Abstract
There is a need for a systematic approach to evaluate patients for potential face transplantation (FT). Ten patients with severe facial defects treated between 1995 and 2017 formed the study group. Data was collected from patient charts and clinical, radiological and laboratory examinations. Facial deficiencies were subdivided into four different categories: anatomical region (10 facial subunits), facial function, aesthetic defect (range 0–9-worst), and impact on health-related quality of life (HRQoL) (15D questionnaire, range 0–1). Immunological status and possible contraindications were also evaluated. Defect aetiology consisted of burns (4), ballistic injury (3), blunt injury (1), blast injury (1), and neurofibromatosis type I (1). All patients had central facial deficiencies and 6 patients had 8 to 10 injured facial subunits. All patients had at least partial loss of facial function. The mean aesthetic disfigurement score was 6.4. The median lowering of 15D score was −0.107. None were significantly sensitized although four patients had relative contraindications and one patient had an absolute contraindication for FT. Three patients with a severe overall facial deficiency were considered as potential FT candidates. We herein propose a comprehensive and systematic tool to evaluate potential candidates for FT. This approach includes assessment of 4 key categories: anatomical regions affected, facial function, aesthetics, and HRQoL.
Introduction
Face transplantation (FT) can offer a method of restoring near to normal facial function and aesthetics in order to enhance quality of life [Citation1,Citation2]. In contrast to lifesaving solid organ transplantations, face transplantation is considered life-enhancing, and therefore the major risks associated with obligatory lifelong immunosuppression [Citation2,,,Citation1,] must be balanced with the positive outcome of regained function, when selecting patients for FT [Citation1].
However, whilst there has been a gradual evolution in indications and contraindications for FT with time, there is not yet a clear consensus across clinics worldwide. In practice, most centers would agree upon the indication of a large central facial defect associated with a functional deficit deemed unrepairable by conventional methods, a patient motivated for treatment, able to give informed consent and committed to lifelong follow-up and immunosuppression [5–9],3. Currently, patient selection is performed on a case-by-case basis, and thus far no definite conclusion has been reached regarding which facial defect is severe enough to justify the significant adverse effects of immunosuppressive medication as well as considerable surgical risk of vascularized composite allotransplantation (VCA) Citation1,Citation1,. Thus far, each VCA centre has pursued their own approach to patient selection with only a small number of patients per centre in a field that is still relatively new.
Ever since the pre-FT days the issues of patient selection and psychosocial factors have been debated [10,11]. However, we are still lacking structured tools for patient evaluation. Instead of evaluating each patient in isolation, the aim of this study was to systematically analyze patients with severe facial defects. A potential candidate for facial VCA was a patient identified as having a sufficiently poor health-related quality of life (HRQoL) and posing a significant challenge for reconstruction with conventional methods. Our goal was to establish certain criteria in connection with facial functions and defects to enable a patient to undergo systematic evaluation as a potential candidate for facial VCA. A secondary aim was to consequently better define parameters for success or failure in FT.
Patients and methods
This study was approved by the Local Ethics Committee, Department of Medicine, Hospital District of Helsinki and Uusimaa. Patients treated between 1995 and 2017 in the University Hospital of Helsinki, Department of Plastic Surgery, were evaluated for different types of facial defects and earlier reconstructions. Patients with a severe major facial defect deemed difficult to repair by several experienced head & neck plastic and reconstructive surgeons were selected for the study. Patients with a less severe deformity treatable with conventional reconstructive methods were excluded. Within the hospital catchment area, ten patients matched the inclusion criteria during the study period, and these included two eventual face transplantation recipients [Citation12,Citation13].
The impact of facial deficiencies was assessed by evaluating four different categories (anatomical region affected, facial function (including motor and sensory status), aesthetic defect, and impact on HRQoL). Patient evaluation was performed by analysis of all available medical records, clinical examination and assessment of radiological and laboratory findings. All patients also gave consent to the use of video recordings for the assessment of facial motor function.
The four facial deficiency categories
1. Anatomical zones affected
Ten anatomical regions (1. forehead and scalp, 2. periorbital, 3. nasal, 4. perioral, 5. cheek and ears, 6. chin, 7. neck, 8. intraoral, 9. maxilla, and 10. mandible) were evaluated.
2. Functional deficiencies
Emphasis was placed on functions most often affected and considered most important for quality of life (breathing, mouth opening, dentition, mastication, swallowing, speaking, labial competence, eyelid function).
