ABSTRACT
Background: Rape is a common traumatic event which may result in the development of posttraumatic stress disorder (PTSD), yet few studies have investigated risk biomarkers in sexually traumatised individuals. Adiponectin is a novel cytokine within inflammatory and cardiometabolic pathways with evidence of involvement in PTSD.
Objective: This prospective exploratory study in a sample of female rape survivors investigated the association of single nucleotide polymorphisms (SNPs) in the adiponectin gene (ADIPOQ) and posttraumatic stress symptom (PTSS) severity, and the interaction of these SNPs of interest with childhood trauma in modifying the association with PTSS severity.
Method: The study involved 455 rape-exposed black South African women (mean age (SD), 25.3 years (±5.5)) recruited within 20 days of being raped. PTSS was assessed using the Davidson Trauma Scale (DTS) and childhood trauma was assessed using a modified version of the Childhood Trauma Scale-Short Form Questionnaire. Eight ADIPOQ SNPs (rs17300539, rs16861194, rs16861205, rs2241766, rs6444174, rs822395, rs1501299, rs1403697) were genotyped using KASP. Mixed linear regression models were used to test additive associations of ADIPOQ SNPs and PTSS severity at baseline, 3 and 6 months following rape.
Results: The mean DTS score post-sexual assault was high (71.3 ± 31.5), with a decrease in PTSS severity shown over time for all genotypes. rs6444174TT genotype was inversely associated with baseline PTSS in the unadjusted model (β = −13.6, 95% CI [−25.1; −2.1], p = .021). However, no genotype was shown to be significantly associated with change in PTSS severity over time and therefore ADIPOQ SNP x childhood trauma interaction was not further investigated.
Conclusion: None of the ADIPOQ SNPs selected for investigation in this population were shown to be associated with change in PTSS severity over a 6-month period and therefore their clinical utility as risk biomarkers for rape-related PTSD appears limited. These SNPs should be further investigated in possible gene-gene and gene-environment interactions.
Highlights
Women who are sexually assaulted are at increased risk of PTSD.
The adiponectin gene (ADIPOQ) has been linked to cardiometabolic disease which frequently co-occurs with PTSD.
ADIPOQ variants were not shown to predict PTSD symptom severity post-rape in this population.
Antecedentes: La violación sexual es un evento traumático común que puede resultar en el desarrollo del trastorno de estrés postraumático (TEPT); no obstante, pocos estudios han investigado biomarcadores de riesgo en personas sexualmente traumatizadas. La adiponectina es una citocina recientemente involucrada en vías inflamatorias y cardiometabólicas que tienen evidencia de compromiso en el TEPT.
Objetivo: Este estudio prospectivo exploratorio, realizado en una muestra de mujeres sobrevivientes a violación sexual, investigó la asociación entre polimorfismos de nucleótido único (SNPs por sus siglas en inglés) en el gen de la adiponectina (ADIPOQ) y la severidad de los síntomas de estrés postraumático (SEPT), así como también cómo la interacción de estos SNPs sobre el trauma infantil modifica la asociación con la severidad de los SEPT.
Método: El estudio incluyó a 455 mujeres sudafricanas de raza negra expuestas a una violación sexual (edad promedio de 25,3 años ± 5,5) reclutadas 20 días después de haber sido violadas sexualmente. Los SEPT se evaluaron empleando la Escala de Trauma de Davidson (DTS por sus siglas en inglés) y el trauma infantil se evaluó empleando una versión modificada de la Escala de Trauma Infantil – Cuestionario de versión corta. Se realizó la genotipificación de ocho SNPs del gen ADIPOQ (rs17300539, rs16861194, rs16861205, rs2241766, rs6444174, rs822395, rs1501299, rs1403697) empleando el KASP. Se emplearon modelos de regresión lineal para evaluar las asociaciones aditivas entre los SNPs del gen ADIPOQ y la severidad de los SEPT de base, a los tres y a los seis meses luego de la violación sexual.
Resultados: El promedio del puntaje en la DTS luego de una violación sexual fue alto (71,3 ± 31,5) con una disminución en la severidad de los SEPT evidenciada a lo largo del tiempo para todos los genotipos. El genotipo rs6444174TT se encontró inversamente asociado a los SEPT de base en el modelo no ajustado (β = −13.6, 95% CI [−25.1; −2.1], p = .021). Sin embargo, ningún genotipo mostró estar asociado significativamente con cambios en la severidad de los SEPT a lo largo del tiempo y, por tanto, ya no se investigó la interacción entre los SNPs del gen ADIPOQ y el trauma infantil.
Conclusiones: Ninguno de los SNPs del ADIPOQ elegidos para esta investigación mostraron tener alguna asociación entre los cambios en la severidad de los SEPT en un periodo de seis meses y, por tanto, su utilidad clínica como marcadores de riesgo para el TEPT asociado a violación sexual es limitada. Se debería investigar más estos SNPs para evaluar las posibles interacciones gen-gen y gen-ambiente.
背景:强奸是一种常见的创伤事件,可能导致创伤后应激障碍 (PTSD) 的发展,但很少有研究考查性创伤个体的风险生物标志物。脂联素是炎症和心血管代谢途径中的一种新型细胞因子,有证据表明它参与了 PTSD。
目的:本前瞻性探索性研究在一个女性强奸幸存者样本中,考查了脂联素基因 (ADIPOQ) 中的单核苷酸多态性 (SNP) 与创伤后应激症状 (PTSS) 严重程度的关联,以及这些感兴趣的 SNP 与童年期创伤在修饰 PTSS 严重性相关关联的交互作用。
方法:本研究纳入在被强奸后 20 天内招募的 455 名遭遇强奸的南非黑人女性(平均年龄 (SD),25.3 岁 (±5.5))。 PTSS 使用戴维森创伤量表 (DTS) 进行评估,童年期创伤使用童年期创伤量表 – 简表修订版进行评估。使用 KASP 对八个 ADIPOQ SNP(rs17300539、rs16861194、rs16861205、rs2241766、rs6444174、rs822395、rs1501299、rs1403697)进行基因分型。使用混合线性回归模型在基线、强奸后 3 个月和 6 个月时考查 ADIPOQ SNP 和 PTSS 严重程度的加性关联。
结果:性侵犯后的平均 DTS 得分很高(71.3 ± 31.5),所有基因型的 PTSS 严重程度都随时间降低。在无调节的模型中,rs6444174TT 基因型与基线 PTSS 呈负相关(β = −13.6, 95% CI [−25.1; −2.1], p = .021)。然而,没有显示基因型与 PTSS 严重程度随时间的变化显著相关,因此没有进一步研究 ADIPOQ SNP x 儿童创伤的交互作用。
结论:在该人群中选择用于考查的 ADIPOQ SNP 均未显示与 6 个月期间 PTSS 严重程度的变化相关,因此它们作为强奸相关 PTSD 风险生物标志物的临床效用似乎有限。应该在可能的基因-基因和基因-环境相互作用中进一步研究这些 SNP。
1. Introduction
Posttraumatic stress disorder (PTSD) is disabling and often chronic psychiatric disorder that may develop following exposure to actual or threatened death, serious injury or sexual violence (American Psychiatric Association, Citation2013; Kessler et al., Citation2005). Compared to other traumas, sexual violence, including rape, is associated with the highest risk of developing PTSD (Dworkin, Citation2018; Scott et al., Citation2018). In South Africa, rape is prevalent, and disproportionately affects women (Abrahams et al., Citation2014; Ajayi et al., Citation2021; Steele et al., Citation2019). Despite experiencing the same trauma, post-sexual assault, the severity, and course of PTSD symptoms (PTSS) vary across individuals (Dworkin et al., Citation2021). Most individuals experience severe PTSS in the immediate aftermath of a sexual assault, with most recovery from PTSS occurring within the first 3 months post-sexual assault (Dworkin et al., Citation2021). Variability in individual risk for, and persistence of PTSS is likely due to underlying differences in biological processes, possibly genetically driven. The heritability of PTSD is estimated to range from 30 to 40% (Duncan et al., Citation2018; Koenen, Citation2007; Nugent et al., Citation2008). Although a number of studies have examined psychosocial correlates of PTSS in female assault victims (Mgoqi-Mbalo et al., Citation2017; Nöthling et al., Citation2022; Tiihonen Möller et al., Citation2014), few biological, including genetic studies exploring mechanisms underlying differential susceptibility for PTSD have been conducted (D’Elia et al., Citation2021; Nöthling et al., Citation2021; Thurston et al., Citation2021). A biomarker that could predict PTSD symptom trajectory after sexual trauma may facilitate early detection and intervention for PTSD and may also advance our understanding of underlying biological mechanisms.
