ABSTRACT
Background: Neurophysiological models link dissociation (e.g. feeling detached during or after a traumatic event) to hypoarousal. It is currently assumed that the initial passive reaction to a threat may coincide with a blunted autonomic response, which constitutes the dissociative subtype of post-traumatic stress disorder (PTSD).
Objective: Within this systematic review we summarize research which evaluates autonomic nervous system activation (e.g. heart rate, blood pressure) and dissociation in PTSD patients to discern the validity of current neurophysiological models of trauma-related hypoarousal.
Method: Of 553 screened articles, 28 studies (N = 1300 subjects) investigating the physiological response to stress provocation or trauma-related interventions were included in the final analysis.
Results: No clear trend exists across all measured physiological markers in trauma-related dissociation. Extracted results are inconsistent, in part due to high heterogeneity in experimental methodology.
Conclusion: The current review is unable to provide robust evidence that peri- and post-traumatic dissociation are associated with hypoarousal, questioning the validity of distinct psychophysiological profiles in PTSD.
HIGHLIGHTS
There is no consensus on physiological biomarkers of trauma-related dissociation.
Peri- and post-traumatic dissociation are physiologically distinct from stress reactions in chronic states.
Standardized methodologies may increase the reproducibility and specificity of psychophysiological biomarkers of dissociation.
Antecedentes: Los modelos neurofisiológicos vinculan la disociación (por ejemplo, la sensación de desapego durante o después de un evento traumático) con la hipoactivación. Actualmente se asume que la reacción pasiva inicial ante una amenaza puede coincidir con una respuesta autonómica embotada, lo que constituye el subtipo disociativo del trastorno de estrés postraumático (TEPT).
Objetivo: En esta revisión sistemática resumimos las investigaciones que evalúan la activación del sistema nervioso autónomo (por ejemplo, la frecuencia cardíaca, la presión arterial) y la disociación en pacientes con TEPT para discernir la validez de los modelos neurofisiológicos actuales de la hipoactivación relacionada con el trauma.
Método: De 553 artículos seleccionados, se incluyeron en el análisis final 28 estudios (N=1300 sujetos) que investigaban la respuesta fisiológica a la provocación del estrés o a las intervenciones relacionadas con el trauma.
Resultados: No existe una tendencia clara en todos los marcadores fisiológicos medidos en la disociación relacionada con el trauma. Los resultados extraídos son inconsistentes, en parte debido a la alta heterogeneidad en la metodología experimental.
Conclusión: La presente revisión no puede aportar pruebas sólidas de que la disociación peri y postraumática esté asociada a la hipoactivación, lo que cuestiona la validez de los distintos perfiles psicofisiológicos en el TEPT
背景:神经生理学模型将解离(例如,在创伤事件期间或之后感到超然)与低唤起联系起来。目前假设对威胁的初始被动反应可能与迟钝的自主反应同时发生,这构成了创伤后应激障碍 (PTSD) 的解离亚型。
目的:在本系统综述中,我们总结了评估 PTSD 患者自主神经系统激活(例如心率、血压)和解离的研究,以认清当前创伤相关低唤起神经生理模型的有效性。
方法:在 553 篇筛选出的文章中,28 项研究(N = 1300 名受试者)考查了对应激刺激或创伤相关干预的生理反应,并被纳入最终分析。
结果:在创伤相关解离中,所有测量的生理标志物都没有明显的趋势。提取的结果不一致,部分原因是实验方法的高度异质性。
结论:本综述无法提供稳健的证据表明创伤前后解离与低唤起有关,质疑了不同心理生理特征在 PTSD 中的有效性。
1. Introduction
‘Does anyone know of research documenting large heart-rate decrease during episodes of psychological dissociation?’. This question, posted in ResearchGate (2016), incited an ongoing debate in the psychotraumatology community by calling into question the accepted notion that dissociation is related to psychophysiological hypoarousal (Iffland et al., Citation2020; Pole, Citation2007). This article thus seeks to provide a systematic and comprehensive overview on autonomic markers of trauma-related dissociation. In the following, we will (1) briefly summarize key theoretical models related to the psychophysiology of peri- and post-traumatic dissociation, and (2) systematically review existing findings on the relationship between dissociation and the autonomic nervous system (ANS) in PTSD.
1.1. Psychophysiology of post-traumatic stress disorder and dissociation
PTSD is diagnosed when a person experiences autonomic hyperarousal and symptoms of vivid re-experiencing, avoidance, and negative changes in mood and thought within one month of the traumatic incident. Hyperarousal refers to an active stress response to a perceived threat characterized by hypervigilance, exaggerated startle response, irritability, disturbed sleeping patterns, and the inability to concentrate (American Psychiatric Association, Citation2013). Early studies (Orr, Citation1997; Orr et al., Citation2002; Orr & Roth, Citation2000) demonstrated that most PTSD patients experience increased heart rate under stressful conditions. Yet, a subset of traumatized individuals (approximately one third) do not show such hyperreactivity to threat cues. Other studies found autonomic responses in PTSD patients to be either physiologically similar to controls or reduced from baseline (Cuthbert et al., Citation2003; D'Andrea et al., Citation2013; Limberg et al., Citation2011; McTeague & Lang, Citation2012). This divergence from the standard hyperarousal phenotype of PTSD towards a more blunted psychophysiological response was later termed hyporeactivity or hypoarousal (D'Andrea et al., Citation2013; Frewen & Lanius, Citation2006b; Terpou et al., Citation2019). Further research by Lanius and colleagues (Lanius et al., Citation2002; Lanius et al., Citation2005; Lanius et al., Citation2006) employing trauma memory recall supports the idea of two different PTSD profiles and paved the way for the inclusion of a new diagnosis by the American Psychiatric Association (Citation2013) (might need to update citation here). PTSD patients with decreased affective and physiological responses, concomitant with altered brain activity in fronto-limbic circuits were labeled ‘physiological non-responders’ and would later fall into the dissociative subtype of PTSD (American Psychiatric Association, Citation2013; Lanius et al., Citation2010; Lanius et al., Citation2012; Spiegel et al., Citation2011; Terpou et al., Citation2019).
1.2. Peri- and post-traumatic dissociation
Previous metanalyses demonstrate a robust link between dissociative experiencing and exposure to trauma (Rafiq et al., Citation2018; Vonderlin et al., Citation2018). Trauma-related dissociation may be an innate defense mechanism to cope with and detach from trauma or trauma-related cues (Dalenberg & Carlson, Citation2012; Lanius et al., Citation2012). Dissociation is characterized by a sense of psychological detachment (Holmes et al., Citation2005) which can manifest as depersonalization (i.e. feeling disconnected from the surroundings), derealization (i.e. feeling disconnected from the surroundings), emotional numbing (Frewen & Lanius, Citation2006a), analgesia (Ludascher et al., Citation2010; Mickleborough et al., Citation2011; Strigo et al., Citation2010), and immobility (Volchan et al., Citation2011; Volchan et al., Citation2017). One can distinguish between peritraumatic dissociation and post-traumatic dissociation by the timing of dissociation relative to the traumatic event (e.g. during or after). Post-traumatic dissociation includes dissociative reactions towards a real or experimental threat within a short time scale (e.g. within the hour) (Carlson et al., Citation2018). If these acute reactions occur repeatedly over time, they may blend into a chronic state where dissociation is experienced daily (i.e. trait dissociation). Between 6 and 45% of dissociative PTSD patients (Hansen et al., Citation2017) exhibit more severe psychological symptoms (more re-experiencing, higher suicidality, and impaired role functioning) than non-dissociative PTSD patients (e.g. Stein et al., Citation2013). It remains unclear which psychobiological mechanisms underlie or illicit peri- and post-traumatic dissociation. We will subsequently highlight several theories which implicate acute defensive reactions and altered ANS activity as the drivers of dissociation in PTSD.
1.3. The autonomic nervous system
The ANS, consisting of the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS), regulates heart rate, respiratory rate, blood pressure, and galvanic skin response. Broadly speaking, an increase in SNS activity corresponds with physiological arousal, as measured by elevated heart rate, respiratory rate, blood pressure and skin conductance (Bandler et al., Citation2000). Meanwhile, a decrease in SNS activity and/or an increase in PNS activity corresponds with the relative slowing of heart rate and lowering of blood pressure and skin conductance. In addition, reduced SNS activity is associated with increased heart rate variability parameters like the high-frequency band or the root mean square successive, which can represent autonomic hypoarousal (see ). It should be noted, though, that sympathetic and parasympathetic activity are not reciprocal, but interact in a loosely coupled fashion (e.g. Billman, Citation2013; de Geus et al., Citation2019). Thus, blunted physiological responses may reflect parasympathetic hyperactivity, sympathetic hypoactivity or both (Bandler et al., Citation2000), warranting a detailed assessment of quantifiable biomarkers which underlie altered autonomic responses in dissociation (see ). In the following, we will summarize key theoretical models that have shaped the current scientific and clinical dialogue on the psychophysiology of threat processing and trauma-related dissociation.
Table 1. Abbreviations, full names, and short definitions of included physiological parameters.
1.4. Psychophysiological models
1.4.1. Defense cascade model
Incorporating animal research (for an overview see https://fanselowlab.psych.ucla.edu/predatory-imminence-continuum/) and Mobbs’ threat processing model (Mobbs et al., Citation2009), the defense cascade model by Lanius and colleagues (2018) posits that humans under severe threat initially respond with an active defense response (‘fight-and-flight’, characterized by sympathetic dominance and endocannabinoid release) and then exhibit SNS/PNS co-activation (‘tonic immobility’). With a limited chance of escape, humans exhibit a passive defensive response, characterized by depersonalization/derealization symptoms (‘unresponsive immobility’) and opioid release. Simultaneously the heart rate, blood pressure and skeletal muscle tone go down (tonic immobility) (McKinnon et al., Citation2016), which may be mediated by increased top-down modulation of limbic brain regions including the amygdala and periaqueductal gray (Lanius et al., Citation2018; Nicholson et al., Citation2017).
