Abstract
In this study, we investigated the feasibility of using a geometrically designed anterior ramus graft to reconstruct lateral mandibular defects. This was achieved by assessing the anatomical dimensions of the mandibular ramus on computed-tomographies. The design sequence and application of the graft was also demonstrated using one of our cases. The following dimensions were measured; a and b - horizontal length from mid-ramus to the posterior and anterior ramus border respectively, c - longest length of the graft, Mp - width at the centre of the ramus, h - vertical length of the angle at its cross-section, w - horizontal length of the angle at its cross-section, x - cross-sectional area along the mandible angle. A total of 80 mandibular rami were examined. The mean length of a, b, c were 17.3 ± 1.8 mm, 15.9 ± 1.2 mm, 54.6 ± 3.8 mm, respectively. The mean width of Mp was 9.8 ± 1.1 mm. The mean cross section area of Eo-Md (x) was 326.7 ± 67.8 mm2. The average length of h and w were 26.5 ± 3.2 and 15.6 ± 2.1 mm, respectively. The use of virtual surgical planning (VSP) to geometrically design the graft was also described. Together with VSP, the anterior ramus bone graft will allow for reconstruction of the mandible with greater surgical efficiency, reduced complexity and without the need for extra-oral bone harvest. This may be an useful alternative in situations where simpler reconstructive procedures are preferred.
1. Introduction
The posterior mandible is a site where tumours and cysts commonly occur [Citation1,Citation2]. Large or aggressive pathologies may require resection surgeries, resulting in the discontinuity of the jaw. This is especially so for atrophic mandibles. The mandible plays a significant role in facial aesthetics, and is also involved in many functions such as mastication, swallowing and speech [Citation3,Citation4]. The role of reconstructive surgery is therefore critical in restoring the patients to their premorbid state [Citation5].
Autogenous bone grafting is the gold standard material for bone regeneration [Citation6,Citation7]. For mandibular defects, a vascularised fibula flap may be the preferred choice [Citation8,Citation9]. The alternative would be a gap-bridging plate accompanied with a myocutaneous flap. While bridging the gap with only a reconstructive plate is possible, it is associated with many complications and is not amenable to subsequent dental prosthesis [Citation10,Citation11]. Non-vascularised bone grafts harvested from extra-oral sites can also be used to reconstruct mandibular defects in patients [Citation12]. Extra-oral harvests are accompanied by increased morbidity and technical difficulty such as multiple surgical sites, extended duration of the operation, increased risk of infections, disfigurement of harvest sites or even permanent disabilities [Citation13,Citation14].
Longer or more complex surgeries may not be suitable for some patients whose compensatory mechanism to surgical stress is poor. This includes elderly patients, and especially so for those with multiple pre-existing comorbidities. In some situations, the physical condition of the potential harvest sites may impede their use. This may be due to osteoporosis, history of fracture, venous stenosis or previous prosthetic surgery. Also, another common concern for the elderly is the risk of impeding their mobility [Citation12,Citation15,Citation16].
In this article, we investigated the use of non-vascularised anterior ramus grafts to reconstruct lateral mandibular segmental defects. This approach can be an alternative to situations whereby there is a need to simplify the surgery or when extra-oral bone harvests are deemed unsuitable. At the same time, it overcomes the problem of gap-bridging plates without significant increase in risks and surgery time. Using a case as a sample, we have also demonstrated the use of Virtual Surgical Planning (VSP) to maximise the ramus graft for reconstruction.
2. Materials and methods
2.1. Reference points and lines
This study was performed in accordance with the Declaration of Helsinki and was approved by our hospital’s human research ethics committee (Reference number 2017-400-1). 40 patients who have an existing Computed Tomography (CT) scan of the maxilla-mandibular region, taken from January 2015 to January 2017, were used for this study. The inclusion criteria were as follows:
No obvious facial asymmetry
No previous pathology, injury or surgical intervention to mandible ramus
Age >60 years old
Missing at least the first, second and third molars
Has an existing Computed Tomography (CT) scans of the maxilla-mandibular region
Fine cut CT and 3D-reconstructed images were retrieved from the radiology database. Selected images were anonymised. The reference points and lines were first marked on the images ( and ): The lowest point of the sigmoid notch (Sn), the highest point of the antegonial notch (An), the deepest point of the curvature along the external oblique ridge (Eo), the deepest point on the anterior border of the ramus (Ad) and the deepest point on the posterior border of the ramus (Pd).