Among several proteins secreted by adipocytes (fat cells), adiponectin is prominent because of its protective metabolic properties, including insulin-sensitising, antiatherogenic (preventing formation of fatty deposits in arteries) and anti-inflammatory effects (Ohashi et al., Citation2012). Adiponectin is encoded by ADIPOQ which spans approximately 15.8 kb, comprises three exons, two introns and is located on chromosome 3q27 (Maeda et al., Citation1996). Low circulatory levels of adiponectin are considered risk factors for cardiometabolic diseases (CMD) such as Type 2 diabetes mellitus (T2DM) and metabolic syndrome (Kadowaki et al., Citation2006; Lee & Shin, Citation2020; von Frankenberg et al., Citation2017). Likewise, various single nucleotide polymorphisms (SNPs) of ADIPOQ have been linked to MetS, T2DM, and cardiovascular disease (Farooq et al., Citation2018; Vionnet et al., Citation2000; Yuan et al., Citation2016) all common comorbidities in individuals with PTSD (Lihua et al., Citation2020; Scherrer et al., Citation2019). Inflammatory and metabolic mechanisms may play a role in the neurobiology of PTSD (Farr et al., Citation2014; Mellon et al., Citation2018; O’Donovan, Citation2016). Specifically, PTSS severity has been associated with higher levels of inflammation (Fonkoue et al., Citation2020).
Adiponectin can cross the blood–brain barrier and exerts neurotrophic and neuroprotective effects in various brain regions (Bloemer et al., Citation2019; Formolo et al., Citation2022). Adiponectin stimulates hippocampal neurogenesis (Zhang et al., Citation2016) and in a mouse model of PTSD, adiponectin infusions into the dentate gyrus of the hippocampus facilitated context-specific fear extinction learning (Zhang et al., Citation2017). Biomarker studies in human have shown that, compared to healthy controls, individuals with PTSD symptomatology (Na et al., Citation2017) and childhood trauma exposure (Lehto et al., Citation2012) exhibit decreased adiponectin levels. In addition, associations between ADIPOQ gene variants and other neuropsychiatric disorders that are mechanistically linked to PTSD, such as depression, have been shown (Awofala et al., Citation2020; Raheja et al., Citation2017; Wang et al., Citation2015). This makes ADIPOQ an interesting candidate gene to explore in relation to PTSD.
No human genetic study of the association between ADIPOQ and PTSD exists. Genome-wide association studies (GWAS) represent the current most robust and unbiased method to assess associations between genetic variants and complex diseases, such as PTSD (Uffelmann et al., Citation2021). Although PTSD GWASs have led to the identification of several novel risk variants not previously explored using biologically driven candidate-gene approaches, GWASs are not without limitations (Duncan et al., Citation2018; Nievergelt et al., Citation2019; Stein et al., Citation2021). To date, large-scale PTSD GWASs have not identified any genetic variants that exceeded genome wide significance (Duncan et al., Citation2018; Nievergelt et al., Citation2019; Stein et al., Citation2021). Furthermore, although one of the largest PTSD-GWAS to date, published by the Psychiatric Genomics Consortium (PGC)-PTSD, included South African samples in their analyses (N = 387); predominantly, samples analysed were of European ancestry (N = 9954) (Duncan et al., Citation2018). Studies among diverse population groups, specifically of African descent is a well-recognised research need and priority (Dalvie et al., Citation2015; Popejoy & Fullerton, Citation2016). Other limitations of GWASs include examining PTSD as a binary outcome (Duncan et al., Citation2018; Nievergelt et al., Citation2019). PTSD symptom severity and trajectories differ according to the index trauma type (Graham et al., Citation2016; Kessler et al., Citation2017). In addition, no GWAS has included cohorts that have exclusively focused on sexual trauma, and it remains unknown whether genetic risk for PTSD differs by trauma-type (Stein et al., Citation2021). Thus, a gap in knowledge exists regarding the genetic contribution to PTSS in sexually traumatised individuals, specifically of African descent.
This prospective exploratory study investigated (i) whether selected single nucleotide polymorphisms (SNPs) of the ADIPOQ gene are associated with change in PTSD symptom (PTSS) severity over 6 months and, (ii) given previous associations shown between adiponectin levels and childhood trauma (Lehto et al., Citation2012), sought to examine whether these SNPs modify the associations between childhood trauma exposure and PTSS severity in a sample of female rape survivors. Identification of genetic variants that are linked to PTSD development may provide insights into underlying disease mechanisms and assist in identifying individuals at risk early.
2. Materials and methods
2.1. Participants and phenotyping
The data for the present study are derived from the Rape Impact Cohort Evaluation (RICE) study, a large prospective comparative cohort study evaluating the impact of rape on adult women’s physical and mental health and their use of health services in South Africa over a 3-year period (Abrahams et al., Citation2017). The design and selection of participants for the RICE study have previously been described in detail (Abrahams et al., Citation2017). Briefly, rape-exposed participants were enrolled within 20 days post-rape incident, on average 12 days from rape care centres in the KwaZulu Natal Province of South Africa. Assessments were conducted at baseline and every 3 months in the 1st year and every 6 months thereafter. Recruitment was restricted to female participants between 16 and 40 years. Participants were excluded from the RICE study if they were too emotionally distressed at the time of enrolment to provide informed consent, intellectually disables or more than 14 weeks pregnant. For this sub-study, we included a total of 455 rape-exposed (RE) participants for whom baseline DNA as well as physical and mental health information was available for the baseline, 3- and 6-month visits.
2.2. Data collection
At the baseline and follow-up visits, participants completed a set of mental health assessments, including self-reported measures of risk and protective factors for the development of PTSD. Questionnaires were administered by trained research assistants who were supervised by a senior psychiatrist. Participants also underwent a physical examination and provided blood samples.
For this sub-study, we included a total of 455 rape-exposed participants for whom baseline DNA as well as physical and mental health information was available for the baseline, 3- and 6-month visits.
2.2.1. Genetics
2.2.1.1. Genome extraction
Genomic DNA was extracted from 3 ml of whole blood samples collected in an EDTA tube according to the manufacturer's instructions, using the Gentra Puregene Blood kit (Qiagen). Following DNA extraction, DNA quantification (ng/µl) and quality (absorbance ratio: 260/280 and 260/230) were determined using a Nanodrop spectrophotometer (ThermoFisher Scientific). DNA was subsequently diluted to 20 ng/ul.
2.2.1.2. Selection and genotyping of polymorphisms
Eight ADIPOQ SNPs were selected a priori after reviewing the literature: rs16861194, rs16861205, rs17300539, rs2241766, rs6444174, rs822395, rs1402697 and rs1501299. These SNPs were selected based on the following criteria: (1) they capture variations in the major blocks of the gene, (2) are functionally relevant and have been studied by others in relation to cardiometabolic diseases such as diabetes and MetS (all of the selected SNPs) (de Luis et al., Citation2016; Howlader et al., Citation2021; Siitonen et al., Citation2011; Wu et al., Citation2014; Yuan et al., Citation2016), (3) they have previously been associated with neuropsychiatric disorders related to PTSD, such as depression (rs2241766, rs1501299) (Awofala et al., Citation2020; Raheja et al., Citation2017; Wang et al., Citation2015) and (4) they have a minor allele frequency (MAF) ≥5% in African populations (all of the selected SNPs).
The MAF for all SNPs was obtained from Ensemble 1000 genomes project (http://www.ensembl.org/Homo_sampiens/Info/Index). The African (AFR) MAF was determined using published data from seven sub-population, including Yoruba and Esan in Nigeria, Luhya in Webuye, Kenya, Gambian in Western Divisions, Gambia, Mende in Sierra Leone, Americans of African Ancestry in South-West U.S.A. and African Caribbean in Barbados, Supplemental File S1.
The SNPs were genotyped using Kompetitive allele-specific PCR (KASP) genotyping (LGC Biosearch Technologies), according to the manufacturer’s instructions. KASP is a novel, fluorescence-based method of SNP genotyping that only requires a few SNP markers to genotype various samples (He et al., Citation2014). Linkage disequilibrium (LD) was assessed using Haploview version 4.2 (Barrett et al., Citation2005) and haploblocks were determined using the confidence bound method of Gabriel et al (Gabriel et al., Citation2002).