The proposed psychophysiological defense stages correspond to the severity of dissociation. Following a threat, the type of defensive response in PTSD (hyper- vs. hypoarousal) depends on the severity of dissociative symptomatology (Terpou et al., Citation2019). In sum, this model conceptualizes dissociation in terms of depersonalization, derealization and emotional/physical numbing and suggests that the initial peritraumatic reaction (i.e. shock) can become a conditioned response, triggered by trauma cues or stressors (Lanius et al., Citation2018) in accordance with the inescapable shock model (van der Kolk, Citation1988). Following this rationale, the initial psychophysiological response to a trauma (peritraumatic) and to a trauma cue/stressor (acute post-traumatic dissociation) may over time result in a chronic dissociative state. To date, few neurophysiological studies have assessed peritraumatic, acute, and trait dissociation within one cohort (e.g. Bichescu-Burian et al., Citation2017; Hauschildt et al., Citation2011), and longitudinal research is warranted to better understand the hypothesized etiological continuity of passive defensive responding in humans.
1.4.2. Shutdown model
The shutdown model by Schauer and Elbert (Citation2010) closely resembles the defense cascade model, but classifies six distinct stages of threat response. After an initial orienting response (1. ‘freeze’), the body under threat prepares to escape (2. ‘flight’), then attack (3. ‘fight’). If both options fail to eliminate the threat, the body subverts to a state of unresponsive immobility (4. ‘tonic immobility’), and shut-down (5. ‘flag’) which may progress to fainting mediated by disgust (6. ‘faint’) as the body surrenders to the threat (i.e. the attacker). Similar to the defense cascade model (Lanius et al., Citation2010; Lanius et al., Citation2012), the shutdown model describes two distinct response profiles: the Type 1 ‘uproar-PTSD’, characterized by dominant sympathetic activation, and Type 2 ‘shutdown-PTSD’, characterized by dominant parasympathetic activation and including patients who follow the cascade at least until stage 4. The authors propose that the initial threat response informs subsequent physiological reactivity, where the hypersensitized trauma-related fear network is easily reactivated, eliciting the initial psychophysiological defense response. Thus, an individual who exhibits a parasympathetic-dominant reaction (e.g. flag or faint) to the trauma is more likely to experience an acute post-traumatic dissociative response, accompanied by inhibition of the SNS, activation of the PNS, and reduction in heart rate and blood pressure. For instance, a study of PTSD patients demonstrated that experiencing acute dissociation during trauma recall (via personalized trauma audio-scripts) is associated with reduced heart rate (Sack et al., Citation2012). It is unknown whether these individuals experienced a similar initial peritraumatic response. The model does not explicitly state that the (repeated) PNS-dominant physiological reactions to trauma cues share similar features to chronic post-traumatic dissociation. However, the characterization of the shutdown model of PTSD suggests that the stable dissociative response pattern may be a conditioned automatic response to stressors and perceived threat (Schauer & Elbert, Citation2010).
1.4.3. Freeze and tonic immobility
Another model of threat-responding is one in which two alternative pathways lead to tonic immobility (Hagenaars et al., Citation2014; Roelofs, Citation2017). In this mode, the initial orienting response to a threat is followed by either the fight/flight response or the freeze response. The latter is characterized by immobility, bradycardia and increased muscle tonus accompanied by concomitant SNS-PNS activation. The authors describe that rapid shifting between the two defensive behaviors of fight/flight and freeze is possible. If both options fail, the body enters a state of tonic immobility (‘death feigning’ or ‘playing dead’), characterized by motor inhibition, muscle rigidity and the inability to express oneself vocally (Kuiling et al., Citation2019). Tonic immobility (i.e. hypotension or unresponsiveness) is conceptualized as a more passive defensive response as opposed to freezing. In this model freezing is associated with, and is thus measurable via, reduced body sway (see also Volchan et al., Citation2017; Citation2011). Roelofs (Citation2017) emphasizes the lack of evidence on the physiology of tonic immobility in humans but suggests that freezing may be linked to a maladaptive emotional response which precedes the development of PTSD, necessitating further longitudinal research in this field. Importantly, this model does not associate defensive behavior with dissociation, but portrays dissociation and tonic immobility as related but different constructs (Abrams et al., Citation2009; Hagenaars, Citation2016; Lima et al., Citation2010).
1.4.4. Polyvagal theory
The polyvagal theory, developed by Stephen Porges (Citation2003, Citation2007, Citation2011), has received substantial attention in the psychotraumatology field. Porges emphasizes the evolutionary relevance of the vagal nerve in facilitating arousal states, mobilization and immobilization, and social engagement. According to Porges, the vagus nerve can be differentiated into three different branches with evolutionarily selective advantages in regulating autonomic functioning (Citation2003). First, the unmyelinated vagus branch (e.g. the dorsal vagal complex) is associated with immobilization (e.g. fainting, shutdown, dissociation), bradycardia and decreased muscle tone. The second branch, the sympathetic-adrenal branch, is active during mobilization behaviors (e.g. fight/flight) when under attack. The final branch, the myelinated branch (e.g. the ventral vagal complex), regulates cardiac vagal activity and withdrawal via inhibition of the sympathetic branch, which corresponds with decreased arousal, social engagement and (self-)soothing behaviors (Porges, Citation2007). Through this framework, dissociative responding in humans is purely a defensive immobilization response modulated by the dorsal vagal complex and associated with large heart rate decreases. This immobilization response occurs when behaviors initiated by the ventral vagal complex (e.g. social engagement) or the sympathetic-adrenal pathway (e.g. active defensive behavior) are not possible. Indirect support for this model is seen in studies which have proven that trait dissociation is negatively correlated with heart rate during experimentally-induced social stress (Powers et al., Citation2021; Simeon et al., Citation2008).
1.4.5. Window of tolerance
The window of tolerance model (Corrigan et al., Citation2011; Ogden et al., Citation2006; Siegel, Citation1999) states that trauma, particularly prolonged childhood trauma, can result in high autonomic sensitivity towards stressors and an imbalance in the dopaminergic system associated with rapid, uncontrolled shifting between two distress dipoles (also termed ‘biphasic rollercoaster’, Corrigan et al., Citation2011, p. 20). When exposed to trauma cues, patients switch between an SNS-dominated high-arousal state of active defensive responding (e.g. fear, anger, impulsivity, vigilance) and a PNS-dominated low-arousal ‘passive defensive’ state of emotional coping (e.g. submission, depression, and numbing). To manage these extremes, the window of tolerance represents a zone of psychological and autonomic flexibility: a balanced psychophysiological state allowing for flexible shifting, regulation, and integration of emotions, so they become tolerable.
In regards to dissociation, this model hypothesizes a noradrenergic lateral tegmental limbic forebrain – midbrain circuit (Bergmann, Citation2008), which activates the PNS and downregulates affect (both positive and negative), leading to emotional numbing and cognitive dissociation. Indirect support for this hypothesis was found in a study by Krause-Utz and colleagues (Citation2018). This study found a strong positive association between acute dissociation and PNS-mediated heart rate variability when patients (with comorbid PTSD and borderline personality disorder) were instructed to downregulate emotion, but passively viewing affective pictures. Although not explicitly stated, the authors draw a clear link between the initial physiological effects of trauma and subsequent SNS and PNS-mediated responses, with the latter including numbing, collapse, and dissociation.
1.5. Summary and study aim
Taken together, current theories of ANS-mediated defensive behaviors share a common framework, mainly in that dissociation is (a) a defensive reaction to traumatic stressors or reminders, (b) related to parasympathetic dominance, and (c) associated with a muted psychophysiological response (i.e. hypoarousal; Pole, Citation2007). However, these theories differ in their use of terminology, such as the meaning of freezing and its corresponding physiological correlates. In the shutdown model, freezing is part of the orienting response, but Hagenaars and colleagues (Citation2014) distinguish these two physiological states as unique experiences. Additionally, this model does not mention other defensive stages following tonic immobility, as suggested by the shutdown model (e.g. flag) and defense cascade model (e.g. collapsed freeze). On the other hand, congruity across models can be found in their neurophysiological correlates of threat response. Importantly, some of these theories either do not address dissociation at all or fail to specify how they conceptualize dissociation. It remains unclear whether acute dissociation as a defensive response shares similar psychophysiological characteristics with peritraumatic (i.e. during a trauma) or post-traumatic dissociation (e.g. a stable behavioral pattern following the trauma). Another general criticism of the aforementioned models, particularly the polyvagal theory (see Grossman & Taylor, Citation2007), concerns the oversimplification of biological processes and the assumption of similarity between human and animal psychophysiology, due to its basis on partially outdated theoretical conceptualizations of human evolutionary biology (see Barrett, Citation2020, for critical reflection; for instance, the triune brain theory by MacLean, Citation1990)
The current study aims to provide more clarity on the question of whether dissociation in PTSD patients is related to autonomic hypoarousal. To this end, a systematic literature search was conducted on research in human subjects evaluating both psychophysiological markers, (e.g. heart rate, blood pressure, and skin conductance) and dissociative experiences in PTSD patients.
2. Methods
2.1. Search strategy
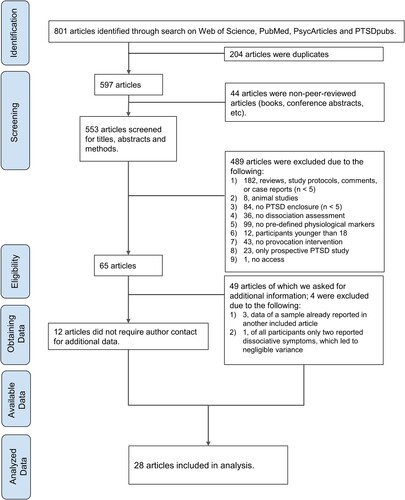
This systematic review was conducted with studies published until 21 March 2022, following the Preferred Reporting Items for Systematic Review and Meta Analysis for Individual Patient Data (PRISMA-IPD; Stewart et al., Citation2015) procedures. 801 articles were identified through searches in Web of Science, PubMed, PsycArticles, and PTSDpubs, using keywords which combined dissociative symptoms according to the dissociative subtype (dissociat*, dissociative, depersonali#ation, dereali#ation, DP/DR), PTSD (PTSD, post-traumatic stress disorder, posttraumatic stress disorder, post-traumatic stress disorder) and ANS-related physiological markers (heart rate, HR, HRV, ECG, electrocardiogram, cardiac, cardiovascular, skin conductance, SC, SCR, electrodermal blood pressure, BP, systolic, diastolic, temperature, eye, pupil size, pupillary dilatation, pupil* diameter, startle response, respiratory sinus arrhythmia, respira*, breath*, ANS, autonomic nervous system, parasympath*, sympath*, PNS, SNS, visceral nervous system). Keywords within one concept were combined with the ‘OR’ command, while the concepts (dissociation, PTSD, and physiological markers) were combined with the ‘AND’ command. Search terms on muscle tension (muscle tone, muscle tension, body sway, electromyography, EMG) were also included. Although skeletal muscles are not part of the ANS, they are of interest as part of the physiologic stress response. However, this search term revealed no studies worthy of inclusion.