Figure 1. Picture on the left (a): the reference points and lines were marked, a and b indicates the smallest width of the posterior and anterior ramus respectively, c indicates the longest length of the planned anterior ramus graft. Picture in the middle (b): Mp is the width of the ramus bone at the mid-point of Sn-An Line. Picture on the right (c): the cross-sectional area of Eo-Md was marked red (x). The tallest and widest part of the cross section were also measured as h and w, respectively.
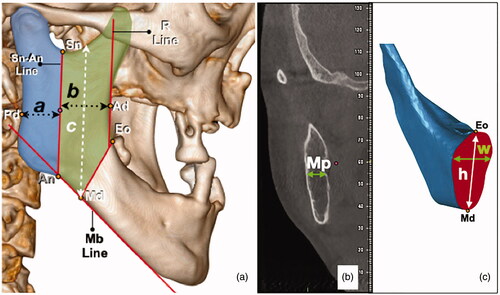
Table 1. Reference points and lines.
The following lines were drawn: Sn-An Line - Line drawn from Sn to An which delineates the anterior and posterior ramus; Mb Line - Line drawn parallel to the inferior border of the mandible; R Line - Line parallel to Sn-An line was drawn passing through Ad. The ramus was arbitrarily delineated from the mandible body by a line from the point Eo, at 150 degrees from the R line. At where this line crosses Mb line was designated as Point Md.
The anterior-posterior length of the anterior and posterior ramus were measured by the perpendicular distance from Sn-An Line to Ad and Pd respectively (a and b). A line was drawn from point md to the sigmoid notch. This line was parallel to the Sn-An Line and the length indicates c. The cross-section (Bucco-lingual) width of the ramus at the midpoint of Sn-An line was also measured (Mp). The cross-section area of Eo-Md (x) was measured using Geomagic Studio 12 (3 D systems, South Carolina). Using the same software, the tallest and widest length of the cross section were measured as h and w respectively.
All measurements were recorded in millimetres (mm). The steps were then repeated again on the contra-lateral side. 2 blinded examiners DY and JH, were involved in the marking of the reference points and measurement of the distances. Inter-examiner calibration was done to ensure 90% agreement in measurements.
2.2. Statistical analysis
Statistical analysis was conducted with SPSS Version 13 software. The significance of the laterality (left or right) to the measurements of a, b, c, Mp, x, h and w were analysed with Student’s t test. Values were considered to be statistically significant when p < 0.05.
3. Results
3.1. Anatomical measurements of a, b, c, Mp, x, h and w
A total of 80 mandibular rami from 40 subjects were involved in the analysis. The mean age of the subjects was 68 years old. The mean length of a, b and c were 17.3 ± 1.8 mm, 15.9 ± 1.2 mm, 54.6 ± 3.8 mm respectively, while the mean width of Mp is 9.8 ± 1.1 mm. Mean cross section area of Eo-Md (x) was 326.7 ± 67.8 mm2. The average length of h and w were found to be 26.5 ± 3.2 mm and 15.6 ± 2.1 mm respectively. For all measurements, there were no significant difference between the left and right side of the mandible (p < 0.05).
3.2. Digitally designed anterior ramus bone graft
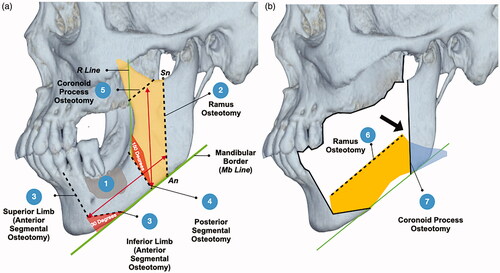
With the study results, we devised a sequence of steps to design the segmental resection and bone graft harvest ().
Figure 2. Top picture (a): design of osteotomy; bottom picture (b): rotation of graft. The grey area shows the pathological defect marked with reference to the CT scans. The yellow area indicates the anterior ramus graft. The blue area indicates the remnant coronoid process after the osteotomy. The green lines are the reference lines (Mb Line and R Line). The black dotted lines are the designed osteotomy lines. The black arrows indicate area of deficiency which can be filled by the remnant coronoid process.
Mark the pathological defect on the simulated 3D model with reference to the CT images
Ramus Osteotomy: Design the osteotomy from the lowest point of the sigmoid notch to the antegonial notch (Sn-An Line)
Anterior segmental osteotomies: The anterior osteotomy consists of two limbs or planes. The inferior limb was X degrees from the border of the mandible (i.e. 30 degrees from Mb Line). The superior limb was continuous from the inferior line and the length of each limb should be adjusted till an adequate safety margin can be achieved at all points.