2.2.1.3. Genotyping quality control (QC) procedures
Quality control evaluations were undertaken for all laboratory procedures. Data were automatically quality control checked on a per SNP basis. No template controls (NTCs) were included on each plate to enable the detection of contamination or non-specific amplification. In addition, SNPs with a MAF ≤ 0.01 or those that deviated from the expected Hardy-Weinberg equilibrium (p < .05) and SNPs with a call rate <90% were removed. After an initial 35 cycles of PCR, all genotypic reaction plates run at LGC were read on a BMG PHERAStar plate reader and visually inspected to assess the progression of the PCR reaction. Plates were then recycled (3 cycles per recycle step) and read after each recycle step. Read data were visually inspected by a laboratory operator after each recycle step until they were satisfied that the PCR reaction had reached endpoint. After genotyping was complete and prior to statistical analysis being performed, genotypes were reviewed by a programmer.
2.2.2. Mental health measures
2.2.2.1. Posttraumatic stress symptoms
The Davidson Trauma Scale (DTS) is a 17-item self-report questionnaire of posttraumatic stress symptoms and was used to determine PTSS severity at all time points (Davidson et al., Citation1997). RE participants completed the DTS in relation to the recent rape event. From the 17 items, participants were first asked to record ‘the trauma that is most disturbing to you’. Participants were then asked, for all items, to ‘consider how often in the last week the symptom troubled you and how severe it was.’ The first five items specifically refer to reexperiencing or avoiding the disturbing event. Reponses were measured on a 5-point Likert scale for symptom frequency ranging from 0 (‘not at all’) to 4 (‘every day’) and symptom severity ranging from 0 (‘not at all distressing’) to 4 (‘extremely distressing’). The DTS total score was computed by adding all item responses together, i.e. both frequency and severity scores, with a possible range of 0–136. A total score of 40 or more is considered indicative of PTSD (Davidson et al., Citation1997). The DTS showed excellent reliability in this study at each timepoint with a Cronbach alpha score of .92 at baseline, .91 at 3 months and .93 at 6 months post-rape.
2.2.2.2. Past trauma
A modified version of the Childhood Trauma Questionnaire (CTQ-SF), revised in South Africa, was used to screen for trauma exposure before the age of 18 years (Bernstein et al., Citation2003; Chirwa et al., Citation2018). The modified version of the CTQ-SF consists of 14 items which are grouped into the following subthemes: (i) witnessing abuse of mother by father or boyfriend, and childhood experiences of (ii) sexual abuse, (iii) physical abuse, (iv) emotional abuse, and (v) parental neglect. All the items were measured on a 4-point scale (1 = never, 2 = sometimes, 3 = often, 4 = very often), total score range between 14 and 56.
A modified version of the Life Events Checklist (LEC) (Nduna et al., Citation2010; Weathers et al., Citation2013) was used to measure direct exposure to nine trauma type using a dichotomous ‘yes/no’ response. The trauma types measured were (1) imprisonment, (2) civil unrest/war, (3) serious injury, (4) being close to death, (5) murder of a family member or friend, (6) unnatural death of a family member or friend, (7) murder of a stranger(s), (8) robbed at gun- or knifepoint, (9) kidnapped and (10) sexual assault. The number of ‘yes’ responses were added together to yield a total score ranging from 0 to 10, indicating the cumulative lifetime trauma load of participants.
2.2.2.3. Depression
The Center for Epidemiologic Studies Depression Scale (CES-D) was administered at all time points and used to screen for the presence of clinically significant depressive symptoms (Radloff, Citation1977). The CES-D is a twenty-item, self-report measure with responses measured on a 4-point Likert scale ranging from 0 (‘rarely or none of the time’) to 3 (‘most or all of the time’), total score range between 0 and 60. A cut-off score of ≥16 has been recommended as indicative of depression caseness with good sensitivity, specificity and high internal consistency (Lewinsohn et al., Citation1997).
2.2.2.4. Previous rape exposure
Women who reported previous (lifetime) exposure to sexual violence, other than the recent rape (within 20 days), were identified with three screening questions at the baseline interview. Participants were asked to indicate whether exposed to one/more of the following: intimate partner rape and/or non-partner rape and/or first sexual intercourse that was forced/rape. Non-partner rape (NPR) was measured using three items: (1) forced to have sex, (2) forced to have sex when under the influence of alcohol/drugs, and (3) gang raped. Intimate partner rape was measured through a single item, i.e. forced to have sex by a current or previous intimate partner.
2.2.3. Physical health
2.2.3.1. Clinical assessments for the presence of medical conditions
The presence of medical conditions (specifically; hypertension, diabetes, and HIV) was determined through self-reported medical history on the administered questionnaires, clinical measurements, and biochemical assessments of blood samples collected by trained personnel.
2.2.3.2. Adiposity-measures
We included body mass index (BMI) as a proxy indicator of generalised obesity. Trained fieldworkers measured height and weight using standard techniques. Weight was measured on a calibrated scale with participants in light clothing, without shoes, and recorded to nearest 0.5 kilograms (kg). Height was measured using a stadiometer in a standing upright position without shoes. BMI was calculated using Quetelet’s formula as weight (kg) divided by height (in square metres) (Nishida et al., Citation2010). The BMI was classified into underweight (<18.5 kg/m2), normal (18–24.9 kg/m2), overweight (25–29.9 kg/m2), obese (≥30 kg/m2).
2.3. Ethics statement
All participants provided written informed consent, and the study protocol was reviewed and approved by the Health Research Ethics Committee at Stellenbosch University in Cape Town, South Africa (N08/02/040 and S17/10/265 for the parent- and this sub-study, respectively) and Human Research Ethics Committee at the South African Medical Research Council (EC019-10/2013). All methods were carried out in accordance with the Declaration of Helsinki 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
3. Statistical analyses
Simple descriptive statistics were computed to summarise patient characteristics and ADIPOQ SNPs using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Deviation from Hardy-Weinberg Equilibrium (HWE) (p < .05) was examined for each SNP using Fisher’s exact test (Hosking et al., Citation2004; Xu et al., Citation2002). The evolution of PTSS up to 6 months was graphically explored using average profile plots for each ADIPOQ SNP.
3.1. Missing data and imputation process
Responses to questionnaires were recorded electronically and an interviewer could not proceed until an item response had been captured. This ensured that there were no missing values at an item level. However, an uploading error resulted in missing value sets for the symptom severity subscale of the DTS which was corrected once it was identified. There were no missing values for the DTS frequency subscale, but 56% (n = 254) of the DTS symptom severity scores were missing at baseline, 46% (n = 209) were missing at 3 months and 37% (n = 127) were missing at 6 months post-rape. In the present study, participants with both severity and frequency scores (i.e. those participants without missing data in the DTS scale) had either the same score for severity as for frequency, or very close to their frequency score. Missing DTS symptom severity values were imputed in the RICE study using a multiple imputation model.
The multiple imputation method used follows a Bayesian iterative Markov chain Monte Carlo (MCMC) procedure which assumes that all the variables in the imputation model have a multivariate normal distribution and use a uniform prior distribution (Carlin & Chib, Citation1995). The imputation model included baseline previous traumatic experiences as covariates. Prior to using multiple imputation, missing data patterns were examined. The DTS overall score was then derived using imputed DTS frequency and severity score and bounded to follow the range of the original scale.
3.2. Genetic association analyses
Linear mixed models were used to evaluate the association between each of the selected ADIPOQ variants and PTSS severity (continuous DTS scores) over time. We considered a range of potential confounding factors which were a priori selected with reference as risk factors for PTSD. To prevent overfitting of the model, each of these variables was individually tested against the PTSS outcome at baseline and those variables displaying a significant association with PTSS at baseline were corrected for in the primary analyses (Supplemental file, S1). Models were adjusted for potential confounding by namely relationship status, depression (CES-D total score), lifetime trauma exposure (LEC total score), and previous rape exposure. An additive genetic model [two copies of the major allele (coded as 0) vs one copy of the major and minor allele (coded as 1) versus two copies of the minor allele (coded as 2)] were used for all models. Depending on the main effects in the linear mixed modelling, we sought to examine interaction effects of the genotype with childhood trauma on PTSS outcome over time (i.e. for SNPs that showed significant differences in the rate of change in PTSS in unadjusted and adjusted mixed linear models). Multiple testing was conducted using the Benjami Hochberg adjustment at 10% false discovery rate and the adjusted p-value (p < .025) was used to detect statistical significance (Benjamini et al., Citation2001). Statistical analyses were conducted using STATA 17.