Of all 801 articles, 204 were duplicates and 44 were not peer reviewed articles. Once removed, this left 553 relevant peer reviewed articles for further evaluation.
All 553 articles were screened by two independent reviewers using the title, abstract, and methods (inter-rater reliability: 0.83). Inclusion criteria were: (a) study participant age equal to or greater than 18, (b) at least 5 patients with a PTSD diagnosis or meeting criteria for PTSD diagnosis included, (c) experimental studies with trauma-related interventions (e.g. recall, script, film) or stress provocation (e.g. Trier Social Stress Test, acoustic startle), (d) with measurable and specific autonomic outcomes, and (e) designed in either a correlative (e.g. dissociation and physiological marker) or comparative (e.g. physiological marker across low dissociation vs. high dissociation subgroups) manner. Exclusion criteria were (a) animal studies, (b) dissociation not assessed, (c) assessment of treatment outcome or baseline/resting state only, and (d) PTSD assessment after experimental intervention (prospective design). In the case of diverging ratings, which did occur for 21 articles, they were assigned by mutual consensus. After the initial screening process, 65 relevant studies remained to be analyzed further. Four studies were excluded in this step for other reasons (see ). For 49 of the remaining 61 studies, authors were contacted to acquire missing patient data or analyses which could support the current review. We contacted authors at least twice and 65% replied. A total of 28 studies were included in the final analysis ( & ; for a list of non-included studies see Supplemental Table S1).
Table 2. Included studies.
2.2. Data extraction
A study coding spreadsheet was utilized to collect data from each included study. Within the coding spreadsheet was data on basic study information (e.g. authors, year, title), sample characteristics (e.g. sample size, mean age, gender, trauma history, PTSD diagnosis or meeting criteria), experimental condition (e.g. traumatic content, stress evoking, acoustic startle), on which value scores were based (e.g. change score – trauma condition minus neutral condition, change score – trauma condition minus baseline, specific trials), as well as indices (e.g. kind and score of effect sizes, significances) of the physiological parameters (see for an overview of all parameters included). It should be noted that, although we focused on physiological activity during an intervention, we also reviewed baseline scores (see Table S2 in the supplement). The spreadsheet also included comments to further define specific trials, as well as notes on our statistical procedures if effect sizes were not directly taken from the original publication.
2.3. Publication bias
Publication bias remains an important issue in psychological and medical research (Head et al., Citation2015; Thornton & Lee, Citation2000). However, Pole (Citation2007), who conducted the most comprehensive meta-analysis on psychophysiological markers and PTSD, argued that studies measuring psychophysiological activity may be less affected by publication bias because they often report multiple physiological measures, increasing the likelihood of non-significant results or results contrary to the hypothesis. Although an earlier meta-analysis on heart rate variability and anxiety disorders in general (Chalmers et al., Citation2014) and PTSD (Nagpal et al., Citation2013) supported this assumption, newer publications reveal publication bias in studies on heart rate variability and PTSD (Ge et al., Citation2020; Schneider & Schwerdtfeger, Citation2020). The influence of publication bias still needs to be carefully considered. Therefore, we did not only screen titles and abstracts for physiological and dissociative measurements, but also the methods section of each of the 553 peer reviewed articles to overcome possible biases in data collection and interpretation. Frequently, dissociation was reported as a descriptive sample characteristic, but not further included into a study’s analysis. If a dissociative physiological measurement was reported in the methods but not results of a study, we asked the authors for additional data or calculations on this parameter.
2.4. Data structuring and presentation
Our examination revealed great heterogeneity between included studies on several characteristics, including experimental condition, physiological parameters, score calculation of physiological parameters, and sample characteristics. Therefore, we have structured this systematic review as follows: articles included are grouped according to the temporal classification of dissociation as trait, peritraumatic, or acute dissociation. Studies that report multiple temporal classifications are included in more than one category (see ). Subsections for each dissociative classification are clustered along with physiological parameters. Within these subsections we also differentiate between stimuli in experimental trauma stimuli and more general stress (See Supplemental materials 4a–e for an additional graphical overview).
We aim to be as precise and accurate as possible in the presentation of its results. Therefore, we have obtained all available effect sizes (r or Cohens d) to elucidate the magnitude of reported differences in dissociation and physiological reactivity. If effect sizes were not available, they were calculated by means and standard deviation via the Psychometrica website (Lenhard & Lenhard, Citation2016). In a few cases, the calculation approach differed from this procedure. This is indicated in Supplemental Table S3. For the study of Kaufman et al. (Citation2002), we were not able to match an appropriate sample size with each physiologic variable and have therefore conservatively based our calculation on the smallest reported sample sizes. Significance levels were taken from the original studies and may therefore differ in their accuracy.
3. Results
3.1. Trait dissociation
3.1.1. General study characteristics
In total, 13 articles were identified that examined cardiovascular or electrodermal reactions to trauma- or stress-related interventions in the context of trait dissociation. Sample sizes varied (n = 14 to n = 134) and a combined 508 participants were studied. Mean age varied from 23.86 years to 46.95 years. Five studies examined female participants only, one study males only, and the percentage of female participants in other studies varied (8% to 95%). In eight studies, all participants had been diagnosed with PTSD (for detailed information see Supplemental Table S3). The remaining articles included some patients with PTSD, non-PTSD trauma-exposed participants and non-exposed controls (one study) (compare for more detailed information).
Table 3. Trait dissociation – Study characteristics and results.
To assess trait dissociation, the majority of the studies used the Dissociative Experiences Scale (DES; Bernstein & Putnam, Citation1986), the most commonly used dissociation self-report questionnaire (Lyssenko et al., Citation2018). Other questionnaires included the Trait Dissociation Questionnaire (TDQ; Murray et al., Citation2002), the Shutdown Dissociation Scale (Shut-D; Schalinski et al., Citation2015), the Multidimensional Inventory of Dissociation (MID; Dell, Citation2006), and the Multiscale Dissociation Inventory (MDI; Briere et al., Citation2005).
To provoke physiologic reactions, seven studies used personalized trauma-related content and one study used videos with trauma-associated content of varying emotional valence. General stress stimuli were used in five studies, including mirror confrontation, the Trier Social Stress Test (TSST), white noise, and acoustic startle probes.
All but two studies examined heart rate (HR). Parameters of heart rate variability (HRV) were assessed in four studies, including root mean square successive difference (RMSSD), high frequency HRV (HF-HRV), low frequency HRV (LF-HRV), the ratio of low frequency to high frequency (LF/HF-ratio) and respiratory sinus arrhythmia (RSA), which can be interpreted as an HRV marker (Berntson et al., Citation1997). Two studies measured systolic (SBP) and diastolic blood pressure (DBP), and one the pre-ejection period (PEP). Five studies measured skin conductance (SC) parameters, which include skin conductance level (SCL), skin conductance response (SCR), and numbers of skin conductance fluctuations (NSCF).
Six studies examined physiological reactions during the experimental condition only. Seven studies either additionally or solely reported a change score, which was corrected by physiological activity during a baseline or neutral condition. In these studies, the baseline or neutral physiological response was subtracted from the experiment response, reducing the amount of interference from the baseline or default activity.
3.1.2. Effects on heart rate
With respect to heart rate, no trend across studies was observed. Of the 11 studies investigating the relationship between trait dissociation severity and HR, only three reported significant results. Using personalized trauma stimuli, only Hyer et al. (Citation1993) found a higher HR in a high dissociative group than in a medium dissociative group under a trauma condition (p < .050, d = 0.34), which was no longer present when the calculation was based on the change score (p > .050, d = 0.10). Neither under the trauma condition (p > .050, d = 0.27) nor based on the change score (p > .050, d = 0.37) were there any differences between the high vs. low dissociation group. Under baseline conditions, HR in the high dissociation group was increased as compared to the medium (p < .010, d = 0.44) and low dissociation groups (p < .050, d = 0.26; see Supplemental Table S2). None of the other studies using personalized trauma reminders (Bichescu-Burian et al., Citation2017; Castro-Chapman et al., Citation2018; Halligan et al., Citation2006; Schmahl et al., Citation2004) or nightmare scripts (Rhudy et al., Citation2008) found a relationship between HR and trait dissociation.
Using general stress stimuli, Simeon et al. (Citation2008) found that trait dissociation correlated negatively and with moderate effect to HR (r = −.48, p < .050) measured during the TSST but not at baseline (r = −.08, p > .050; for baseline see Supplemental Table S2). This negative correlation (r = −.65, p = .009) between trait dissociation and HR during the TSST was confirmed by Powers et al. (Citation2021), who observed this relationship even at baseline (r = −.52, p = .047). The remaining studies reported no significant change in HR to mirror confrontation (Schäflein et al., Citation2018), white noise (Schalinski et al., Citation2013), or fear-potentiated startle (Seligowski et al., Citation2019).
3.1.3. Effects on heart rate variability
Seligowski et al. (Citation2019) demonstrated a positive correlation between RSA and depersonalization/derealization subscales (with MDI depersonalization subscale in the general hospital sample: r = .35, p < .010; with MID depersonalization/derealization mean in the psychiatric sample: r = .33, p < .010) during fear-potentiated startle, but not with overall trait dissociation (in the general hospital sample: r = .16, p > .050; in the psychiatric sample: r = .10, p > .050).
None of the other studies found correlations between HRV and trait dissociation after exposure to trauma related material (Hauschildt et al., Citation2011) or general stress stimuli (Powers et al., Citation2021; Schäflein et al., Citation2018).
3.1.4. Effects on blood pressure
Only one study examined blood pressure during trauma exposure (Schmahl et al., Citation2004), demonstrating a positive correlation between trait dissociation and SBP (change score: r = .65, p = .032), but not DBP (r = .48, p = .137).
One study examining blood pressure under a more general stress stimulus (Simeon et al., Citation2008) showed neither a correlation for SBP (r = −.24, p > .050) nor for DBP (r = −.37, p > .050) during TSST. However, this study demonstrated a correlation between trait dissociation and SBP at baseline (r = −.54, p < .010), but not with DBP (r = −.24, p > .050; for baseline see Supplemental Table S2).