Posterior Segmental Osteotomy: The posterior segmental osteotomy was drawn at 180-X degrees to the anterior border of the ramus (i.e. 150 degrees from R Line) The osteotomy should be kept at least 1cm away from the pathological defect.
Coronoid Process Osteotomy: The distance from the anterior segmental osteotomies to the ramus osteotomy determines the height of coronoid process osteotomy. The angle of the osteotomy was parallel to the border of the mandible (Mb Line).
Graft Rotation: The graft was then flipped along its long axis (Medial surface rotated to face laterally) and rotated to fit into the defect. The bone surface from the ramus osteotomy should face occlusal, while the bone surface from the coronoid osteotomy should contact the remaining posterior ramus. Alternatively, the graft rotation can be simulated first and the coronoid osteotomy can be determined by the area of overlap.
The remnant coronoid process can be used to fill up deficient areas at the anterior and posterior portions of the graft.
3.2.1. Clinical application
Our centre has successfully applied this novel reconstructive approach into our clinical practice. Six patients have underwent simultaneous resection and reconstruction with an anterior ramus bone graft. The period of follow-up ranges from 1 to 2 years. The profile of the patients are summarised in .
Table 2. Clinical application.
We illustrated the clinical application using one of our cases. The patient was a 68 year old Chinese gentleman who underwent mandibular reconstruction using this method. In July 2018, he was diagnosed with a left mandibular gingival squamous cell carcinoma. Although a marginal mandibulectomy was adequate to clear the disease, a segmental resection was more appropriate in view of his atrophic mandible. Due to the laborious nature of his occupation, the patient refused any form of tissue harvest from his limbs. In order to circumvent the need for extra-oral bone harvest, reduce treatment cost and to reconstruct the mandibular bone continuity, we offered the option of an anterior ramus bone graft to reconstruct the mandible. He was counselled on the advantages and disadvantages of this procedure. Most importantly, we emphasized that the ideal option for oncological cases would be a vascularised bone graft as free bone graft are prone to failure if radiotherapy was deemed to be necessary post-operatively. However, the patient was insistent on not having any operations on his limbs.
3.2.2. Virtual surgical planning and 3D printing
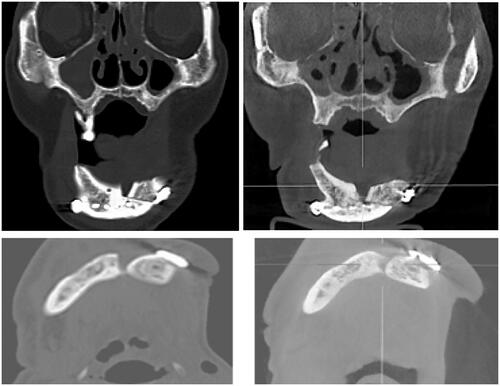
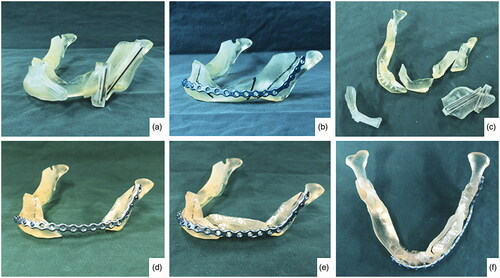
Virtual Surgical Planning (VSP) was done using Proplan CMF 2.0 Software (Materialise, Belgium). The principles of the geometric design was applied on the planning. The fit of the anterior ramus graft was also simulated. Area of deficiency was noted be mainly at the interface between the coronoid osteotomy site and the posterior ramus (). Surgical cutting guides were fabricated so as to ensure accurate angulation of the osteotomies. A reconstruction plate was bent on a printed 3 D model of the pre-operative mandible. The same model was cut following the surgical guides and the fit of the reconstruction plate and the anterior ramus graft was tested ().
Figure 3. The upper left picture (a) shows the 3D simulation of the unoperated mandible. Pictures (b–d) demonstrates the rotation of the graft. Gray – area to be resected; yellow – anterior ramus graft; blue – remnant coronoid bone; pink – remaining native bone. The red arrow points to the pathological defect.