4. Results
4.1. Sociodemographic characteristics of participants
The clinical characteristics of the study sample are presented in . The mean age of the 455 RE females included in this study was 25.3(5.5) years. Most participants were unemployed (73.6%, n = 335), and 48.6% (n = 221) tested positive for HIV infection at baseline. The mean PTSS score at baseline was high for the sample; 71.3(31.5). More than half of the participants (51.4%, n = 234) indicated a past rape experience other than the most recent rape event.
Table 1. General characteristics of the study cohort (N = 455).
4.2. Genetics
A total of eight SNPs in ADIPOQ (rs16861194, rs16861205, rs17300539, rs2241766, rs6444174, rs822395, rs1403697 and rs1501299) were genotyped for each participant. rs1501299 and rs1403697 were found to not be in HWE and were therefore excluded from further analysis. SNP information including observed MAF and HWE p-values are shown in . The linkage disequilibrium (LD) plot for the six ADIPOQ polymorphisms in HWE is presented in . No ADIPOQ haploblocks were identified, and thus no haplotype analysis was performed.
Figure 1. Linkage disequilibrium (LD) map. Linkage disequilibrium for the 8 ADIPOQ polymorphisms investigated. D’ values are depicted in the diamonds, with darker colours depicting stronger LD. The LD map was created using the confidence interval of LD, implemented in Haploview version 4.2.
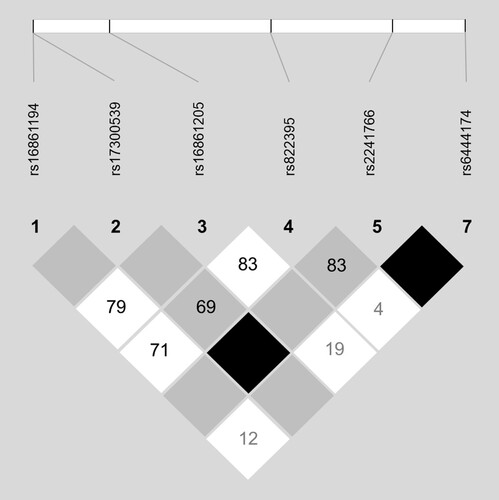
Table 2. Characteristics of the eight SNPs genotyped in 455 participants.
4.3. Associations between ADIPOQ SNPs and PTSS
The genotype distribution of the selected SNPs and corresponding PTSS scores at baseline, 3- and 6-month follow-up is shown in . Most of the improvement in PTSS severity occurred within the first 3 months followed by a slower rate of improvement between 3 and 6 months for all genotypes, except for participants with rs17300539GA and rs644174CC genotypes, who had a non-significant increase in PTSS severity (mean DTS increase score of 0.5, p = .954; 1.4, p = .806) between 3 and 6 months follow up. A graphical depiction of the PTSS trajectory over 6 months for all SNPs is presented in Supplemental file, S2. The association between the 6 SNPs (those in HWE) and PTSS scores was investigated using a linear mixed model at 3- and 6-month follow-up (). The rs6444174CC genotype was significantly associated with PTSS at baseline (β = −13.6, 95% CI [−25.2; −2.1], p = .021). None of the genotypes were significantly associated with PTSS in the unadjusted or adjusted models at follow-up.
Table 3. Genotype distribution of the selected ADIPOQ SNPs with PTSS severity at baseline, 3 and 6 months.
Table 4. Linear mixed model of ADIPOQ SNPs and posttraumatic stress symptom (PTSS) severity at 3- and 6-month follow up.
4.4. Gene × environment interaction analyses
We did not conduct further analysis investigating ADIPOQ SNP × childhood trauma interaction effects on PTSS, due to the absence of significant associations in linear mixed models.
5. Discussion
In the present study, the association between selected ADIPOQ SNPs and PTSS severity was explored longitudinally among adult female rape survivors. Consistent with previous research, we found a high prevalence of PTSS in this cohort in the days following the rape, with the majority of PTSS recovery occurring within the first 3 months post-sexual assault (Dworkin et al., Citation2021). At the baseline assessment, the mean DTS score was markedly higher than the recommended cut-off for clinically significant PTSS. Although two of the examined SNPs (rs17300539GA and rs6444174CC) were associated with non-significant increases in PTSS severity between 3 and 6 months; none of the six ADIPOQ SNPs were shown to be associated with PTSS severity over time.
To the best of our knowledge, this is the first study to investigate the association between ADIPOQ and PTSD. Adiponectin is best known for its associations with cardiometabolic diseases (Chen et al., Citation2012; Davis et al., Citation2015; Ghadge et al., Citation2018). Genetic variations in ADIPOQ have been shown to be associated with obesity, T2DM, hypertension and related phenotypes in various populations (Farooq et al., Citation2018; Wu et al., Citation2014; Yuan et al., Citation2016). High rates of cardiometabolic disease among individuals with PTSD suggest that there may be mechanistic links underlying these disorders. This is of relevance among victims of sexual assault who show an increased risk for both PTSD and cardiometabolic disease longitudinally (Ba & Bhopal, Citation2017; Dworkin, Citation2018; Oshodi et al., Citation2016). To date, ADIPOQ has not been linked with PTSD in PTSD-GWAS (Duncan et al., Citation2018; Gelernter et al., Citation2019; Nievergelt et al., Citation2019; Stein et al., Citation2021), however given differences in ethnicity, trauma types, trauma severity and gender distribution between currently published GWAS and our study, we undertook to investigate this gene in our population. In addition, GWAS cannot identify all genetic determinants of complex traits (Tam et al., Citation2019).
There are several potential reasons why our analysis did not reveal any significant association between examined SNPs and PTSS in this cohort. First, the relatively small sample may have increased risk of a Type II error which could have masked an association or decreased statistical power. Only a limited number of ADIPOQ SNPs were examined in this study; the investigated SNPs were not functional SNPs and thus unlikely to represent the causal SNPs. Gene-gene and gene-environment interactions may also contribute to PTSD risk, which were not accounted for in the present study. ADIPOQ SNP x dietary interactions have been shown to influence risk of MetS (Coltell et al., Citation2021; Ferguson et al., Citation2010). Epistatic (gene-gene) interactions are considered to play a major role in susceptibility to complex diseases (Nagel, Citation2005; Wei et al., Citation2014). It has been proposed that epistasis could explain some of the ‘missing heritability’ undetected by PTSD-GWAS (Manolio et al., Citation2009; Zhang et al., Citation2019). There is a strong body of evidence showing that PTSD is associated with a dysregulated HPA-axis resulting in altered cortisol levels (Dunlop & Wong, Citation2019; Yehuda et al., Citation2000). The FK506 binding protein 5 (FKBP5) is a major regulatory protein of the HPA axis, which binds to glucocorticoid receptors and modulates glucocorticoid sensitivity (Fani et al., Citation2016; Zannas et al., Citation2016). SNPs in the FKBP5 gene have been shown to be associated with reduced cortisol-levels (Velders et al., Citation2011) and PTSD (Kang et al., Citation2019; Zhang et al., Citation2020). Interestingly, cortisol has been shown to have an inhibitory effect on adiponectin (Fallo et al., Citation2004; Fernandez-Real et al., Citation2005; Gavrila et al., Citation2003). Given this reported negative correlation between circulating adiponectin and cortisol levels, the potential intermolecular epistatic interactions between ADIPOQ and FKBP5 may be avenue of further study to identify individuals at risk for PTSD. However, investigation of such interactions would require a much larger sample size to attain optimal statistical power (Murcray et al., Citation2011). Future research could seek to determine whether epigenetic modification of the ADIPOQ locus influences PTSD risk. Of interest, the promotor region of ADIPOQ contains consensus sequences for the glucocorticoid receptor and could, therefore, be subject to environmental modifications in response to stress (Comuzzie et al., Citation2001). Lastly, rare variants, which were not investigated in the present study, may also affect disease risk. Replication of this analysis in a much larger sample is required to conclusively rule out an association between the investigated ADIPOQ SNPs and rape-related PTSD.