Mimic muscle function was tested using the Sunnybrook facial scale analysis [Citation14,Citation15]. For the previously transplanted patients, the analysis was performed via historical video-recorded material prior to transplantation. Basic facial movements such as forehead wrinkling, facial grimacing, smile and mouth-puckering were also evaluated.
Sensory function was assessed in six facial regions: forehead and scalp, periorbital, perioral, cheek and ears, chin and neck with light touch discrimination using a static monofilament and well-localized 10 mm two-point touch discrimination. Sensation was graded: normal, impaired, and severely impaired or absent.
3. Aesthetic evaluation
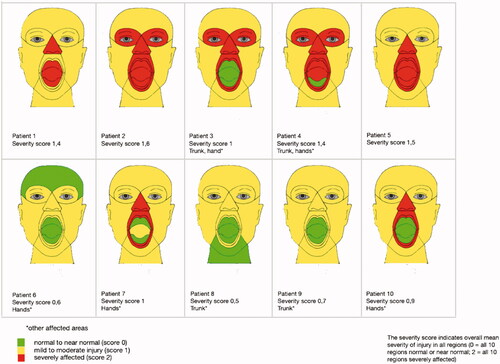
Twenty board-certificated plastic surgeons assessed patient photographs and awarded a score from 1 to 9 (1 being normal and 9 the most severe aesthetic defect) [Citation16]. The score was also transformed to a 3-grade (green-yellow-red) colour score.
4. Health-Related Quality of life
The patients’ HRQoL was measured using the generic 15D and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Head and Neck Module (EORTC QLQ-HN35) [Citation17,Citation18] (range 35–130 points, a lower score indicating a better QoL). The single index score (15D score) represents the overall HRQoL on a 0–1 scale (1 = full health, 0 = being dead). Each patient’s 15D score was compared to the mean score of patient’s gender- and age-group in the general population. The minimum clinically important change or difference in the 15D score has been estimated to be ±0.015. The changes or differences in the 15D scores can be classified as follows: >0.035 -much better, 0.015–0.035 -slightly better, >–0.015 and <0.015 -no change, –0.035 to –0.015 slightly worse and <–0.035 -much worse [Citation19].
Comorbidities and immunological status
Potential contraindications for face transplant and comorbidities were recorded. These included: malignancies, progressive chronic medical disease, neurological diseases and psychiatric disorders. In order to predict the immunological suitability of a potential donor, human leukocyte antigen antibodies (HLA-ab) were measured from a routine venous blood sample and cPRA (calculated panel reactive antibodies) were estimated by comparing the patients’ HLA-ab findings to HLA antigen frequencies in the Finnish reference population. One Lambda Labscreen® mixed and single antigen beads with Luminex® were used for HLA antibody screening and identification with the use of HLA Fusion software (One Lambda Inc., Canoga Park, CA). A normalized Mean Fluorescence Intensity (MFI) cut-off point of 1000 was used for positivity in single antigen analyses.
In order to better visualize and summate the data, all categories and sub-categories were also given a three-grade colour score (normal-green, impaired-yellow, and severely impaired or non-functional-red). The functional facial defect category was awarded an overall colour score based on analyses for all functional parameters and their median value.
Results
Demographic data ()
Patients consisted of three females and seven males. The median age at the time of assessment was 41 years (range 26–66) and at the time of injury 29 years (range 22–57, excluding one patient with a congenital defect and one patient with unknown age at injury). Aetiology consisted of trauma in 9 patients and congenital neurofibromatosis type 1 (NF1) in one patient. Multiple reconstruction operations were performed in all patients and the median number of reconstructive operations was 10 and a median number of 3 microvascular flaps were used in five patients ().
Table 1. Demographic data.
Table 2. Functional impairment evaluation.
Table 3. Scores for aesthetic and quality of life impairment.
Table 4. Immunological status and potential contraindications.
1. Anatomical defects ()
Four patients had maxillary injuries, associated with a mandible deformity in three. All had severe bony injuries. The central facial region was affected in all patients and severely affected in 7 patients. Intraoral injuries were present in five patients and four were graded as severe.
2. Functional facial defects ()
Oral function
Mouth opening was affected in seven patients and labial competence affected in all patients. This was most commonly caused by scarring. Three ballistic injury patients were edentulous and the NF1 patient had incomplete dentition. Eating was compromised in four patients and swallowing impaired in all three of the ballistic injury patients. However, none of the patients had a permanent gastrostomy.