3.1.5. Effects on skin conductance
No study utilizing personalized trauma stimuli found a relationship between SC and trait dissociation (Bichescu-Burian et al., Citation2017; Rhudy et al., Citation2008; Schmahl et al., Citation2004; Vermes et al., Citation2020).
Under non-personalized stress stimuli, Seligowski et al. (Citation2019) showed a moderate correlation between SCR and trait dissociation during a fear-potentiated startle paradigm. These results varied according to the sample population and presented stimuli. In the general hospital sample, trait dissociation correlated negatively with the SCR response to the danger signal (r = −.39, p < .010), but not the SCR response to the safety signal (r = −.28, p > .050). On the contrary, in the psychiatric hospital sample, trait dissociation correlated positively with the SCR to the safety signal (r = .38, p < .010), but not the SCR to the danger signal (r = .20, p > .050).
3.2. Peritraumatic dissociation
3.2.1. General study characteristics
In total, 8 articles were identified that examined cardiovascular or electrodermal reactions to trauma- or stress-related interventions in the context of peritraumatic dissociation. Sample sizes varied (n = 19 to n = 200) and 581 participants were studied. Mean age varied from 18.74 years to 61.0 years. Three studies examined female participants only, one study males only, and the percentage of female participants in the other studies varied (12% to 69%). In two studies, all participants had been diagnosed with PTSD (for detailed information see Supplemental Table S3). The remaining articles included some patients with PTSD and non-PTSD trauma-exposed participants (compare for more detailed information).
Table 4. Peritraumatic dissociation – Study characteristics and results.
To assess peritraumatic dissociation, almost all included studies used the Peritraumatic Dissociative Experience Questionnaire (PDEQ; Marmar et al., Citation1994; Marmar et al., Citation1997). Several modifications to this instrument were made: four studies used the 10-item self-report version, and three studies used a rater-administrated version with varied item length. One study used the State Dissociation Questionnaire (SDQ; Murray et al., Citation2002) to assess peritraumatic experiences like derealization, depersonalization, detachment, altered time sense, and emotional numbing during the traumatic experience (Halligan et al., Citation2003).
To provoke physiological reactions, five studies used personalized intrusive content. One study of combat veterans used standardized audiovisual combat scenes as well as scripts describing combat situations. One study used videos with trauma-associated content of varying emotional valence. Only one study used more general stressors in the form of acoustic startle probes.
Six articles examined HR. HRV was assessed in two studies as RMSSD, HF-HRV, LF-HRV and RSA. Two studies measured SBP and DBP, of which one also introduced the cardiovascular response discordance (CRD), a subtraction of standardized BP scores from standardized HR scores. One study measured PEP and four examined SC via SCL and SCR.
Four studies examined physiological reaction during experimental conditions, while four reported baseline or neutral condition-corrected change scores (either alone or in combination).
3.2.2. Effects on heart rate
The relationships between peritraumatic dissociation and HR during trauma exposure were mixed, although there was a tendency towards a positive correlation. Sledjeski and Delahanty (Citation2012) report a positive correlation between peritraumatic dissociation and HR (r = .34, p = .034), and a negative correlation between peritraumatic dissociation and the PEP score (r = −.42, p = .008), which corresponds to a faster HR. Additionally, Hetzel-Riggin and Wilber (Citation2010) demonstrate a large positive correlation between peritraumatic dissociation and HR in a PTSD subsample during the trauma condition (r = .52, p = .023). However, with a change score this correlation is non-significant (r = .11, p = .661). On the other hand, Bichescu-Burian et al. (Citation2017) used a change score to demonstrate a large negative correlation between peritraumatic dissociation and HR during an intervention (r = −.54, p = .010). No effects of experimentally-induced peritraumatic dissociation on HR were observed by Halligan et al. (Citation2006), Pole et al. (Citation2006), and Kaufman et al. (Citation2002).
3.2.3. Effects on heart rate variability
There was no correlation with peritraumatic dissociation and the assessed HRV parameters in the study by Hauschildt et al. (Citation2011) or with RSA in the study by Sledjeski and Delahanty (Citation2012).
3.2.4. Effects on blood pressure
At baseline, Pole et al. (Citation2006) found lower SBP (p < .050, d = −1.09), and DBP (p < .050, d = −1.15) for the high dissociation group compared to the low dissociation group (for baseline see Supplemental Table S2). This effect persisted while participants thought about their trauma for SBP (p < .050, d = −1.19), but not for DBP in the same condition (p > .050, d = −0.68). Neither SBP (p > .050, d = −0.75) nor DBP (p > .050, d = −0.39) differed while talking about the trauma condition. The authors of this study additionally calculated the CRD. In this regard, they found that in the high dissociation group HR was elevated relative to their associated trauma SBP, which was not observed in the low dissociation group during both the trauma and baseline conditions (for baseline see Supplemental Table S2). Kaufman et al. (Citation2002) found no effects of peritraumatic dissociation on BP.
3.2.5. Effects on skin conductance
No study found associations between SCL and peritraumatic dissociation when participants were exposed to personalized trauma stimuli (Hetzel-Riggin & Wilber, Citation2010; Kaufman et al., Citation2002). However, there is some evidence of elevated SCR in relation to peritraumatic dissociation. Hetzel-Riggin and Wilber (Citation2010) found a positive correlation between SCR and peritraumatic dissociation in a sample of female assault victims (r = .24, p < .050) but not in the subset who had a diagnosis of PTSD (r = .19, p = .434). Contrarily, during an intervention Bichescu-Burian et al. (Citation2017) found no effects of peritraumatic dissociation on SCR amplitude or frequency.
Using general stress stimuli, Ladwig et al. (Citation2002) found increased SCR in participants with high levels of dissociation as compared to participants with no/low dissociation during an acoustic startle probe (p = .017, d = 0.42). Furthermore, when dissociation was high, the presence or absence of PTSD did not affect SCR (p = .878, d = 0.17).
3.3. Acute dissociation
3.3.1. General study characteristics
In total, 15 articles examined cardiovascular or electrodermal reactions to trauma- or stress-related interventions in the context of acute state dissociation. Sample sizes varied (n = 14 to n = 61). A combined 482 participants were studied. Mean age varied from 18.8 years to 42.4 years. One study examined predominantly male participants, seven studies examined female participants only, and the percentage of female participants in the other studies was high (65% to 95%).
In five studies all participants were diagnosed with PTSD. Another five studies included trauma-exposed participants of which some met criteria for PTSD (for detailed information see Supplemental Table S3). In contrast to trait and peritraumatic dissociation, there are more studies on acute dissociative reactions during symptom provocation in BPD patients. This is expected, as dissociation is classified as a pathological reaction in BPD (American Psychiatric Association, Citation2013). However, most of the BPD patients in the included studies report a history of trauma and/or PTSD symptoms. This is to be expected due to the high comorbidity of the two disorders (Jowett et al., Citation2020). Accordingly, four studies assessed acute dissociation in patients with BPD, where all or a relevant proportion of the participants reported comorbid PTSD. However, one study reported correlations in an overall sample of BPD-patients with and without comorbid PTSD, as well as healthy controls (compare for more detailed information).
Table 5. Acute dissociation – Study characteristics and results.
To evaluate acute dissociation, four studies used the Dissociation-Tension Scale-acute (DSS-acute; Stiglmayr et al., Citation2003; Stiglmayr et al., Citation2010), consisting of dissociation-related items from the DES (Bernstein & Putnam, Citation1986), somatoform dissociation from the Somatoform Dissociation Questionnaire (SDQ-20; Nijenhuis et al., Citation1996), and 1–3 items of inner tension. Two studies used the related short version, the DSS-4 (Stiglmayr et al., Citation2009). Two studies used the dissociation subscale of the Responses to Script-Driven Imagery Scale (RSDI-SSD; Hopper, Frewen, Sack et al., Citation2007). This self-report rating was developed to assess post-traumatic reactions during experimental trauma confrontation on the subscales of re-experiencing, avoidance, and dissociation. Three studies used the Clinician-Administered Dissociative States Scale (CADSS; Bremner et al., Citation1998), which is rated by the subject and by an observer, either on a 4-point scale or as a dichotomy (present vs. absent). One study used the Dissociation State Scale (DSS), a self-rating adaptation of the CADSS (Bremner et al., Citation1998), and one study modified the PDEQ (Marmar et al., Citation1997) into an 8-item version they termed the State Dissociation Scale (SDS). Malta et al. (Citation2021) designed a study-specific Dissociation Scale, including 3 items assessing dissociation related experiences (e.g. losing sense of time, losing track of events, and feeling emotionally numb) on a 7-point scale.
To provoke physiological reactions to trauma stimuli, six studies used personalized intrusive content and one study used videos with trauma-associated content of varying emotional valence. Malta et al. (Citation2021) used a novel approach, incorporating virtual reality simulations of combat stressors. Seven studies used more general stress stimuli, including the TSST, white noise, acoustic startle probes, mirror confrontation, idiographic aversive scripts with startle probes, emotion regulation (with pictures of varying emotional valence) and aversive differential delay conditioning. Regarding the results of the latter, we focused on stress-eliciting interventions and therefore only report the psychophysiology for the initial reactivity within this conditioning paradigm.
Eight studies examined HR, one of which focused on HR acceleration and HR deceleration (defined as peak and lowest HR) following the experimental stimulus. Five studies assessed HRV parameters, including RMSSD, HF-HRV, LF-HRV and LF/HF-ratio. Three studies examined BP, one PEP and one finger pulse volume (FPV). Six studies examined SC parameters, including SCL, SCR and NSCF.
3.3.2. Effects on heart rate
Regarding personalized trauma stimuli, only one study (Sack et al., Citation2012) showed a moderate negative correlation between maximum HR in the trauma condition and acute dissociation (r = −.30, p < .050), also reflected by the baseline corrected change scores (r = −.23, p < .050). Additionally, they used median splitting of the RSDI-SSD to divide the full sample into high and low acute dissociation groups and, subsequently, into high re-experiencing and low re-experiencing groups. Focusing here on the high re-experiencing group, those with high dissociation exhibited significantly lower maximum HR than those with low dissociation (p = .002, d = −1.11) a finding confirmed by significantly different HR change scores across groups (p = .002, d = −1.11). Other studies (Bichescu-Burian et al., Citation2017; Chou et al., Citation2018; Lanius et al., Citation2002; Malta et al., Citation2021; Schmahl et al., Citation2004) found no correlation between HR and alterations in acute dissociative states. Lanius et al. (Citation2002) found no differences in HR between trauma-exposed patients without PTSD and PTSD patients with comorbid Dissociative Disorder Not Otherwise Specified (DDNOS), but the authors interpreted this as the absence of an increase in HR that would be expected in PTSD patients.