6. Strengths and limitations
Findings from this study should be interpreted in the context of several unavoidable limitations. First, this study used a candidate gene approach to assess the role of ADIPOQ variants in predicting PTSS development following rape. To data, candidate gene studies have largely been underpowered due to small sample sizes and GWAS approaches are increasingly favoured in PTSD genetics research. Nevertheless, we believe our study has merit and utility given that it was conducted among sexually traumatised individuals of African ancestry, both understudied populations in genetic research. Second, we were unable to account for the genomic ancestral substructure of this sample as this information was not available (Tian et al., Citation2008). Third, the sample was small providing limited statistical power for detecting the effect of adiponectin genotype on PTSD status. Fourth, our study results may not be readily generalisable to male, other ethnic population groups or other trauma types. The strengths of our study include its prospective design, comprehensive assessment of known risk and predictive factors in a well characterised and a relatively understudied population in psychiatric genetics and a homogenous index trauma. Findings are considered preliminary, and replication is needed in large, well characterised samples exposed to other types of interpersonal trauma.
7. Conclusions
ADIPOQ polymorphisms were not associated with PTSS in this population of rape-exposed black South African women. These findings suggest that these SNPs may not be clinically helpful to predict PTSS severity post-sexual assault. Replications studies in larger, mixed sex trauma samples followed over a longer time course (> 6 months) are warranted as is the examination of other ADIPOQ variants. The role of these SNPs in possible gene-gene and gene-environment interactions also remains to be established.
Supplemental Material
Download MS Word (226 KB)Acknowledgements
Thank you to Ryan Tran for assistance with images and Natasha Kitchin for management of genetic samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are being used to address additional study aims and cannot be shared publicly at this stage. The study collaborative team will review individual requests to access data.
Additional information
Funding
References
- Abrahams, N., Devries, K., Watts, C., Pallitto, C., Petzold, M., Shamu, S., & García-Moreno, C. (2014). Worldwide prevalence of non-partner sexual violence: A systematic review. Lancet, 383(9929), 1648–1654. https://doi.org/10.1016/S0140-6736(13)62243-6
- Abrahams, N., Seedat, S., Lombard, C., Kengne, A. P., Myers, B., Sewnath, A., Mhlongo, S., Ramjee, G., Peer, N., Garcia-Moreno, C., & Jewkes, R. (2017). Study protocol for a longitudinal study evaluating the impact of rape on women’s health and their use of health services in South Africa. BMJ Open, 7(9), e017296. https://doi.org/10.1136/bmjopen-2017-017296
- Ajayi, A. I., Mudefi, E., & Owolabi, E. O. (2021). Prevalence and correlates of sexual violence among adolescent girls and young women: Findings from a cross-sectional study in a South African university. BMC Women’s Health, 21(1), 1–9. https://doi.org/10.1186/S12905-021-01445-8/TABLES/4
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.).
- Awofala, A. A., Ogundele, O. E., & Adekoya, K. O. (2020). ADIPOQ gene is linked to emotional eating behaviour in young Nigerian adults independent of psychological traits. Bulletin of the National Research Centre, 44(1), 195. https://doi.org/10.1186/s42269-020-00450-5
- Ba, I., & Bhopal, R. S. (2017). Physical, mental and social consequences in civilians who have experienced war-related sexual violence: A systematic review (1981–2014). Public Health, 142, 121–135. https://doi.org/10.1016/j.puhe.2016.07.019
- Barrett, J. C., Fry, B., Maller, J., & Daly, M. J. (2005). Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics, 21(2), 263–265. https://doi.org/10.1093/bioinformatics/bth457
- Benjamini, Y., Drai, D., Elmer, G., Kafkafi, N., & Golani, I. (2001). Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research, 125(1–2), 279–284. https://doi.org/10.1016/S0166-4328(01)00297-2
- Bernstein, D. P., Stein, J. A., Newcomb, M. D., Walker, E., Pogge, D., Ahluvalia, T., Stokes, J., Handelsman, L., Medrano, M., Desmond, D., & Zule, W. (2003). Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse & Neglect, 27(2), 169–190. https://doi.org/10.1016/S0145-2134(02)00541-0. Retrieved February 8, 2019, from http://www.ncbi.nlm.nih.gov/pubmed/12615092
- Bloemer, J., Pinky, P. D., Smith, W. D., Bhattacharya, D., Chauhan, A., Govindarajulu, M., Hong, H., Dhanasekaran, M., Judd, R., Amin, R. H., Reed, M. N., & Suppiramaniam, V. (2019). Adiponectin knockout mice display cognitive and synaptic deficits. Frontiers in Endocrinology, 10, 819. https://doi.org/10.3389/fendo.2019.00819
- Carlin, B. P., & Chib, S. (1995). Bayesian model choice via Markov chain Monte Carlo methods. Journal of the Royal Statistical Society: Series B (Methodological), 57(3), 473–484. https://doi.org/10.1111/j.2517-6161.1995.tb02042.x. http://www.jstor.org/stable/2346151
- Chen, S. J., Yen, C. H., Huang, Y. C., Lee, B. J., Hsia, S., & Lin, P. T. (2012). Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS One, 7(9), 1–5. https://doi.org/10.0.5.91/journal.pone.0045693
- Chirwa, E. D., Sikweyiya, Y., Addo-Lartey, A. A., Ogum Alangea, D., Coker-Appiah, D., Adanu, R. M. K., & Jewkes, R. (2018). Prevalence and risk factors of physical or sexual intimate violence perpetration amongst men in four districts in the central region of Ghana: Baseline findings from a cluster randomised controlled trial. PLoS One, 13(3), e0191663. https://doi.org/10.1371/journal.pone.0191663
- Coltell, O., Ortega-Azorín, C., Sorlí, J. V., Portolés, O., Asensio, E. M., Saiz, C., Barragán, R., Estruch, R., & Corella, D. (2021). Circulating adiponectin and its association with metabolic traits and type 2 diabetes: Gene-diet interactions focusing on selected gene variants and at the genome-wide level in high-cardiovascular risk Mediterranean subjects. Nutrients, 13(2). https://doi.org/10.3390/nu13020541
- Comuzzie, A. G., Funahashi, T., Sonnenberg, G., Martin, L. J., Jacob, H. J., Black, A. E. K., Maas, D., Takahashi, M., Kihara, S., Tanaka, S., Matsuzawa, Y., Blangero, J., Cohen, D., & Kissebah, A. (2001). The genetic basis of plasma variation in adiponectin, a global endophenotype for obesity and the metabolic syndrome. The Journal of Clinical Endocrinology & Metabolism, 86(9), 4321–4325. https://doi.org/10.1210/jcem.86.9.7878
- Dalvie, S., Koen, N., Duncan, L., Abbo, C., Akena, D., Atwoli, L., Chiliza, B., Donald, K. A., Kinyanda, E., Lochner, C., Mall, S., Nakasujja, N., Newton, C. R., Ramesar, R., Sibeko, G., Teferra, S., Stein, D. J., & Koenen, K. C. (2015). Large scale genetic research on neuropsychiatric disorders in African populations is needed. EBioMedicine, 2(10), 1259–1261. https://doi.org/10.1016/j.ebiom.2015.10.002
- Davidson, J. R. T., Book, S. W., Colket, J. T., Tupler, L. A., Roth, S., David, D., Hertzberg, M., Mellman, T., Beckham, J. C., Smith, R. D., Davison, R. M., Katz, R., & Feldman, M. E. (1997). Assessment of a new self-rating scale for post-traumatic stress disorder. Psychological Medicine, 27(1), 153–160. https://doi.org/10.1017/S0033291796004229