Breathing was impaired in seven patients with loss of the nasal airway and patient 2 required a permanent tracheostomy. Speech was partially impaired in two patients and unintelligible in three patients. In the three patients with a ballistic injury, speech impairment was due to reduced tongue mobility and abnormal oral cavity anatomy.
Periorbital function
One patient was blind, and one patient had only monocular vision. Seven patients had impaired lid function, most commonly from ectropium secondary to scarring.
Sensation
Impaired sensation most commonly affected the midface (periorbitally in 8 patients and periorally in all patients). Sensation was also impaired in the cheeks and ears in all 10 patients and over the chin in 8 patients.
Mimic muscles
Forehead movement was impaired or absent in 3 patients, facial grimacing was impaired or absent in 7 patients, smile was impaired or absent in 9 patients and lip puckering was impaired or absent in 8 patients. Applying the Sunnybrook scores (data available from 7 patients) there was moderate to severe impairment in six patients (Supplemental Table 1).
3. Aesthetic evaluation ()
The mean aesthetic score was 6.4 (range 3.8–8.5).
4. Quality of life ()
HRQoL data were available from 7 patients. EORTC QLQ-HN35 scores ranged from 47 to 82. The mean 15D score was 0.780. This is 0.164 lower than the mean 15D score in the age-and gender-matched general population suggesting a severely impaired mean HRQoL. This applied to all but one patients. The patients were on average markedly worse off in all 15D dimensions except two (sleeping and excretion).
Comorbidities and potential contraindications ()
Mental health issues were documented in four patients, problems with alcohol consumption in two patients, and liver cirrhosis in one patient. Seven of the 10 patients had additional injuries related to their facial injury. All five facial burn patients had suffered burns also to their torsos and extremities. Two of the latter patients had severe hand injuries and were considered as potential candidates for hand transplantation The NF1 patient also had significant incapacitating tumours affecting his torso and extremities.
Immunological status ()
HLA-ab and PRA data were available for eight patients. The calculated PRA varied between 0 and 23% (Class I antibodies). None of the patients demonstrated any Class II antibodies. None of the three patients with major burns were sensitized at a clinically relevant level (PRA 0–1%) and this was in spite of multiple blood transfusions.
Summary of all 4 categories and overall score ()
All 4 categories were each awarded an ‘overall category score’ and then an ‘overall final score’ was concluded. The same 3 grade colour scores were applied, consisting of normal to near normal (green), moderate deficiency (yellow), or severe deficiency (red). Three patients were ultimately awarded an overall severe deficiency score, and each of these patients had three or four categories scoring ‘severe’ (including the two face transplant patients from Helsinki, patients 1 and 2). None of the 10 patients were highly immunized. One patient had an absolute contraindication for FT (cirrhosis).
Table 5. Summary of the four categories.
Discussion
At present there is no consensus on which parameters are required when evaluating and selecting candidates for FT. Furthermore, there exists no consensus on how to define success or failure in FT. The complex and subtle interplay between facial anatomy, function and aesthetics helps explain the difficulty thus far in defining the parameters necessary for evaluation in patient selection for FT.
Our study provides the first attempt in the literature to perform a comprehensive analysis of the different aspects of facial disfigurement and their combined effect on patients in the context of FT. Herein we created a systematic method for evaluating 10 patients with severe facial disfigurement. Four categories were analyzed in all patients: the anatomical zones of facial injury, functional deficiencies, the aesthetic appearance, and HRQoL. We applied a simple three colour code system to assist interpretation of the results. The overall impacts of facial disfigurement was then graded in order to estimate whether or not an individual patient would be a suitable candidate for FT.
Previously it has been stated that FT is indicated in adults with a severe facial defect and functional deficits that cannot be adequately addressed with conventional reconstruction techniques [Citation8–10]. At present this includes: a person with significant loss of periorbital and perioral tissues or an extensive loss of facial structures. This definition is broad and does not define which functions are considered essential in this respect nor does it take into account the psychosocial effects of the deficit in a specific person. In view of the fact that FT is such a major procedure associated with high risks and that the immunosuppressive treatment may have severe side effects, there is a need to develop a universal instrument to aid patient selection. In addition, in order to define whether a FT has succeeded and has been beneficial for the patient, we need to be able to demonstrate improvements in well-defined parameters that have been evaluated prior to FT.