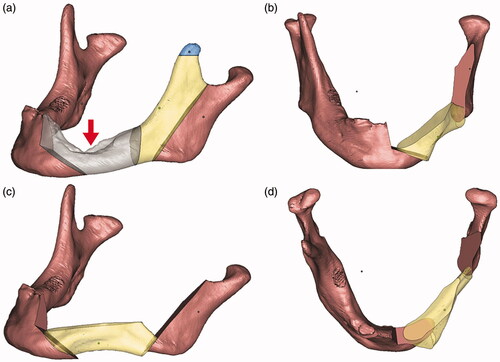
Figure 4. Picture on the upper left corner (a), fitting of the surgical guides; (b) pre-bending of reconstruction plate according to the contour of the 3D printed pre-operative mandible; (c) the 3D printed mandible was cut according to the guides; (d) temporary fixation of the reconstruction plate to the mandible; (e and f) fitting of the anterior ramus graft.
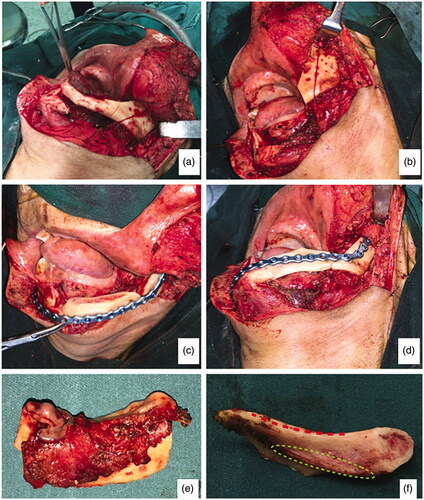
3.2.3. Surgical procedure
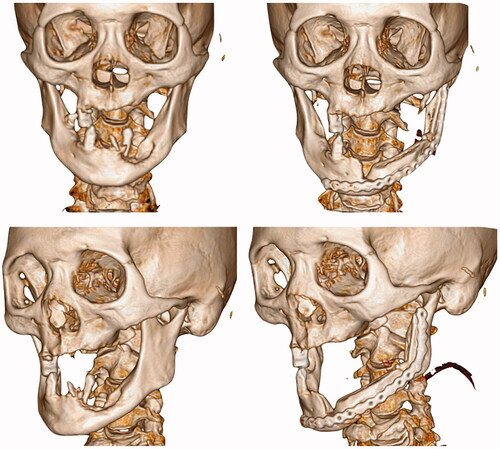
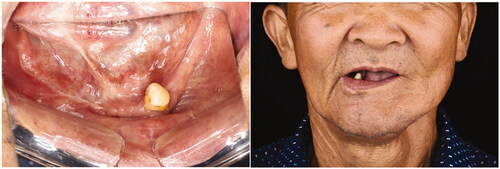
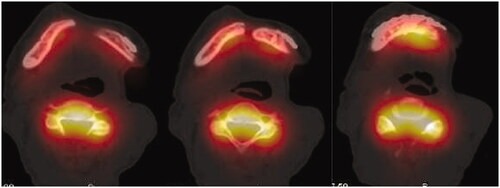
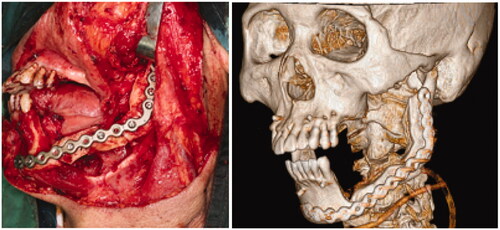
In early August 2018, the patient was admitted for his treatment under general anaesthesia ( and ). The total surgery time was 2.5 h and the reconstruction process only required 20 min. Blood loss was minimal at 100 ml. The post-operative recovery was uneventful, and the patient was discharged 1 week after the surgery. The patient was reviewed every 3 months. After 1 year, there were no signs of recurrence. Radiographic investigations showed bony bridge formation between the graft and native bone. The bone scans also suggest that the grafted bone is viable (). The patient remains satisfied with the outcome of the surgery and was able to return to his previous occupation. shows the another case (left mandibular gingival SCC) which also underwent reconstruction of mandible by anterior ramus bone graft.
Figure 5. (a) Osteotomy lines; (b) the mandibular segmental defect; (c and d) superior and inferior view of reconstructed mandible; (e) Resected pathology; (f) the red dashed line demonstrate the natural curvature of the anterior border of the ramus – upon rotation, this will form inferior border of the reconstructed mandible. The internal oblique ridge is marked within a green dashed circle.