- Davis, S. K., Gebreab, S. Y., Xu, R., Riestra, P., Khan, R. J., Sumner, A. E., Hickson, D., & Bidulescu, A. (2015). Association of adiponectin with type 2 diabetes and hypertension in African American men and women: The Jackson heart study. BMC Cardiovascular Disorders, 15(1). https://doi.org/10.1186/s12872-015-0005-5
- de Luis, D. A., Izaola, O., de La Fuente, B., Primo, D., Ovalle, H. F., Romero, E., & Fernandez Ovalle, H. (2016). Rs1501299 polymorphism in the adiponectin gene and their association with total adiponectin levels, insulin resistance and metabolic syndrome in obese subjects. Annals of Nutrition and Metabolism, 69(3–4), 226–231. https://doi.org/10.1159/000453401
- D’Elia, A. T. D., Juruena, M. F., Coimbra, B. M., Mello, M. F., Mello, A. F., Rafati, F., Sharif Nia, H., Khoshnood, Z., & Allen, K.-A. (2021). Posttraumatic stress disorder (PTSD) and depression severity in sexually assaulted women: Hypothalamic-pituitary-adrenal (HPA) axis alterations. BMC Psychiatry, 21(1), 1–12. https://doi.org/10.1186/s12888-020-02964-8
- Duncan, L. E., Ratanatharathorn, A., Aiello, A. E., Almli, L. M., Amstadter, A. B., Ashley-Koch, A. E., Baker, D. G., Beckham, J. C., Bierut, L. J., Bisson, J., Bradley, B., Chen, C.-Y., Dalvie, S., Farrer, L. A., Galea, S., Garrett, M. E., Gelernter, J. E., Guffanti, G., Hauser, M. A., … Koenen, K. C. (2018). Largest GWAS of PTSD (N = 20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Molecular Psychiatry, 23(3), 666–673. https://doi.org/10.1038/mp.2017.77
- Dunlop, B. W., & Wong, A. (2019). The hypothalamic-pituitary-adrenal axis in PTSD: Pathophysiology and treatment interventions. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 89, 361–379. https://doi.org/10.1016/j.pnpbp.2018.10.010
- Dworkin, E. R. (2018). Risk for mental disorders associated with sexual assault: A meta-analysis. Trauma, Violence, and Abuse. https://doi.org/10.1177/1524838018813198
- Dworkin, E. R., Jaffe, A. E., Bedard-Gilligan, M., & Fitzpatrick, S. (2021). PTSD in the year following sexual assault: A meta-analysis of prospective studies. Trauma, Violence, & Abuse. https://doi.org/10.1177/15248380211032213
- Fallo, F., Scarda, A., Sonino, N., Paoletta, A., Boscaro, M., Pagano, C., Federspil, G., & Vettor, R. (2004). Effect of glucocorticoids on adiponectin: A study in healthy subjects and in Cushing’s syndrome. European Journal of Endocrinology, 150(3), 339–344. https://doi.org/10.1530/eje.0.1500339
- Fani, N., King, T. Z., Shin, J., Srivastava, A., Brewster, R. C., Jovanovic, T., Bradley, B., & Ressler, K. J. (2016). Structural and functional connectivity in posttraumatic stress disorder: Associations with FKBP5. Depression and Anxiety, 33(4), 300–307. https://doi.org/10.1002/da.22483
- Farooq, R., Majid, S., Ahmad Bhat, S., Amin, S., Hayat Bhat, M., Ahmad Wani, H., & Ahmad Shah, P. (2018). Association of adiponectin gene polymorphism with type 2 diabetes and metabolic syndrome. Translational Metabolic Syndrome Research, 1, 39–47. https://doi.org/10.1016/j.tmsr.2018.09.002
- Farr, O. M., Sloan, D. M., Keane, T. M., & Mantzoros, C. S. (2014). Stress- and PTSD-associated obesity and metabolic dysfunction: A growing problem requiring further research and novel treatments. Metabolism, 63(12), 1463–1468. https://doi.org/10.1016/j.metabol.2014.08.009
- Ferguson, J. F., Phillips, C. M., Tierney, A. C., Pérez-Martínez, P., Defoort, C., Helal, O., Lairon, D., Planells, R., Shaw, D. I., Lovegrove, J. A., Gjelstad, I. M., Drevon, C. A., Blaak, E. E., Saris, W. H., Leszczyńska-Gołąbek, I., Kiec-Wilk, B., Risérus, U., Karlström, B., Miranda, J. L.-, & Roche, H. M. (2010). Gene-nutrient interactions in the metabolic syndrome: Single nucleotide polymorphisms in ADIPOQ and ADIPOR1interact with plasma saturated fatty acids to modulate insulin resistance. The American Journal of Clinical Nutrition, 91(3), 794–801. https://doi.org/10.3945/ajcn.2009.28255
- Fernandez-Real, J. M., Pugeat, M., López-Bermejo, A., Bornet, H., & Ricart, W. (2005). Corticosteroid-binding globulin affects the relationship between circulating adiponectin and cortisol in men and women. Metabolism, 54(5), 584–589. https://doi.org/10.1016/j.metabol.2004.11.015
- Fonkoue, I. T., Marvar, P. J., Norrholm, S., Li, Y., Kankam, M. L., Jones, T. N., Vemulapalli, M., Rothbaum, B., Bremner, J. D., Le, N.-A., & Park, J. (2020). Symptom severity impacts sympathetic dysregulation and inflammation in post-traumatic stress disorder (PTSD). Brain, Behavior, and Immunity, 83, 260–269. https://doi.org/10.1016/j.bbi.2019.10.021
- Formolo, D. A., Cheng, T., Yu, J., Kranz, G. S., & Yau, S. Y. (2022). Central adiponectin signaling – a metabolic regulator in support of brain plasticity. Brain Plasticity, 1–18. https://doi.org/10.3233/BPL-220138
- Gabriel, S. B., Schaffner, S. F., Nguyen, H., Moore, J. M., Roy, J., Blumenstiel, B., Higgins, J., DeFelice, M., Lochner, A., Faggart, M., & Liu-Cordero, S. N. (2002). The structure of haplotype blocks in the human genome. Science, 296(5576), 2225–2229. https://doi.org/10.1126/science.1069424
- Gavrila, A., Peng, C. K., Chan, J. L., Mietus, J. E., Goldberger, A. L., & Mantzoros, C. S. (2003). Diurnal and ultradian dynamics of serum adiponectin in healthy men: Comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. The Journal of Clinical Endocrinology & Metabolism, 88(6), 2838–2843. https://doi.org/10.1210/jc.2002-021721
- Gelernter, J., Sun, N., Polimanti, R., Pietrzak, R., Levey, D. F., Bryois, J., Lu, Q., Hu, Y., Li, B., Radhakrishnan, K., Aslan, M., Cheung, K.-H., Li, Y., Rajeevan, N., Sayward, F., Harrington, K., Chen, Q., Cho, K., Pyarajan, S., … Stein, M. B. (2019). Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nature Neuroscience, 22(9), 1394–1401. https://doi.org/10.1038/s41593-019-0447-7
- Ghadge, A. A., Khaire, A. A., & Kuvalekar, A. A. (2018). Adiponectin: A potential therapeutic target for metabolic syndrome. Cytokine & Growth Factor Reviews, 39, 151–158. https://doi.org/10.1016/j.cytogfr.2018.01.004
- Graham, J., Legarreta, M., North, L., DiMuzio, J., McGlade, E., & Yurgelun-Todd, D. (2016). A preliminary study of DSM–5 PTSD symptom patterns in veterans by trauma type. Military Psychology, 28(2), 115–122. https://doi.org/10.1037/mil0000092
- He, C., Holme, J., & Anthony, J. (2014). SNP genotyping: The KASP assay. Methods in Molecular Biology, 1145, 75–86. https://doi.org/10.1007/978-1-4939-0446-4_7
- Hosking, L., Lumsden, S., Lewis, K., Yeo, A., McCarthy, L., Bansal, A., Riley, J., Purvis, I., & Xu, C.-F. (2004). Detection of genotyping errors by Hardy-Weinberg equilibrium testing. European Journal of Human Genetics, 12(5), 395–399. https://doi.org/10.1038/sj.ejhg.5201164
- Howlader, M., Sultana, M. I., Akter, F., MdM, H., & Hossain, M. M. (2021). Adiponectin gene polymorphisms associated with diabetes mellitus: A descriptive review. Heliyon, 7(8), e07851. https://doi.org/10.1016/j.heliyon.2021.e07851
- Kadowaki, T., Yamauchi, T., Kubota, N., Hara, K., Ueki, K., & Tobe, K. (2006). Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. Journal of Clinical Investigation, 116(7), 1784–1792. https://doi.org/10.1172/JCI29126
- Kang, J. I., Kim, T. Y., Chung, H. G., Choi, J. H., Hwang, E. H., & Kim, S. J. (2019). SA57 – genetic and epigenetic involvement of the FKPB5 gene in posttraumatic stress disorder after combat trauma. European Neuropsychopharmacology, 29, S853–S854. https://doi.org/10.1016/j.euroneuro.2017.08.129