Category 1. Affected anatomical zones
Patients with a severe facial deformity often have panfacial injuries or large areas of their face injured. Soft-tissues with or without bony injuries may be involved. There may be additional iatrogenic facial injuries that occur during attempts at conventional reconstruction. The Boston group have previously reported an inclusion criteria for FT consisting of a defect comprising over 25% of the face and/or loss of one of the major facial features, such as the nose, lip(s), or eyelid(s) [Citation1]. There has also been some debate regarding whether or not patients should be subjected to a full or partial FT. The Boston group has advocated preserving all of the patient’s functional tissue and only removing and restoring what is non-functional [Citation18]. From the perspective of the aesthetic outcome, a partial FT is often more discernible and might not succeed in restoring a near to normal appearance. Moreover, if the aesthetic outcome is considered to be one parameter of success it would then be advisable to perform a full FT in a patient with a large portion of their face injured [Citation19]. The flip side is that the latter would increase the stakes in view of possible early or late graft failure. Our approach has been to preserve all functioning tissue in view of this being a high-risk procedure. In addition, a back-up plan should be in place in the event of acute or early graft loss [Citation20].
Seven of our patients had additional injuries affecting other parts of their body. These patients might benefit from additional vascular composite allotransplantations (VCA) in addition to FT. The possibility of an additional VCA whilst already on immunosuppressive medication could be considered an additional factor that supports the decision to proceed with FT. However, all simultaneous face and upper extremity transplantations have so far suffered serious complications [Citation21] and thus the current consensus has recommended against performing simultaneous transplants. In spite of the latter, there have been very recent media reports of a (thus far) successful double hand and face transplantation in New York, US [Citation22].
Category 2. Functional deficit
We analyzed several facial functions, including labial continence, periorbital function, mimic muscle movements, and sensation. All patients had a reduced Sunnybrook scale score and variable severity of impaired facial mimic function. The Sunnybrook scale has been used previously by the Boston group in FT patients and is at present the most suitable instrument to use in the absence of any scales evaluating bilateral facial function deficiency [Citation23]. It has been shown that facial function suitable for social interaction can be restored at least partially after FT [Citation23–26].
In this study, facial sensation was impaired in all patients. A full or close to full recovery of sensation after FT has been documented by several groups [Citation27]. Therefore, sensation should be included in the evaluation.
Severe functional injuries to the central face are overrepresented in this patient population. There are reports in the literature regarding improvements in oral competence, eating, breathing and speaking after FT [Citation24–27]. Therefore, oral functional impairment should be included in the evaluation.
Category 3. Aesthetic deficit
To assess the aesthetic deficit, we used the single-item, nine-point Likert scale that measures the degree of disfigurement previously described by Katz et al. [Citation16]. The mean aesthetic score in our group of patients was 6.4. Interestingly, both of our FT patients scored 7, hence neither being awarded the worst aesthetic score. Facial disfigurement led to a reduction in the number of social contacts for all 10 patients in this study. The face is hence an important mediator in social communication and even though FT should not be performed for solely aesthetic reasons, there is still an important need for these patients to look as normal as possible to facilitate successful integration into society. Furthermore, appearance enhancement has been proven to improve quality of life [Citation16].
Category 4. Quality of life
All VCA teams that have reported their FT results have included QoL instruments in their evaluation; however, there have been several different instruments used [Citation1]. There exists no validated HRQoL-measurement scale for facial trauma patients. The FaCE Scale and Face-Q are not yet validated in the Finnish language [Citation29,Citation30]. We used a head and neck cancer-oriented measurement [Citation17,Citation18], since we consider this to most resemble the study patient group as well as the generic HRQoL instrument 15D, which has been widely used and validated and can be used to estimate the overall changes in HRQoL [Citation17,Citation31–33]. The 15D has good psychometric properties and provides 15D data from a large, representative sample of the general population. There was severe impairment in the generic HRQoL in 9 of the 10 patients. It is our opinion that a significantly reduced HRQoL score is a prerequisite for consideration of FT selection. Indeed, there is a need for a FT-specific facial disfigurement QoL assessment instrument.