4. Discussion
The anatomical measurements of the ramus have shown that it is an appropriate site for harvesting bone grafts for the reconstruction of lateral segmental mandibular defects. While there are previous studies in the literature on the use of ramus grafts, these are mainly limited to smaller harvests intended for alveolar bone reconstruction. The use of an intra-oral graft allows the patient to be mobilised earlier by avoiding a separate surgery on the limbs, and thus reduces the chance of developing bed sores and venous thrombosis. These outcomes may be beneficial to elderly patients. We have successfully applied this approach in 6 elderly patients (Age ranged from 63 to 70). The surgical duration lasted between 130 and 166 min. Of which, the reconstruction time lasted only 20–35 min. The short surgery time was facilitated by the use of the surgical guides and pre-bent reconstruction plates. With appropriate planning, minimal to no adjustments were required, therefore, reducing both the surgical stress and duration. No complications occurred in any of the 6 patients. Follow ups of 1–2 years did not reveal any signs of graft infection, graft failure or recurrence. All patients remained satisfied with outcomes. We attributed the outcomes to proper case selection, with the criteria as listed in . Apart from primary closure, it is also crucial to instill proper oral hygiene and limit functional activity to prevent wound breakdown.
Table 3. Proposed criteria for case selection.
The curvature of the anterior border of the ramus matches well to the inferior border of the mandible. Therefore after the graft rotation, the curvature and the continuity of the mandible can be recreated. Apart from the morphology, the quantity of the graft is equally important. The cross-section area, width and height of the anterior ramus (x, h, and w) are comparable to the fibula and anterior iliac crest bone graft [Citation17,Citation18]. These dimensions are crucial because the graft must be big enough to tolerate masticatory stresses and provide enough surface area to ensure good contact with the native bone [Citation19]. The remaining posterior ramus segment must also be sufficiently thick to support the functional of the mandible and to provide sufficient space for fixation of a reconstructive plate. In this study, the posterior ramus was measured to be 17.3 mm. This is comparable to the thickness of a fibula bone graft, which was reported to be approximately 15 mm thick [Citation20] However, it is important to note that unlike the fibula bone, the thickness of the posterior ramus is not consistent throughout its entire length. Furthermore, functional stresses are also not equally distributed and tends to greatest at the condylar region [Citation21]. Therefore, it is also important to not reduce the dimensions of the condylar region by keeping the ramus osteotomy within the Sn-An Line. Removal of the coronoid and detachment of the temporalis muscle as part of the harvesting procedure will also help to reduce functional load on the remaining ramus.
The ideal goal of mandibular reconstruction would include oral rehabilitation with implant-supported dental prostheses. The mean mid-ramus width and anterior-posterior length of the anterior ramus were measured to be 9.8 and 15.9 mm, respectively. This would allow for the placement of wide diameter dental implants of 10 mm in length. The harvested anterior ramus also offers a good balance of cortical and cancellous bone, which provides for adequate implant stability and vascularity for osseointegration [Citation22,Citation23]. The intramembranous lineage of the ramus may provide several advantages over autogenous graft of endochondral origin. Intramembranous grafts have been shown to result in higher rates of revascularisation and healing. Furthermore, the rate of resorption of these grafts were found to be much slower when compared to bone from endochondral origin [Citation24,Citation25]. This is especially important as the dental implants are usually placed 6 months later. However, the placement in this graft will require further validation.
There are several caveats to this approach. Firstly, the ramus of the mandible was halved and its effect on the masticatory strength remains to be investigated. There may also be concerns whether the remaining ramus will be prone to fracture. However, given the experience from fibula bone grafts, it was deemed to be unlikely. Secondly, despite that the grafts were taken from the same surgical site of the resection, they were still non-vascularised bone grafts. In the absence of a pedicle, non-vascularised grafts are at greater risks of resorption, necrosis and infection. In view of this, vascularised flaps are usually considered in patients who are prone to infections and those who had or will undergo radiotherapy. These are typically patients with oncologic disease. While free bone graft techniques are mainly used in benign pathologies, we have managed to show successful use of this graft in oncologic patients. In order to reduce the likelihood of complications, criteria indicated in should be fulfilled, i.e. the length of defect to be reconstructed should not exceed more than 6 cm [Citation12]. There are also potential for the anterior ramus graft to be vascularised by keeping a significant amount of periosteum and muscles attached (i.e. temporalis or medial pterygoid muscles).