For general stress stimuli, no correlation was found between HR and acute dissociation (D'Andrea et al., Citation2013; Schäflein et al., Citation2018).
3.3.3. Effects on heart rate variability
Studies using personalized trauma stimuli demonstrate inconsistent and contradictory findings on HRV changes in acute dissociation. Sack et al. (Citation2012) found no correlation between the RMSSD acute dissociation in the overall sample (r = −.21, p < .050), but did find differences in RMSSD across participants with high vs low dissociation (p = .029, d = −0.80). More specifically, the high dissociation group exhibited a smaller decrease in RMSSD from the neutral to the trauma condition, reflecting less PNS adaptability. Chou et al. (Citation2018) found a negative correlation between dissociation and LF/HF-ratio (r = −.53, p < .050), reflecting increased parasympathetic activity, but no correlation with HF-HRV (r = .16, p > .050) or LF-HRV (r = −.28, p > .050). Hauschildt et al. (Citation2011) found no correlation between HRV and acute dissociation.
Regarding general stress stimuli, Schäflein et al. (Citation2018) found no correlation between acute dissociation and RMSSD in the mirror confrontation task. Krause-Utz et al. (Citation2018) also demonstrated no effects of acute dissociation on HF-HRV while participants watched pictures with varying emotional valence. However, when participants were asked to downregulate their emotions while viewing, there was a robust correlation between acute dissociation and HF-HRV for negative pictures (r = .66, p = .025) but not for positive pictures (r = .32, p = .614).
3.3.4. Effects on blood pressure and finger pulse volume
There was no correlation between acute dissociation and BP during trauma confrontation (Schmahl et al., Citation2004) or stress provocation (Duesenberg et al., Citation2019; Metz et al., Citation2020).
However, Sack et al. (Citation2012) found a positive correlation between acute dissociation and FPV (r = .33, p < .010). This effect was not observed in participants with high re-experiencing when comparing high vs. low dissociation (p > .050, d = −0.79).
3.3.5. Effects on skin conductance
With respect to studies using personalized trauma stimuli, no effects of acute dissociation on SC were observed (Bichescu-Burian et al., Citation2017; Schmahl et al., Citation2004; Vermes et al., Citation2020).
When general stress stimuli were utilized, findings were inconclusive. In a mixed sample including BPD patients with and without comorbid PTSD as well as healthy controls, Barnow et al. (Citation2012) demonstrated a positive correlation between acute dissociation and SCL change score (r = .14, p = .001). Neither D'Andrea et al. (Citation2013) nor Ebner-Priemer et al. (Citation2009) found group differences in SCL at baseline (for baseline see Supplemental Table S2). Regarding SCR, D'Andrea et al. (Citation2013) demonstrated that SCR was moderately decreased in the high dissociation group (p = .040, d = −0.57).
4. Discussion
We systematically reviewed 28 articles (n = 1300 subjects) with the hope of identifying the psychophysiological correlates of trait, peritraumatic, and acute dissociation. Due to conflicting results within and between studies, we found no clear pattern or trend for physiological markers of trauma-related dissociation. Studies on trait dissociation demonstrated contradicting physiological reactions. Two studies using TSST (Powers et al., Citation2021; Simeon et al., Citation2008) found a decrease in heart rate during stress induction which was associated with trait dissociation, whereas two other studies (Hyer et al., Citation1993; Schmahl et al., Citation2004) demonstrated hyperactive autonomic responses to traumatic content. These inconsistencies allude that the type of intervention and stress arousal (social stress vs. traumatic stress) may drive specific physiological responses. However, this divergence was only observed in relation to chronic trait dissociation. For peritraumatic dissociation, one study depicted a significantly decreased heart rate in comorbid PTSD-BPD patients (Bichescu-Burian et al., Citation2017). Other studies showed a slight trend towards hyperarousal associated with the retrospectively assessed dissociative reactions experienced during the initial traumatic event (Hetzel-Riggin & Wilber, Citation2010; Ladwig et al., Citation2002; Sledjeski & Delahanty, Citation2012). Contrary to this, several studies investigating acute post-traumatic dissociation depicted a tendency towards a hypoaroused autonomic reactivity (Chou et al., Citation2018; D'Andrea et al., Citation2013; Krause-Utz et al., Citation2018; Sack et al., Citation2012), suggesting that peri- and post-traumatic dissociation may be physiologically distinct from each other.
According to the defense cascade (Lanius et al., Citation2018) and shutdown models (Schauer & Elbert, Citation2010), individuals who experience dissociation during the initial traumatic event should bypass the tonic immobility state and enter a hypoaroused state. Additionally, an individual’s initial physiologic response to a traumatic event will determine their subsequent responses to trauma cues and stressors (see Introduction). Current research assessing peritraumatic and/or acute dissociation has not yet provided sufficient support for this idea. As previously stated, only a single study on peritraumatic dissociation demonstrated a significantly decreased heart rate, reflecting a hypoaroused autonomic state (Bichescu-Burian et al., Citation2017). Contrarily, several other studies on peritraumatic dissociation found a tendency towards increased arousal. With regards to acute post-traumatic dissociation, findings were similarly inconsistent, although there was some evidence of an attenuated response (based on heart rate, heart rate variability and skin conductance) (Chou et al., Citation2018; D'Andrea et al., Citation2013; Krause-Utz et al., Citation2018; Sack et al., Citation2012). Based on our assessment of the reviewed studies, we conclude that peritraumatic dissociation and acute posttraumatic dissociation are neither associated with PNS-mediated hypoarousal, nor follow the proposed physiologic trajectory in which the initial passive response to a trauma predicts one’s responses to subsequent stressors or trauma reminders. At this point, there is insufficient evidence to prove a consistent association beyond spurious findings, raising the question: why do well-prepared experimental studies fail to extract psychophysiological markers of dissociation?
One possible answer might lie in the inconsistent assessment of psychophysiological parameters across studies. Outcomes may be influenced by varying adaptation times, measurement durations, baseline conditions, artifact editing, and post-experiment processing. For instance, a review (Beauchaine et al., Citation2019) reported high inconsistencies across studies which used the RSA as a measure of transpathological dimensions, which they attribute to a lack of methodological standardization. Contrarily, psychophysiological markers of PTSD have been extensively investigated and respected for their easy and affordable implementation into clinical, ambulatory, and laboratory research (see Pineles & Orr, Citation2018). Additionally, if PTSD is examined without consideration of dissociative symptoms, there is consistent support for increased autonomic activity while resting and across experimental paradigms, as measured by HR, HRV parameters, and SC (Ge et al., Citation2020; Nagpal et al., Citation2013; Schneider & Schwerdtfeger, Citation2020). However, when the dissociative subtype of PTSD is extracted, the psychophysiological fingerprint remains elusive.
A recent extensive systematic review focused on the psychophysiological markers (among others) of pathological dissociation in a broader, transdiagnostic view (Roydeva & Reinders, Citation2021). This review confirmed our findings, citing inconclusive results due to most studies reporting no effects. The authors further concluded that none of the reviewed physiological measures could be recommended as a biomarker of pathological dissociation (Roydeva & Reinders, Citation2021). The same is true for markers of post-traumatic dissociation at the level of brain activation or anatomy. A review on this topic failed to establish a clear trend across studies (Lotfinia et al., Citation2020). Although analyses on resting-state brain activation tentatively support the idea of an overmodulated fear network (see defense cascade and shutdown models) through reports of altered functional connectivity between prefrontal and subcortical areas in dissociative PTSD patients (Nicholson et al., Citation2017; Nicholson et al., Citation2019; Rabellino et al., Citation2018), experimental studies are lacking. The few experimental neuroimaging studies to date (Daniels et al., Citation2012; Felmingham et al., Citation2008; Hopper, Frewen, van der Kolk et al., Citation2007; Mertens et al., Citation2022) either report inconsistent brain regions associated with trauma-related dissociation or fail to present any associated neural regions at all. Ultimately, this undermines the proposed neurobiological basis (e.g. Lanius et al., Citation2010; Terpou et al., Citation2019) of the defense cascade model and the dissociative subtype as a whole.
Other factors which may influence the assessment of valid objective markers of dissociation are the inconsistent conceptualization and measurement of dissociation. Within this review alone, we include studies with 14 unique measures of dissociation, which each emphasize different aspects of dissociation. The resulting sum scores are therefore driven by a spectrum of symptoms, from those considered less pathological (e.g. absorption) to those considered more pathological (e.g. identity fragmentation). When correlating such a sum score with psychophysiological measures, any true effect of depersonalization and derealization could be masked by the influence of these other symptoms. Hence, heterogeneity in our results may be driven by the diversity of dissociation assessment techniques.
Additionally, the meaning of hypoarousal is not consistent in the dissociation literature. Most of the aforementioned models defined hypoarousal as equivalent to parasympathetic dominance; however physiological research shows that alterations in heart rate or heart rate variability may be steered by both SNS and PNS activity (de Geus et al., Citation2019). Multimodal assessments incorporating psychophysiological measures reflective of both SNS and PNS activation are thus highly recommended to disentangle these complex interactions (e.g. as in Schmahl et al., Citation2004; Seligowski et al., Citation2019).
The initial pilot studies describing alternating physiological reactions to individualized trauma scripts in chronic PTSD patients (Lanius et al., Citation2001; Lanius et al., Citation2002) interpreted the data as supportive for two distinct subtypes ‘each representing unique pathways to chronic stress- related psychopathology’ (Lanius et al., Citation2006, p. 710). In these studies, patients with elevated heart rates (70%) were characterized as intrusive subtypes whereas the 30% without elevated heart rates were characterized as dissociative subtypes, reflective of derealization or depersonalization. Here, dissociative hypoarousal was characterized as the absence of the PTSD-pathognomonic hyperarousal state. It should be noted that these findings were based on small samples (N ≤ 10). Another study from the same research group found that although some dissociative PTSD patients had unchanged (or not increased) HR during trauma exposure, there was no clinical difference between these patients and those who exhibited decreased or increased HR (Lanius et al., Citation2005). Meanwhile, other studies employed the term ‘active suppression’ to reflect significant heart rate deceleration (D'Andrea et al., Citation2013) or reduced heart rate reactivity (Sack et al., Citation2012) concurrent with parasympathetic dominance (Lanius et al., Citation2018; Schauer & Elbert, Citation2010). Due to these inconsistencies in language, we implore future researchers in this field to clearly state whether they define hypoarousal as the absence of an increase in HR, a relative decrease in HR within-subjects (e.g. from baseline or within tasks), or a decrease in HR between-groups (e.g. compared to trauma-exposed controls).