Aspects other than just the facial disfigurement need to also be taken into account during the decision-making process. Patients with severe comorbidities should in general be excluded. In addition, a thorough psychological and psychiatric evaluation is essential to rule out any psychiatric disorders that may jeopardize the postoperative recovery as well as reduce the patient’s compliance with immunosuppressive medication. The immunological status of the patient also needs to be evaluated. Preformed HLA-antibodies present a risk for subsequent antibody-mediated rejection and needs to be assessed in view of donor specific antibodies present at the time of FT [Citation34]. Interestingly, all three burn study patients had received multiple blood products as well as cadaveric skin substitutes during their acute burn management, but yet had no clinically relevant HLA-antibodies present. The highest PRA scores were recorded in the ballistic injury patients who had received blood products before 2001, prior to when all red cell products in Finland were changed to leucodepleted products [Citation35].
Regarding the selection of our two FT recipients, both patients had all facial zones affected including both the maxilla and mandible [Citation36,Citation37]. Both had a severely affected nasal area and intraoral area. The second patient additionally had severely affected periorbital regions and lips. They also had impaired facial function, a severe aesthetic disfigurement and had a severely reduced HRQoL. Their overall scores, considering all parameters, indicated a severe deficiency. A third patient (patient 4) was also assessed to have an overall severe deficiency based on 3 severely impaired categories. However, this patient has declined a FT.
Conclusion
The aim of this study was to evaluate patients with severe facial disfigurement and to create a systematic instrument to analyze their suitability for FT. We emphasize that there are several aspects to consider including QoL, social well-being, functional, anatomical, and aesthetic features. All of these parameters intend to address the sequelae of a severe facial deformity. Other factors such as compliance and comorbidities may also influence the final decision. Furthermore, it is a matter for debate how to weight these different parameters. Is reduced quality of life as important as functional deficiency? Hence, we are reluctant to define a precise cut off score for inclusion or exclusion. Our colour grading score system intends to simplify and enable visual assessment of the global impact of the deformity.
The number of FT patients worldwide is growing steadily but long-term outcome data is only gradually accumulating. A valid preoperative evaluation chart would also help when evaluating the overall outcome of FT. In summary, we propose a systematic and comprehensive tool for FT candidates, which considers 4 fundamental parameters: the anatomical facial subunits affected, wide ranging functional analyses, the aesthetic deficit, and HRQoL evaluation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aycart MA, Kiwanuka H, Krezdorn N, et al. Quality of Life after Face Transplantation: Outcomes, Assessment Tools, and Future Directions. Plast Reconstr Surg. 2017;139(1):194–203.
- Shanmugarajah K, Hettiaratchy S, Clarke A, et al. Clinical outcomes of facial transplantation: a review. Int J Surg. 2011;9(8):600–607.
- Reske A, Reske A, Metze M. Complications of immunosuppressive agents therapy in transplant patients. Minerva Anestesiol. 2015;81(11):1244–1261.
- Coffman KL. Psychiatric evaluation of the face transplant candidate. Curr Opin Organ Transplant. 2015;20(2):222–228.
- Wo L, Bueno E, Pomahac B. Facial transplantation: worth the risks? A look at evolution of indications over the last decade. Curr Opin Organ Transplant. 2015;20(6):615–620.
- Siemionow M, Ozturk C. Face transplantation: outcomes, concerns, controversies, and future directions. J Craniofac Surg. 2012;23(1):254–259.
- Pomahac B, Diaz-Siso JR, Bueno EM. Evolution of indications for facial transplantation. J Plast Reconstr Aesthet Surg. 2011;64(11):1410–1416.
- Gordon CR, Siemionow M, Coffman K, et al. The Cleveland Clinic FACES Score: a preliminary assessment tool for identifying the optimal face transplant candidate. J Craniofac Surg. 2009;20(6):1969–1974.
- Losee JE, Fletcher DR, Gorantla VS. Human facial allotransplantation: patient selection and pertinent considerations. J Craniofac Surg. 2012;23(1):260–264.
- Clarke A, Butler PE. Patient selection for facial transplantation II: Psychological considerations. Int J Surg. 2004;2(2):116–117.
- Summerton I, Agha RA. Sociological considerations in face transplantation. Int J Surg. 2004;2(2):82–83.
- Lassus P, Lindford A, Vuola J, et al. The Helsinki Face Transplantation: Surgical aspects and 1-year outcome. J Plast Reconstr Aesthet Surg. 2018;71(2):132–139.
- Lindford AJ, Mäkisalo H, Jalanko H, et al. The Helsinki approach to face transplantation. J Plast Reconstr Aesthet Surg. 2019;72(2):173–180.
