Guidance for workers; [cited 2017 Oct 30]. Available from: http://apps.who.int/iris/bitstream/10665/77771/1/9789241503402_eng.pdf?ua=1.
- Yotsu RR, Nakanaga K, Hoshino Y, et al. Buruli ulcer and current situation in Japan: a new emerging cutaneous Mycobacterium infection. J Dermatol. 2012;39(7):587–593.
- En J, Goto M, Nakanaga K, et al. Mycolactone is responsible for the painlessness of Mycobacterium ulcerans infection (Buruli ulcer) in a murine study. Infect Immun. 2008;76(5):2002–2007.
- George KM, Chatterjee D, Gunawardana G, et al. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283(5403):854–857.
- Nakanaga K, Yotsu RR, Hoshino Y, et al. Buruli ulcer and mycolactone-producing mycobacteria. Jpn J Infect Dis. 2013;66(2):83–88.
- Adusumilli S, Mve-Obiang A, Soarer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo. Cell Microbiol. 2005;7(9):1295–1304.
- Baron L, Paatero AO, Morel JD, et al. Mycolactone subverts immunity by selectively blocking the Sec61 translocon. J Exp Med. 2016;213(13):2885–2896.
- George KM, Barker LP, Welty DM, et al. Partial purification and characterization of biological effects of a lipid toxin produced by Mycobacterium ulcerans. Infect Immun. 1998;66(2):587–593.
- Marsollier L, Brodin P, Jackson M, et al. Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS Pathog. 2007;3(5):e62.
- Tsukagoshi S, Dehn TC. Buruli ulcer in a nine-month-old boy. Ann R Coll Surg Engl. 2012;94(7):e215-216.
- Ackumey MM, Gyapong M, Pappoe M, Maclean CK, Weiss MG. Socio-cultural determinants of timely and delayed treatment of Buruli ulcer: implications for disease control. Infect Dis Poverty. 2012;1(1):6.
- Alferink M, van der Werf TS, Sopoh GE, et al. Perceptions on the effectiveness of treatment and the timeline of Buruli ulcer influence pre-hospital delay reported by healthy individuals. PLoS Negl Trop Dis. 2013;7(1):e2014.
- de Zeeuw J, Omansen TF, Douwstra M, et al. Persisting social participation restrictions among former Buruli ulcer patients in Ghana and Benin. PLoS Negl Trop Dis. 2014;8(11):e3303. Erratum in: PLoS Negl Trop Dis. 2014 Dec;8(12):e3418. Stientstra, Ymkje [corrected to Stienstra, Ymkje]
- Trubiano JA, Lavender CJ, Fyfe JA, et al. The incubation period of Buruli ulcer (Mycobacterium ulcerans infection). PLoS Negl Trop Dis. 2013;7(10):e2463.
- Stinear T, Ross BC, Davies JK, et al. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J Clin Microbiol. 1999;37(4):1018–1023.
- Yeboah-Manu D, Danso E, Ampah K, et al. Isolation of Mycobacterium ulcerans from swab and fine-needle-aspiration specimens. J Clin Microbiol. 2011;49(5):1997–1999.
- Converse PJ, Nuermberger EL, Almeida DV, et al. Treating Mycobacterium ulcerans disease (Buruli ulcer): from surgery to antibiotics, is the pill mightier than the knife? Future Microbiol. 2011;6(10):1185–1198.
- O’Brien DP, Hughes AJ, Cheng AC, et al. Outcomes for Mycobacteriun ulcerans infection with combined surgery and antibiotic therapy: findings from a south-eastern Australia case series. Med J Aust. 2007;186(2):58–61.
- Johnson PD, Hayman JA, Quek TY, et al. Consensus recommendations for the diagnosis, treatment and control of Mycobacterium ulcerans infection (Bairnsdale or Buruli ulcer) in Victoria, Australia. Med J Aust. 2007;186(2):64–68.
- O’Brien DP, Ford N, Vitoria M, et al. Management of BU-HIV co-infection. Trop Med Int Health. 2014;19(9):1040–1047.
- Knipper P, Zilliox R, Johnson C, et al. Ulcère de Buruli et chirurgie plastique au dispensaire. Ann Chir Plast Esth. 2004;49(3):265–272.
- Radford AJ. The surgical management of lesions of ulcerans infections due to Mycobacterium ulcerans, revisited. Trans R Soc Trop Med Hyg. 2009;103(10):981–984.
- Ouattara D, Meningaud JP, Kaba L, et al. [Treatment of Buruli ulcer desease by excision and skin graft]. Ann Chir Plast Esthet. 2004;49(1):11–16.
- Adu EJ. Management of contractures: a five-year experience at Komfo Anokye Teaching Hospital in Kumasi. Ghana Med J. 2011;45(2):66–72.
- Murase C, Kono M, Nakanaga K, et al. Buruli ulcer successfully treated with negative-pressure wound therapy. JAMA Dermatol. 2015;151(10):1137–1139.
- Kibadi AK. Relapses after surgical treatment of Buruli ulcer in Africa. Bull Soc Pathol Exot. 2006;99(4):230–235.
- Barogui Y, Johnson RC, van der Werf TS, et al. Functional limitations after surgical or antibiotic treatment for Buruli ulcer in Benin. Am J Trop Med Hyg. 2009;81(1):82–87.
- O'Brien DP, Walton A, Hughes AJ, et al. Risk factors for recurrent Mycobacterium ulcerans disease after exclusive surgical treatment in an Australian cohort. Med J Aust. 2013;198(8):436–439.
- Adu E, Ampadu E, Acheampong D. Surgical management of Buruli ulcer disease: a four-year experience from four endemic districts in Ghana. Ghana Med J. 2011;45(1):4–9.
- Velding K, Klis SA, Abass KM, et al. Wound care in Buruli ulcer disease in Ghana and Benin. Am J Trop Med Hyg. 2014;91(2):313–318.
- Guifo ML, Essiene A, Ngo Nsoga M, et al. Surgical management of chronic wounds in a Buruli ulcer endemic area in Central Africa. World J Surg. 2016;40(5):1041–1046.