4.1. Limitations and strengths
Several limitations are present in the current review. First, this qualitative review did not include a quantitative meta-analysis of the reviewed studies’ findings. Although the search resulted in sufficient data to be quantitatively reviewed, it was not deemed sensible due to the high heterogeneity in sample characteristics (e.g. PTSD severity, gender, comorbidity), experimental interventions, dissociation assessment tools, and physiological measures. With this variation, a meta-analysis would run the risk of hastily simplifying a complex phenomenon. Thus, a systematic overview of these heterogenetic studies seemed to be more appropriate.
This review specifically focused on post-traumatic dissociation and therefore concentrated on search terms according to derealization/depersonalization. However, except for the RSDI-SSD, none of the reported instruments solely measured derealization/depersonalization. Therefore, no clear distinction could be made between physiological reactions specific to derealization/depersonalization vs. those associated with ‘compartmentalization’ (Holmes et al., Citation2005). Hence, these results can only be interpreted to a limited extent. Additionally, we have limited information on the differences between dissociators vs non-dissociators with PTSD to verify the postulated opposing subtypes. Only one study on these opposing groups was identified within in each included dissociative modality (e.g. acute, trait, and peritraumatic dissociation). We observed lower autonomic activity in people with high acute dissociation (Sack et al., Citation2012), higher HR in those with high trait dissociation (Hyer et al., Citation1993), and no physiologic effect in those with high peritraumatic dissociation (Kaufman et al., Citation2002).
One of the main limitations to the reviewed studies is the low sample sizes and thus limited statistical power to detect subtle changes in sensitive measures of psychophysiological activity. Additionally, the heterogeneity of physiological indices and dissociation measures employed across studies impedes our ability to make any direct comparisons or generalizations. Specifically, only five studies included at least two types of dissociation modalities (e.g. assessed both peritraumatic and acute dissociation). Most studies related dissociation to mean scores of psychophysiological measurements. In future studies, temporal changes in physiology should be included to highlight any fluctuations or trends that may support current models of defense response (e.g. progression of an initial increased HR followed by reduced HR/hypotension as suggested by the defense cascade model).
This review has several strengths and provides novel insight into the psychophysiology of dissociation in PTSD. One strength is that we included only experimental studies that employed symptom provocation or stress-induction paradigms. This is of significance as all defense response models underscore the importance of dissociation as a defensive reaction to a clear stressor or trauma reminder. To our knowledge, this is the first review to summarize the relationship between dissociation and experimentally induced acute physiological responses. Furthermore, we categorize findings according to distinct dissociation modalities, thus permitting a clear comparison of physiological responses across peri-, acute, and chronic post-traumatic dissociation. In doing so, we begin to disentangle dissociation-related psychophysiological reactions from the moment the trauma occurs, gets reactivated, and develops into a chronic pattern.
4.2. Conclusion and future outlook
In sum, the current review investigates psychophysiological correlates of dissociation in PTSD through a well-defined and narrow scope. From the extracted literature, we find (a) an association between a hyperactive defensive response and peritraumatic dissociation, (b) an association between a hypoactive defensive response and acute post-traumatic dissociation, and (c) a possible impact of the type of stressor on physiologic responses in chronic trait dissociation. However, there is no evidence for a clear link between a hypoaroused ANS and peri- and post-traumatic dissociation across studies. Furthermore, it should be noted that none of the studied physiological parameters are pathognomonic predictors of trauma-related dissociation as similar reactive patterns have been observed across various psychiatric disorders (see Chalmers et al., Citation2014 for anxiety; see Koenig et al., Citation2016 for BPD). As recommended by Roydeva and Reinders (Citation2021), future research should aim to increase the specificity of biomarkers to PTSD-related dissociation, for instance by testing the robustness of these psychophysiological correlates across other psychopathologies (e.g. depression or anxiety).
To overcome the gap between theoretical models and empirical evidence, studies are needed which have clear inclusion criteria (e.g. PTSD or post-traumatic stress symptoms), control for PTSD severity, and use longitudinal designs to assess symptom variation. Particularly, studies which assess peritraumatic dissociation as early as possible after a trauma will reduce the risk of retrospective bias. Ambulatory assessment strategies like ecological sampling (e.g. wireless HR monitoring) may overcome the necessity of provoking PTSD and dissociation symptoms. These findings would be far more applicable to the PTSD patient population.
Supplemental Material
Download MS Word (34.9 KB)Supplemental Material
Download MS Word (22.6 KB)Supplemental Material
Download MS Excel (34.4 KB)Supplemental Material
Download MS Word (203.8 KB)Acknowledgments
The authors would like to thank Christina Schulte for taking part in the rating process as independent rater and Margaret Engstrom for language proofreading. The authors express our gratitude to all authors of the original publications for supporting this work by providing additional information, calculations, and their interest in this research topic.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
Supplementary material, including a data file with the effect sizes and detailed descriptions of the studies reported (see Supplementary Table 3), can be found in the online version, at https://doi.org/10.1080/20008066.2022.2132599
.Additional information
Funding
References
- Abrams, M. P., Carleton, R. N., Taylor, S., & Asmundson, G. J. (2009). Human tonic immobility: Measurement and correlates. Depress and Anxiety, 26(6), 550–556. https://doi.org/10.1002/da.20462
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing. https://doi.org/10.1176/appi.books.9780890425596
- Bandler, R., Keay, K. A., Floyd, N., & Price, J. (2000). Central circuits mediating patterned autonomic activity during active vs. Passive emotional coping. Brain Research Bulletin, 53(1), 95–104. https://doi.org/10.1016/s0361-9230(00)00313-0
- Barnow, S., Limberg, A., Stopsack, M., Spitzer, C., Grabe, H. J., Freyberger, H. J., & Hamm, A. (2012, Apr). Dissociation and emotion regulation in borderline personality disorder. Psychological Medicine, 42(4), 783–794. https://doi.org/10.1017/S0033291711001917
- Barrett, L. F. (2020). Seven and a half lessons about the brain (1st ed.). Houghton Mifflin Harcourt.
- Beauchaine, T. P., Bell, Z., Knapton, E., McDonough-Caplan, H., Shader, T., & Zisner, A. (2019). Respiratory sinus arrhythmia reactivity across empirically based structural dimensions of psychopathology: A meta-analysis. Psychophysiology, 56(5), e13329. https://doi.org/10.1111/psyp.13329
- Bergmann, U. (2008). She’s come undone: A neurobiological exploration of dissociative disorders. In C. Forgash & M. Copeley (Eds.), Healing the heart of trauma and dissociation: with EMDR and ego state therapy (pp. 61–90). Springer.
- Bernstein, E. M., & Putnam, F. W. (1986, Dec). Development, reliability, and validity of a dissociation scale. The Journal of Nervous and Mental Disease, 174(12), 727–735. https://doi.org/10.1097/00005053-198612000-00004
- Berntson, G. G., Bigger, J. T., Jr., Eckberg, D. L., Grossman, P., Kaufmann, P. G., Malik, M., Nagaraja, H. N., Porges, S. W., Saul, J. P., Stone, P. H., & van der Molen, M. W. (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. https://doi.org/10.1111/j.1469-8986.1997.tb02140.x
- Bichescu-Burian, D., Steyer, J., Steinert, T., Grieb, B., & Tschoke, S. (2017, Mar). Trauma-related dissociation: Psychological features and psychophysiological responses to script-driven imagery in borderline personality disorder. Psychophysiology, 54(3), 452–461. https://doi.org/10.1111/psyp.12795
- Billman, G. E. (2013). The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Frontiers in Physiology, 4, 1–5. https://doi.org/10.3389/fphys.2013.00026
- Bremner, J. D., Krystal, J. H., Putnam, F. W., Southwick, S. M., Marmar, C., Charney, D. S., & Mazure, C. M. (1998, Jan). Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). Journal of Traumatic Stress, 11(1), 125–136. https://doi.org/10.1023/A:1024465317902
- Briere, J., Weathers, F. W., & Runtz, M. (2005, Jun). Is dissociation a multidimensional construct? Data from the multiscale dissociation inventory. Journal of Traumatic Stress, 18(3), 221–231. https://doi.org/10.1002/jts.20024
- Carlson, E. B., Waelde, L. C., Palmieri, P. A., Macia, K. S., Smith, S. R., & McDade-Montez, E. (2018, Jan). Development and validation of the dissociative symptoms scale. Assessment, 25(1), 84–98. https://doi.org/10.1177/1073191116645904