- Neely JG, Cherian NG, Dickerson CB, et al. Sunnybrook facial grading system: reliability and criteria for grading. Laryngoscope. 2010;120(5):1038–1045.
- Kanerva M, Poussa T, Pitkaranta A. Sunnybrook and House-Brackmann Facial Grading Systems: intrarater repeatability and interrater agreement. Otolaryngol Head Neck Surg. 2006;135(6):865–871.
- Katz MR, Irish JC, Devins GM, Rodin GM, et al. Reliability and validity of an observer-rated disfigurement scale for head and neck cancer patients. Head Neck. 2000;22(2):132–141.
- Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33(5):328–336.
- Sherman AC, Simonton S, Adams DC, et al. Assessing quality of life in patients with head and neck cancer: cross-validation of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Head and Neck module (QLQ-H&N35). Arch Otolaryngol Head Neck Surg. 2000;126(4):459–467.
- Alanne S, Roine RP, Räsänen P, et al. Estimating the minimum important change in the 15D scores. Qual Life Res. 2015;24(3):599–606.
- Pomahac B, Bueno EM, Sisk GC, et al. Current principles of facial allotransplantation: the Brigham and Women's Hospital Experience. Plast Reconstr Surg. 2013;131(5):1069–1076.
- Dorafshar AH, Bojovic B, Christy MR, et al. Total face, double jaw, and tongue transplantation: An evolutionary concept. Plast Reconstr Surg. 2013;131(2):241–251.
- Santanelli di Pompeo F, Longo B, Giovanoli P, et al. Facial Transplantation: Nonimmune-Related Hyperacute Graft Failure-The Role of Perfusion Injury: A Case Report. Ann Plast Surg. 2021;86(4):469–475.
- Carty MJ, Hivelin M, Dumontier C, Talbot SG, et al. Lessons learned from simultaneous face and bilateral hand allotransplantation. Plast Reconstr Surg. 2013;132(2):423–432.
- https://edition.cnn.com/2021/02/03/us/face-and-double-hand-transplant/index.html.
- Aycart MA, Perry B, Alhefzi M, et al. Surgical Optimization of Motor Recovery in Face Transplantation. J Craniofac Surg. 2016;27(2):286–292.
- Roche NA, Blondeel PN, Vermeersch HF, et al. Long-Term Multifunctional Outcome and Risks of Face Vascularized Composite Allotransplantation. J Craniofac Surg. 2015;26(7):2038–2046.
- Lantieri L, Grimbert P, Ortonne N, et al. Face transplant: long-term follow-up and results of a prospective open study. Lancet. 2016;388(10052):1398–1407.
- Khalifian S, Brazio PS, Mohan R, et al. Facial transplantation: the first 9 years. Lancet. 2014;384(9960):2153–2163.
- Fischer S, Kueckelhaus M, Pauzenberger R, et al. Functional outcomes of face transplantation. Am J Transplant. 2015;15(1):220–233.
- Bramstedt KA. A Lifesaving View of Vascularized Composite Allotransplantation: Patient Experience of Social Death Before and After Face, Hand, and Larynx Transplant. J Patient Exp. 2018;5(2):92–100. Vol.
- Kahn JB, Gliklich RE, Boyev KP, et al. Validation of a patient-graded instrument for facial nerve paralysis: the FaCE scale. Laryngoscope. 2001;111(3):387–398.
- Klassen AF, Cano SJ, Scott A, et al. Measuring patient-reported outcomes in facial aesthetic patients: development of the FACE-Q. Facial Plast Surg. 2010;26(4):303–309.
- Kaukola L, Snäll J, Lindqvist C, et al. Health-related quality of life after surgical treatment of mandibular fracture. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(4):402–407.
- Kaukola L, Snäll J, Roine R, et al. Health-related quality of life of patients with zygomatic fracture. Med Oral Patol Oral Cir Bucal. 2017;22(5):e636–e642.
- Rajantie H, Kaukola L, Snäll J, et al. Health-related quality of life in patients surgically treated for orbital blow-out fracture: a prospective study. Oral Maxillofac Surg. 2020;Dec 5. Online ahead of print.
- Chandraker A, Arscott R, Murphy GF, et al. The management of antibody-mediated rejection in the first presensitized recipient of a full-face allotransplant. Am J Transplant. 2014;14(6):1446–1452.
- Capraro L, Kuitunen A, Vento AE, et al. Universal leukocyte reduction of transfused red cells does not provide benefit to patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2007;21(2):232–236