- Kessler, R. C., Aguilar-Gaxiola, S., Alonso, J., Benjet, C., Bromet, E. J., Cardoso, G., Degenhardt, L., de Girolamo, G., Dinolova, R. V., Ferry, F., Florescu, S., Gureje, O., Haro, J. M., Huang, Y., Karam, E. G., Kawakami, N., Lee, S., Lepine, J.-P., Levinson, D., … Koenen, K. C. (2017). Trauma and PTSD in the WHO world mental health surveys. European Journal of Psychotraumatology, 8(sup5), Article 1353383. https://doi.org/10.1080/20008198.2017.1353383
- Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., & Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry, 62(6), 593–602. https://doi.org/10.1001/archpsyc.62.6.593
- Koenen, K. C. (2007). Genetics of posttraumatic stress disorder: Review and recommendations for future studies. Journal of Traumatic Stress, 20(5), 737–750. https://doi.org/10.1002/jts.20205
- Lee, K. W., & Shin, D. (2020). Prospective associations of serum adiponectin, leptin, and leptin-adiponectin ratio with incidence of metabolic syndrome: The Korean genome and epidemiology study. International Journal of Environmental Research and Public Health, 17(9), 3287. https://doi.org/10.3390/IJERPH17093287
- Lehto, S. M., Elomaa, A. P., Niskanen, L., Herzig, K.-H., Tolmunen, T., Viinamäki, H., Koivumaa-Honkanen, H., Huotari, A., Honkalampi, K., Valkonen-Korhonen, M., Sinikallio, S., Ruotsalainen, H., & Hintikka, J. (2012). Serum adipokine levels in adults with a history of childhood maltreatment. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 37(2), 217–221. https://doi.org/10.1016/j.pnpbp.2012.01.016
- Lewinsohn, P. M., Seeley, J. R., Roberts, R. E., & Allen, N. B. (1997). Center for epidemiologic studies depression scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and Aging, 12(2), 277–287. https://doi.org/10.1037/0882-7974.12.2.277
- Lihua, M., Tao, Z., Hongbin, M., Hui, W., Caihong, J., & Xiaolian, J. (2020). Metabolic syndrome risk in relation to posttraumatic stress disorder among trauma-exposed civilians in Gansu province, China. Medicine, 99(1), e18614. https://doi.org/10.1097/MD.0000000000018614
- Maeda, K., Okubo, K., Shimomura, I., Funahashi, T., Matsuzawa, Y., & Matsubara, K. (1996). cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose most abundant gene transcript 1). Biochemical and Biophysical Research Communications, 221(2), 286–289. https://doi.org/10.1006/bbrc.1996.0587
- Manolio, T. A., Collins, F. S., Cox, N. J., Goldstein, D. B., Hindorff, L. A., Hunter, D. J., McCarthy, M. I., Ramos, E. M., Cardon, L. R., Chakravarti, A., Cho, J. H., Guttmacher, A. E., Kong, A., Kruglyak, L., Mardis, E., Rotimi, C. N., Slatkin, M., Valle, D., Whittemore, A. S., … Visscher, P. M. (2009). Finding the missing heritability of complex diseases. Nature, 461(7265), 747–753. https://doi.org/10.1038/nature08494
- Mellon, S. H., Gautam, A., Hammamieh, R., Jett, M., & Wolkowitz, O. M. (2018). Metabolism, metabolomics, and inflammation in posttraumatic stress disorder. Biological Psychiatry, 83(10), 866–875. https://doi.org/10.1016/j.biopsych.2018.02.007
- Mgoqi-Mbalo, N., Zhang, M., & Ntuli, S. (2017). Risk factors for PTSD and depression in female survivors of rape. Psychological Trauma: Theory, Research, Practice, and Policy, 9(3), 301–308. https://doi.org/10.1037/tra0000228
- Murcray, C. E., Lewinger, J. P., Conti, D. V., Thomas, D. C., & Gauderman, W. J. (2011). Sample size requirements to detect gene-environment interactions in genome-wide association studies. Genetic Epidemiology, 35(3), 201–210. https://doi.org/10.1002/gepi.20569
- Na, K. S., Kim, E. K., & Park, J. T. (2017). Decreased plasma adiponectin among male firefighters with symptoms of post-traumatic stress disorder. Journal of Affective Disorders, 221, 254–258. https://doi.org/10.1016/j.jad.2017.06.015
- Nagel, R. L. (2005). Epistasis and the genetics of human diseases. Comptes Rendus Biologies, 328(7), 606–615. https://doi.org/10.1016/j.crvi.2005.05.003
- Nduna, M., Jewkes, R. K., Dunkle, K. L., Shai, N. P. J., & Colman, I. (2010). Associations between depressive symptoms, sexual behaviour and relationship characteristics: A prospective cohort study of young women and men in the eastern cape, South Africa. Journal of the International AIDS Society, 13(1), 44. https://doi.org/10.1186/1758-2652-13-44
- Nievergelt, C. M., Maihofer, A. X., Klengel, T., Atkinson, E. G., Chen, C.-Y., Choi, K. W., Coleman, J. R. I., Dalvie, S., Duncan, L. E., Gelernter, J., Levey, D. F., Logue, M. W., Polimanti, R., Provost, A. C., Ratanatharathorn, A., Stein, M. B., Torres, K., Aiello, A. E., Almli, L. M., … Koenen, K. C. (2019). International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nature Communications, 10(1), 1–16. https://doi.org/10.1038/s41467-019-12576-w
- Nishida, C., Ko, G. T., & Kumanyika, S. (2010). Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO expert consultation on waist circumference and waist–hip ratio. European Journal of Clinical Nutrition, 64(1), 2–5. https://doi.org/10.1038/ejcn.2009.139
- Nöthling, J., Abrahams, N., Jewkes, R., Mhlongo, S., Lombard, C., Hemmings, S. M. J., & Seedat, S. (2022). Risk and protective factors affecting the symptom trajectory of posttraumatic stress disorder post-rape. Journal of Affective Disorders, 309, 151–164. https://doi.org/10.1016/j.jad.2022.04.032
- Nöthling, J., Abrahams, N., Toikumo, S., Suderman, M., Mhlongo, S., Lombard, C., Seedat, S., & Hemmings, S. M. J. (2021). Genome-wide differentially methylated genes associated with posttraumatic stress disorder and longitudinal change in methylation in rape survivors. Translational Psychiatry, 11(1), 594. https://doi.org/10.1038/s41398-021-01608-z
- Nugent, N. R., Amstadter, A. B., & Koenen, K. C. (2008). Genetics of post-traumatic stress disorder: Informing clinical conceptualizations and promoting future research. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 148C(2), 127–132. https://doi.org/10.1002/ajmg.c.30169
- O’Donovan, A. (2016). PTSD is associated with elevated inflammation: Any impact on clinical practice? Evidence Based Mental Health, 19(4), 120–120. https://doi.org/10.1136/eb-2016-102376
- Ohashi, K., Ouchi, N., & Matsuzawa, Y. (2012). Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie, 94(10), 2137–2142. https://doi.org/10.1016/j.biochi.2012.06.008
- Oshodi, Y., Macharia, M., Lachman, A., & Seedat, S. (2016). Immediate and long-term mental health outcomes in adolescent female rape survivors. Journal of Interpersonal Violence, 35, 252–267. https://doi.org/10.1177/0886260516682522
- Popejoy, A. B., & Fullerton, S. M. (2016). Genomics is failing on diversity. Nature, 538(7624), 161–164. https://doi.org/10.1038/538161a
- Radloff, L. S. (1977). The CES-D scale. Applied Psychological Measurement, 1(3), 385–401. https://doi.org/10.1177/014662167700100306
- Raheja, U., Karim, N. N., Ryan, K. A., Nijjar, G. V., Shuldiner, A. R., Mitchell, B. D., & Postolache, T. T. (2017). 556 – adiponectin gene polymorphism and seasonality in the old order Amish. Biological Psychiatry, 81(10), S225–S225. https://doi.org/10.1016/j.biopsych.2017.02.1164
- Scherrer, J. F., Salas, J., Norman, S. B., Schnurr, P. P., Chard, K. M., Tuerk, P., Schneider, F. D., van den Berk-Clark, C., Cohen, B. E., Friedman, M. J., & Lustman, P. J. (2019). Association between clinically meaningful posttraumatic stress disorder improvement and risk of type 2 diabetes. JAMA Psychiatry, 76(11), 1159–1166. https://doi.org/10.1001/jamapsychiatry.2019.2096