- Castro-Chapman, P. L., Orr, S. P., Berg, J., Pineles, S. L., Yanson, J., & Salomon, K. (2018, Mar). Heart rate reactivity to trauma-related imagery as a measure of PTSD symptom severity: Examining a new cohort of veterans. Psychiatry Research, 261, 574–580. https://doi.org/10.1016/j.psychres.2018.01.024
- Chalmers, J. A., Quintana, D. S., Abbott, M. J., & Kemp, A. H. (2014). Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Frontiers in Psychiatry, 5, 1–5. https://doi.org/10.3389/fpsyt.2014.00080
- Chou, C. Y., La Marca, R., Steptoe, A., & Brewin, C. R. (2018). Cardiovascular and psychological responses to voluntary recall of trauma in posttraumatic stress disorder. European Journal of Psychotraumatology, 9(1), Article 1472988. https://doi.org/10.1080/20008198.2018.1472988
- Corrigan, F. M., Fisher, J. J., & Nutt, D. J. (2011). Autonomic dysregulation and the window of tolerance model of the effects of complex emotional trauma. Journal of Psychopharmacology, 25(1), 17–25. https://doi.org/10.1177/0269881109354930
- Cuthbert, B. N., Lang, P. J., Strauss, C., Drobes, D., Patrick, C. J., & Bradley, M. M. (2003). The psychophysiology of anxiety disorder: Fear memory imagery. Psychophysiology, 40(3), 407–422. https://doi.org/10.1111/1469-8986.00043
- D'Andrea, W., Pole, N., DePierro, J., Freed, S., & Wallace, D. B. (2013, Oct). Heterogeneity of defensive responses after exposure to trauma: Blunted autonomic reactivity in response to startling sounds. International Journal of Psychophysiology, 90(1), 80–89. https://doi.org/10.1016/j.ijpsycho.2013.07.008
- Dalenberg, C., & Carlson, E. B. (2012). Dissociation in posttraumatic stress disorder part II: How theoretical models fit the empirical evidence and recommendations for modifying the diagnostic criteria for PTSD. Psychological Trauma: Theory, Research, Practice, and Policy, 4(6), 551–559. https://doi.org/10.1037/a0027900
- Daniels, J. K., Coupland, N. J., Hegadoren, K. M., Rowe, B. H., Densmore, M., Neufeld, R. W., & Lanius, R. A. (2012). Neural and behavioral correlates of peritraumatic dissociation in an acutely traumatized sample. The Journal of Clinical Psychiatry, 73(4), 420–426. https://doi.org/10.4088/JCP.10m06642
- de Geus, E. J. C., Gianaros, P. J., Brindle, R. C., Jennings, J. R., & Berntson, G. G. ((2019, Feb). Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology, 56(2), e13287. https://doi.org/10.1111/psyp.13287
- Dell, P. F. (2006). The multidimensional inventory of dissociation (MID): A comprehensive measure of pathological dissociation. Journal of Trauma & Dissociation, 7(2), 77–106. https://doi.org/10.1300/J229v07n02_06
- Duesenberg, M., Wolf, O. T., Metz, S., Roepke, S., Fleischer, J., Elias, V., Renneberg, B., Otte, C., & Wingenfeld, K. (2019). Psychophysiological stress response and memory in borderline personality disorder. European Journal of Psychotraumatology, 10(1), Article 1568134. https://doi.org/10.1080/20008198.2019.1568134
- Ebner-Priemer, U. W., Mauchnik, J., Kleindienst, N., Schmahl, C., Peper, M., Rosenthal, M. Z., Flor, H., & Bohus, M. (2009). Emotional learning during dissociative states in borderline personality disorder. Journal of Psychiatry & Neuroscience: JPN, 34(3), 214–222. https://www.jpn.ca/content/34/3/214.full
- Felmingham, K., Kemp, A. H., Williams, L., Falconer, E., Olivieri, G., Peduto, A., & Bryant, R. (2008). Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychological Medicine, 38(12), 1771–1780. https://doi.org/10.1017/S0033291708002742
- Frewen, P. A., & Lanius, R. A. (2006a). Neurobiology of dissociation: Unity and disunity in mind-body-brain. Psychiatric Clinics of North America, 29(1), 113–128. https://doi.org/10.1016/j.psc.2005.10.016
- Frewen, P. A., & Lanius, R. A. (2006b). Toward a psychobiology of posttraumatic self-dysregulation: Reexperiencing, hyperarousal, dissociation, and emotional numbing. Annals of the New York Academy of Sciences, 1071(1), 110–124. https://doi.org/10.1196/annals.1364.010
- Ge, F., Yuan, M., Li, Y., & Zhang, W. (2020, Jan). Posttraumatic stress disorder and alterations in resting heart rate variability: A systematic review and meta-analysis. Psychiatry Investigation, 17(1), 9–20. https://doi.org/10.30773/pi.2019.0112 [Record #149 is using a reference type undefined in this output style.]
- Grossman, P., & Taylor, E. W. (2007). Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology, 74(2), 263–285. https://doi.org/10.1016/j.biopsycho.2005.11.014
- Hagenaars, M. A. (2016). Tonic immobility and PTSD in a large community sample. Journal of Experimental Psychopathology, 7(2), 246–260. https://doi.org/10.5127/jep.051915
- Hagenaars, M. A., Oitzl, M., & Roelofs, K. (2014). Updating freeze: Aligning animal and human research. Neuroscience & Biobehavioral Reviews, 47, 165–176. https://doi.org/10.1016/j.neubiorev.2014.07.021
- Halligan, S. L., Michael, T., Clark, D. M., & Ehlers, A. (2003, Jun). Posttraumatic stress disorder following assault: The role of cognitive processing, trauma memory, and appraisals. Journal of Consulting and Clinical Psychology, 71(3), 419–431. https://doi.org/10.1037/0022-006x.71.3.419
- Halligan, S. L., Michael, T., Wilhelm, F. H., Clark, D. M., & Ehlers, A. (2006, Oct). Reduced heart rate responding to trauma reliving in trauma survivors with PTSD: Correlates and consequences. Journal of Traumatic Stress, 19(5), 721–734. https://doi.org/10.1002/jts.20167
- Hansen, M., Ross, J., & Armour, C. (2017, Apr 15). Evidence of the dissociative PTSD subtype: A systematic literature review of latent class and profile analytic studies of PTSD. Journal of Affective Disorders, 213, 59–69. https://doi.org/10.1016/j.jad.2017.02.004
- Hauschildt, M., Peters, M. J., Moritz, S., & Jelinek, L. (2011, Dec). Heart rate variability in response to affective scenes in posttraumatic stress disorder. Biological Psychology, 88(2-3), 215–222. https://doi.org/10.1016/j.biopsycho.2011.08.004
- Head, M. L., Holman, L., Lanfear, R., Kahn, A. T., & Jennions, M. D. (2015, Mar). The extent and consequences of p-hacking in science. PLOS Biology, 13(3), e1002106. https://doi.org/10.1371/journal.pbio.1002106
- Hetzel-Riggin, M. D., & Wilber, E. L. (2010). To dissociate or suppress? Predicting automatic vs. conscious cognitive avoidance. Journal of Trauma & Dissociation, 11(4), 444–457. https://doi.org/10.1080/15299732.2010.495376
- Holmes, E. A., Brown, R. J., Mansell, W., Fearon, R. P., Hunter, E. C., Frasquilho, F., & Oakley, D. A. (2005). Are there two qualitatively distinct forms of dissociation? A review and some clinical implications. Clinical Psychology Review, 25(1), 1–23. https://doi.org/10.1016/j.cpr.2004.08.006
- Hopper, J. W., Frewen, P. A., Sack, M., Lanius, R. A., & van der Kolk, B. A. (2007). The responses to script-driven imagery scale (RSDI): Assessment of state posttraumatic symptoms for psychobiological and treatment research. Journal of Psychopathology and Behavioral Assessment, 29(4), 249–268. https://doi.org/10.1007/s10862-007-9046-0
- Hopper, J. W., Frewen, P. A., van der Kolk, B. A., & Lanius, R. A. (2007). Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. Journal of Traumatic Stress, 20(5), 713–725. https://doi.org/10.1002/jts.20284
- Hyer, L. A., Albrecht, J. W., Boudewyns, P. A., Woods, M. G., & Brandsma, J. (1993). Dissociative experiences of Vietnam veterans with chronic posttraumatic stress disorder. Psychological Reports, 73(2), 519–530. https://doi.org/10.2466/pr0.1993.73.2.519
- Iffland, B., Klein, F., Rosner, R., Renneberg, B., Steil, R., & Neuner, F. (2020, Jan). Cardiac reactions to emotional words in adolescents and young adults with PTSD after child abuse. Psychophysiology, 57(1), e13470. https://doi.org/10.1111/psyp.13470
- Jowett, S., Karatzias, T., & Albert, I. (2020, Sep). Multiple and interpersonal trauma are risk factors for both post-traumatic stress disorder and borderline personality disorder: A systematic review on the traumatic backgrounds and clinical characteristics of comorbid post-traumatic stress disorder/borderline personality disorder groups versus single-disorder groups. Psychology and Psychotherapy: Theory, Research and Practice, 93(3), 621–638. https://doi.org/10.1111/papt.12248
- Kaufman, M. L., Kimble, M. O., Kaloupek, D. G., McTeague, L. M., Bachrach, P., Forti, A. M., & Keane, T. M. (2002). Peritraumatic dissociation and physiological response to trauma-relevant stimuli in Vietnam combat veterans with posttraumatic stress disorder. The Journal of Nervous and Mental Disease, 190(3), 167–174. https://doi.org/10.1097/00005053-200203000-00005
- Koenig, J., Kemp, A. H., Feeling, N. R., Thayer, J. F., & Kaess, M. (2016). Resting state vagal tone in borderline personality disorder: A meta-analysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 64, 18–26. https://doi.org/10.1016/j.pnpbp.2015.07.002
- Krause-Utz, A., Walther, J.-C., Lis, S., Schmahl, C., & Bohus, M. (2018). Heart rate variability during a cognitive reappraisal task in female patients with borderline personality disorder: The role of comorbid posttraumatic stress disorder and dissociation. Psychological Medicine, 49(11), 1810–1821. https://doi.org/10.1017/s0033291718002489
- Kuiling, J. M. E., Klaassen, F., & Hagenaars, M. A. (2019). The role of tonic immobility and control in the development of intrusive memories after experimental trauma. Memory, 27(6), 772–779. https://doi.org/10.1080/09658211.2018.1564331
- Ladwig, K.-H., Marten-Mittag, B., Deisenhofer, I., Hofmann, B., Schapperer, J., Weyerbrock, S., Erazo, N., & Schmitt, C. (2002). Psychophysiological correlates of peritraumatic dissociative responses in survivors of life-threatening cardiac events. Psychopathology, 35(4), 241–248. https://doi.org/10.1159/000063825