5. Conclusion
There are clinical situations where an anterior ramus bone graft may be preferred or when the most ideal treatment is not accepted by the patient i.e. for elderly patients where long surgeries are not recommended or who decline harvesting bone from a separate site. In these situations, this novel approach will be a viable alternative. The use of digital technology to plan the surgery will allow the potential of the graft to be maximized. Further investigations on the validation of the use of dental implants in this graft will be useful. (3589 words)
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Robinson RA. Diagnosing the most common odontogenic cystic and osseous lesions of the jaws for the practicing pathologist. Mod Pathol. 2017;30(s1):S96–s103.
- Dunfee BL, Sakai O, Pistey R, et al. Radiologic and pathologic characteristics of benign and malignant lesions of the mandible. Radiographics: a review publication of the. Radiographics. 2006;26(6):1751–1768.
- Vigliante CE. Anatomy and functions of the muscles of facial expression. Oral Maxillofac Surg Clin North Am. 2005;17(1):1–15.
- Laine FJ, Smoker WR. Oral cavity: anatomy and pathology. Semin Ultrasound CT Mr. 1995;16(6):527–545.
- Villaret AB, Cappiello J, Piazza C, et al. Quality of life in patients treated for cancer of the oral cavity requiring reconstruction: a prospective study. Acta otorhinolaryngologica Italica. 2008;28(3):120–125.
- Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10 (Suppl 2):S96–S101.
- Kao ST, Scott DD. A review of bone substitutes. Oral Maxillofac Surg Clin North Am. 2007;19(4):513–521, vi.
- Shingaki S, Nomura T, Takada M, et al. Squamous cell carcinomas of the mandibular alveolus: analysis of prognostic factors. Oncology. 2002;62(1):17–24.
- Niu LX, Feng ZE, Wang DC, et al. Prognostic factors in mandibular gingival squamous cell carcinoma: A 10-year retrospective study. Int J Oral Maxillofac Surg. 2017;46(2):137–143.
- Klotch DW, Prein J. Mandibular reconstruction using AO plates. Am J Surg. 1987;154(4):384–388.
- Maurer P, Eckert AW, Kriwalsky MS, et al. Scope and limitations of methods of mandibular reconstruction: a long-term follow-up. British J Oral Maxillofac Surg. 2010;48(2):100–104.
- Moura LB, Carvalho PH, Xavier CB, et al. Autogenous non-vascularized bone graft in segmental mandibular reconstruction: a systematic review. Int J Oral Maxillofac Surg. 2016;45(11):1388–1394.
- Nkenke E, Weisbach V, Winckler E, et al. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: a prospective study. Int J Oral Maxillofac Surg. 2004;33(2):157–163.
- Fernandes R. Fibula free flap in mandibular reconstruction. Atlas Oral Maxillofac Surg Clin North Am. 2006;14(2):143–150.
- Akbay E, Aydogan F. Reconstruction of isolated mandibular bone defects with non-vascularized corticocancellous bone autograft and graft viability. Auris Nasus Larynx. 2014;41(1):56–62.
- Okoturo E. Non-vascularised iliac crest bone graft for immediate reconstruction of lateral mandibular defect. Oral Maxillofac Surg. 2016;20(4):425–429.
- Lin K, Xia L, Li H, et al. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials. 2013;34(38):10028–10042.
- Stern A, Barzani G. Autogenous bone harvest for implant reconstruction. Dent Clin North Am. 2015;59(2):409–420.
- van Eijden TM. Biomechanics of the mandible. Crit Rev Oral Biol Med. 2000;11(1):123–136.
- Horiuchi K, Hattori A, Inada I, et al. Mandibular reconstruction using the double barrel fibular graft. Microsurgery. 1995;16(7):450–454.
- Liu YF, Wang R, Baur DA, et al. A finite element analysis of the stress distribution to the mandible from impact forces with various orientations of third molars. J Zhejiang Univ Sci B. 2018;19(1):38–48.
- Mohlhenrich SC, Heussen N, Ayoub N, et al. Three-dimensional evaluation of the different donor sites of the mandible for autologous bone grafts. Clin Oral Invest. 2015;19(2):453–458.
- Schubert W, Kobienia BJ, Pollock RA. Cross-sectional area of the mandible. J Oral Maxillofac Surg. 1997;55(7):689–692.
- Wong RW, Rabie AB. A quantitative assessment of the healing of intramembranous and endochondral autogenous bone grafts. Eur J Orthodont. 1999;21(2):119–126.
- Mertens C, Decker C, Seeberger R, et al. Early bone resorption after vertical bone augmentation-a comparison of calvarial and iliac grafts. Clin Oral Implants Res. 2013;24(7):820–825.