- Scott, K. M., Koenen, K. C., King, A., Petukhova, M. V., Alonso, J., Bromet, E. J., Bruffaerts, R., Bunting, B., de Jonge, P., Haro, J. M., Karam, E. G., Lee, S., Medina-Mora, M. E., Navarro-Mateu, F., Sampson, N. A., Shahly, V., Stein, D. J., Torres, Y., Zaslavsky, A. M., & Kessler, R. C. (2018). Post-traumatic stress disorder associated with sexual assault among women in the WHO world mental health surveys. Psychological Medicine, 48(1), 155–167. https://doi.org/10.1017/S0033291717001593
- Siitonen, N., Pulkkinen, L., Lindström, J., Kolehmainen, M., Eriksson, J. G., Venojärvi, M., Ilanne-Parikka, P., Keinänen-Kiukaanniemi, S., Tuomilehto, J., & Uusitupa, M. (2011). Association of ADIPOQ gene variants with body weight, type 2 diabetes and serum adiponectin concentrations: The Finnish diabetes prevention study. BMC Medical Genetics, 12(1), 1–13. https://doi.org/10.1186/1471-2350-12-5
- Steele, S. J., Abrahams, N., Duncan, K., Woollett, N., Hwang, B., O’Connell, L., van Cutsem, G., & Shroufi, A. (2019). The epidemiology of rape and sexual violence in the platinum mining district of Rustenburg, South Africa: Prevalence, and factors associated with sexual violence. PLoS One, 14(7), e0216449. https://doi.org/10.1371/journal.pone.0216449
- Stein, M. B., Levey, D. F., Cheng, Z., Wendt, F. R., Harrington, K., Pathak, G. A., Cho, K., Quaden, R., Radhakrishnan, K., Girgenti, M. J., Ho, Y.-L. A., Posner, D., Aslan, M., Duman, R. S., Zhao, H., Polimanti, R., Concato, J., & Gelernter, J. (2021). Genome-wide association analyses of post-traumatic stress disorder and its symptom subdomains in the million veteran program. Nature Genetics, 53(2), 174–184. https://doi.org/10.1038/s41588-020-00767-x
- Tam, V., Patel, N., Turcotte, M., Bossé, Y., Paré, G., & Meyre, D. (2019). Benefits and limitations of genome-wide association studies. Nature Reviews Genetics, 20(8), 467–484. https://doi.org/10.1038/s41576-019-0127-1
- Thurston, R. C., Jakubowski, K. P., Wu, M., Aizenstein, H. J., Chang, Y., Derby, C. A., Koenen, K. C., Barinas-Mitchell, E., & Maki, P. M. (2021). Sexual assault and white matter hyperintensities among midlife women. Brain Imaging and Behavior, 16, 773–780. https://doi.org/10.1007/s11682-021-00536-2
- Tian, C., Gregersen, P. K., & Seldin, M. F. (2008). Accounting for ancestry: Population substructure and genome-wide association studies. Human Molecular Genetics, 17(R2), R143–R150. https://doi.org/10.1093/hmg/ddn268
- Tiihonen Möller, A., Bäckström, T., Söndergaard, H. P., & Helström, L. (2014). Identifying risk factors for PTSD in women seeking medical help after rape. PLoS One, 9(10), e111136. https://doi.org/10.1371/journal.pone.0111136
- Uffelmann, E., Huang, Q. Q., Munung, N. S., de Vries, J., Okada, Y., Martin, A. R., Martin, H. C., Lappalainen, T., & Posthuma, D. (2021). Genome-wide association studies. Nature Reviews Methods Primers, 1(1), 59. https://doi.org/10.1038/s43586-021-00056-9
- Velders, F. P., Kuningas, M., Kumari, M., Dekker, M. J., Uitterlinden, A. G., Kirschbaum, C., Hek, K., Hofman, A., Verhulst, F. C., Kivimaki, M., Van Duijn, C. M., Walker, B. R., & Tiemeier, H. (2011). Genetics of cortisol secretion and depressive symptoms: A candidate gene and genome wide association approach. Psychoneuroendocrinology, 36(7), 1053–1061. https://doi.org/10.1016/j.psyneuen.2011.01.003
- Vionnet, N., Hani, E. H., Dupont, S., Gallina, S., Francke, S., Dotte, S., De Matos, F., Durand, E., Leprêtre, F., Lecoeur, C., Gallina, P., Zekiri, L., Dina, C., & Froguel, P. (2000). Genomewide search for type 2 diabetes-susceptibility genes in French whites: Evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. American Journal of Human Genetics, 67(6), 1470–1480. https://doi.org/10.1086/316887
- von Frankenberg, A. D., Reis, A. F., Gerchman, F., von Frankenberg, A. D., Reis, A. F., & Gerchman, F. (2017). Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: A literature review. Archives of Endocrinology and Metabolism, 61(6), 614–622. https://doi.org/10.1590/2359-3997000000316
- Wang, Q., Zhu, X. C., Liu, H., Ran, M. S., & Fang, D. Z. (2015). A longitudinal study of the association of adiponectin gene rs1501299 with depression in Chinese Han adolescents after Wenchuan earthquake. Journal of Affective Disorders, 175, 86–91. https://doi.org/10.1016/j.jad.2014.12.056
- Weathers, F. W., Blake, D. D., Schnurr, P. P., Kaloupek, D. G., Marx, B. P., & Keane, T. M. (2013). The life events checklist for DSM-5 (LEC-5) – extended [measurement instrument]. http://www.ptsd.va.gov
- Wei, W. H., Hemani, G., & Haley, C. S. (2014). Detecting epistasis in human complex traits. Nature Reviews Genetics, 15(11), 722–733. https://doi.org/10.1038/nrg3747
- Wu, J., Liu, Z., Meng, K., & Zhang, L. (2014). Association of adiponectin gene (ADIPOQ) rs2241766 polymorphism with obesity in adults: A meta-analysis. PLOS ONE, 9(4), e95270. https://doi.org/10.1371/journal.pone.0095270
- Xu, J., Turner, A., Little, J., Bleecker, E. R., & Meyers, D. A. (2002). Positive results in association studies are associated with departure from Hardy-Weinberg equilibrium: Hint for genotyping error? Human Genetics, 111(6), 573–574. https://doi.org/10.1007/s00439-002-0819-y
- Yehuda, R., Bierer, L. M., Schmeidler, J., Aferiat, D. H., Breslau, I., & Dolan, S. (2000). Low cortisol and risk for PTSD in adult offspring of holocaust survivors. American Journal of Psychiatry, 157(8), 1252–1259. https://doi.org/10.1176/appi.ajp.157.8.1252
- Yuan, H. P., Sun, L., Li, X. H., Cappello, V., Marchetti, L., Parlanti, P., Landi, S., Tonazzini, I., Cecchini, M., Piazza, V., & Gemmi, M. (2016). Association of adiponectin polymorphism with metabolic syndrome risk and adiponectin level with stroke risk: A meta-analysis. Scientific Reports, 6(1), 1–10. https://doi.org/10.1038/s41598-016-0001-8
- Zannas, A. S., Wiechmann, T., Gassen, N. C., & Binder, E. B. (2016). Gene-stress-epigenetic regulation of FKBP5: Clinical and translational implications. Neuropsychopharmacology, 41(1), 261–274. https://doi.org/10.1038/npp.2015.235
- Zhang, D., Wang, X., & Lu, X. Y. (2016). Adiponectin exerts neurotrophic effects on dendritic arborization, spinogenesis, and neurogenesis of the dentate gyrus of male mice. Endocrinology, 157(7), 2853–2869. https://doi.org/10.1210/en.2015-2078
- Zhang, D., Wang, X., Wang, B., Garza, J. C., Fang, X., Scherer, P. E., Brenner, R., & Lu, X.-Y. (2017). Adiponectin regulates contextual fear extinction and intrinsic excitability of dentate gyrus granule neurons through AdipoR2 receptors. Molecular Psychiatry, 22(7), 1044–1055. https://doi.org/10.1038/mp.2016.58
- Zhang, K., Li, G., Wang, L., Cao, C., Fang, R., Luo, S., & Liu, P. (2019). An epistasis between dopaminergic and oxytocinergic systems confers risk of post-traumatic stress disorder in a traumatized Chinese cohort. Scientific Reports, 9(1), 19252. https://doi.org/10.1038/s41598-019-55936-8
- Zhang, L., Hu, X. Z., Yu, T., Chen, Z., Dohl, J., Li, X., Benedek, D. M., Fullerton, C. S., Wynn, G., Barrett, J. E., Li, M., Russell, D. W., & Ursano, R. J. (2020). Genetic association of FKBP5 with PTSD in US service members deployed to Iraq and Afghanistan. Journal of Psychiatric Research, 122, 48–53. https://doi.org/10.1016/j.jpsychires.2019.12.014