- Lanius, R. A., Bluhm, R., Lanius, U., & Pain, C. (2006). A review of neuroimaging studies in PTSD: Heterogeneity of response to symptom provocation. Journal of Psychiatric Research, 40(8), 709–729. https://doi.org/10.1016/j.jpsychires.2005.07.007
- Lanius, R. A., Boyd, J. E., McKinnon, M. C., Nicholson, A. A., Frewen, P., Vermetten, E., Jetly, R., & Spiegel, D. (2018). A review of the neurobiological basis of trauma-related dissociation and Its relation to cannabinoid- and opioid-mediated stress response: A transdiagnostic, translational approach. Current Psychiatry Reports, 20(12), 1–14. https://doi.org/10.1007/s11920-018-0983-y
- Lanius, R. A., Brand, B., Vermetten, E., Frewen, P. A., & Spiegel, D. (2012). The dissociative subtype of posttraumatic stress disorder: Rationale, clinical and neurobiological evidence, and implications. Depression and Anxiety, 29(8), 701–708. https://doi.org/10.1002/da.21889
- Lanius, R. A., Vermetten, E., Loewenstein, R. J., Brand, B., Schmahl, C., Bremner, J. D., & Spiegel, D. (2010). Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry, 167(6), 640–647. https://doi.org/10.1176/appi.ajp.2009.09081168
- Lanius, R. A., Williamson, P. C., Bluhm, R. L., Densmore, M., Boksman, K., Neufeld, R. W. J., Gati, J. S., & Menon, R. S. (2005). Functional connectivity of dissociative responses in posttraumatic stress disorder: A functional magnetic resonance imaging investigation. Biological Psychiatry, 57(8), 873–884. https://doi.org/10.1016/j.biopsych.2005.01.011
- Lanius, R. A., Williamson, P. C., Boksman, K., Densmore, M., Gupta, M., Neufeld, R. W. J., Gati, J. S., & Menon, R. S. (2002). Brain activation during script-driven imagery induced dissociative responses in PTSD: A functional magnetic resonance imaging investigation. Biological Psychiatry, 52(4), 305–311. https://doi.org/10.1016/s0006-3223(02)01367-7
- Lanius, R. A., Williamson, P. C., Densmore, M., Boksman, K., Gupta, M. A., Neufeld, R. W., Gati, J. S., & Menon, R. S. (2001). Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. American Journal of Psychiatry, 158(11), 1920–1922. https://doi.org/10.1176/appi.ajp.158.11.1920
- Lenhard, W., & Lenhard, A. (2016). Berechnung von Effektstärken [Calculation of effect sizes]. In Psychometrica. https://www.psychometrica.de/effektstaerke.html
- Lima, A. A., Fiszman, A., Marques-Portella, C., Mendlowicz, M. V., Coutinho, E. S., Maia, D. C., Berger, W., Rocha-Rego, V., Volchan, E., Mari, J. J., & Figueira, I. (2010, Mar). The impact of tonic immobility reaction on the prognosis of posttraumatic stress disorder. Journal of Psychiatric Research, 44(4), 224–228. https://doi.org/10.1016/j.jpsychires.2009.08.005
- Limberg, A., Barnow, S., Freyberger, H. J., & Hamm, A. O. (2011). Emotional vulnerability in borderline personality disorder is cue specific and modulated by traumatization. Biological Psychiatry, 69(6), 574–582. https://doi.org/10.1016/j.biopsych.2010.10.024
- Lotfinia, S., Soorgi, Z., Mertens, Y., & Daniels, J. (2020). Structural and functional brain alterations in psychiatric patients with dissociative experiences: A systematic review of magnetic resonance imaging studies. Journal of Psychiatric Research, 128, 5–15. https://doi.org/10.1016/j.jpsychires.2020.05.006
- Ludascher, P., Valerius, G., Stiglmayr, C., Mauchnik, J., Lanius, R. A., Bohus, M., & Schmahl, C. (2010). Pain sensitivity and neural processing during dissociative states in patients with borderline personality disorder with and without comorbid posttraumatic stress disorder: A pilot study. Journal of Psychiatry and Neuroscience, 35(3), 177–184. https://doi.org/10.1503/jpn.090022
- Lyssenko, L., Schmahl, C., Bockhacker, L., Vonderlin, R., Bohus, M., & Kleindienst, N. (2018, Jan 1). Dissociation in psychiatric disorders: A meta-analysis of studies using the dissociative experiences scale. American Journal of Psychiatry, 175(1), 37–46. https://doi.org/10.1176/appi.ajp.2017.17010025
- MacLean, P. D. (1990). The triune brain in evolution: Role in paleocerebral functions. Springer Science & Business Media.
- Malta, L. S., Giosan, C., Szkodny, L. E., Altemus, M. M., Rizzo, A. A., Silbersweig, D. A., & Difede, J. (2021). Development of a virtual reality laboratory stressor. Virtual Reality, 25(2), 293–302. https://doi.org/10.1007/s10055-020-00455-5
- Marmar, C. R., Weiss, D. S., & Metzler, T. J. (1997). The peritraumatic dissociative experiences questionnaire. In J. P. Wilson & T. M. Keane (Eds.), Assessing psychological trauma and PTSD (pp. 412–428). The Guilford Press.
- Marmar, C. R., Weiss, D. S., Schlenger, W. E., Fairbank, J. A., Jordan, B. K., Kulka, R. A., & Hough, R. L. (1994, Jun). Peritraumatic dissociation and posttraumatic stress in male Vietnam theater veterans. American Journal of Psychiatry, 151(6), 902–907. https://doi.org/10.1176/ajp.151.6.902
- McKinnon, M. C., Boyd, J. E., Frewen, P. A., Lanius, U. F., Jetly, R., Richardson, J. D., & Lanius, R. A. (2016, Sep). A review of the relation between dissociation, memory, executive functioning and social cognition in military members and civilians with neuropsychiatric conditions. Neuropsychologia, 90, 210–234. https://doi.org/10.1016/j.neuropsychologia.2016.07.017
- McTeague, L. M., & Lang, P. J. (2012). The anxiety spectrum and the reflex physiology of defense: From circumscribed fear to broad distress. Depression and Anxiety, 29(4), 264–281. https://doi.org/10.1002/da.21891
- Mertens, Y. L., Manthey, A., Sierk, A., Walter, H., & Daniels, J. K. (2022, Jun 10). Neural correlates of acute post-traumatic dissociation: A functional neuroimaging script-driven imagery study. BJPsych Open, 8(4), e109. https://doi.org/10.1192/bjo.2022.65
- Metz, S., Duesenberg, M., Hellmann-Regen, J., Wolf, O. T., Roepke, S., Otte, C., & Wingenfeld, K. (2020, Nov). Blunted salivary cortisol response to psychosocial stress in women with posttraumatic stress disorder. Journal of Psychiatric Research, 130, 112–119. https://doi.org/10.1016/j.jpsychires.2020.07.014
- Mickleborough, M. J., Daniels, J. K., Coupland, N. J., Kao, R., Williamson, P. C., Lanius, U. F., Hegadoren, K., Schore, A., Densmore, M., Stevens, T., & Lanius, R. A. (2011). Effects of trauma-related cues on pain processing in posttraumatic stress disorder: An fMRI investigation. Journal of Psychiatry & Neuroscience, 36(1), 6–14. https://doi.org/10.1503/jpn.080188
- Mobbs, D., Marchant, J. L., Hassabis, D., Seymour, B., Tan, G., Gray, M., Petrovic, P., Dolan, R. J., & Frith, C. D. (2009). From threat to fear: The neural organization of defensive fear systems in humans. Journal of Neuroscience, 29(39), 12236–12243. https://doi.org/10.1523/JNEUROSCI.2378-09.2009
- Murray, J., Ehlers, A., & Mayou, R. A. (2002, Apr). Dissociation and post-traumatic stress disorder: Two prospective studies of road traffic accident survivors. British Journal of Psychiatry, 180(4), 363–368. https://doi.org/10.1192/bjp.180.4.363
- Nagpal, M. L., Gleichauf, K., & Ginsberg, J. P. (2013). Meta-analysis of heart rate variability as a psychophysiological indicator of posttraumatic stress disorder. Trauma & Treatment, 03(182), 1–8. https://doi.org/10.4172/2167-1222.1000182
- Nicholson, A. A., Densmore, M., McKinnon, M. C., Neufeld, R. W. J., Frewen, P. A., Theberge, J., Jetly, R., Richardson, J. D., & Lanius, R. A. (2019, Sep). Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: A multimodal neuroimaging approach. Psychological Medicine, 49(12), 2049–2059. https://doi.org/10.1017/S0033291718002866
- Nicholson, A. A., Friston, K. J., Zeidman, P., Harricharan, S., McKinnon, M. C., Densmore, M., Neufeld, R. W. J., Theberge, J., Corrigan, F., Jetly, R., Spiegel, D., & Lanius, R. A. (2017, Nov). Dynamic causal modeling in PTSD and its dissociative subtype: Bottom-up versus top-down processing within fear and emotion regulation circuitry. Human Brain Mapping, 38(11), 5551–5561. https://doi.org/10.1002/hbm.23748
- Nijenhuis, E. R., Spinhoven, P., Van Dyck, R., Van der Hart, O., & Vanderlinden, J. ((1996, Nov). The development and psychometric characteristics of the Somatoform Dissociation Questionnaire (SDQ-20). The Journal of Nervous and Mental Disease, 184(11), 688–694. https://doi.org/10.1097/00005053-199611000-00006
- Ogden, P., Minton, K., & Pain, C. (2006). Trauma and the body: A sensorimotor approach to psychotherapy. W. W. Norton & Company.
- Orr, S. P. (1997). Psychophysiologic reactivity to trauma-related imagery in PTSD. Diagnostic and theoretical implications of recent findings. In R. Yehuda & A. C. McFarlane (Eds.), Psychobiology of posttraumatic stress disorder (pp. 114–124). New York Academy of Sciences.
- Orr, S. P., Metzger, L. J., & Pitman, R. K. (2002). Psychophysiology of post-traumatic stress disorder. Psychiatric Clinics of North America, 25(2), 271–293. https://doi.org/10.1016/s0193-953x(01)00007-7
- Orr, S. P., & Roth, W. T. (2000). Psychophysiological assessment: Clinical applications for PTSD. Journal of Affective Disorders, 61(3), 225–240. https://doi.org/10.1016/s0165-0327(00)00340-2
- Pineles, S. L., & Orr, S. P. (2018). The psychophysiology of PTSD. In C. B. Nemeroff & C. R. Marmar (Eds.), Post-traumatic stress disorder (pp. 393–408). Oxford University Press. https://doi.org/10.1093/med/9780190259440.003.0022
- Pole, N. (2007, Sep). The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin, 133(5), 725–746. https://doi.org/10.1037/0033-2909.133.5.725
- Pole, N., Cumberbatch, E., Taylor, W. M., Metzler, T. J., Marmar, C. R., & Neylan, T. C. (2006). Comparisons between high and low peritraumatic dissociators in cardiovascular and emotional activity while remembering trauma. Journal of Trauma & Dissociation, 6(4), 51–67. https://doi.org/10.1300/J229v06n04_04
- Porges, S. W. (2003, Dec). Social engagement and attachment: A phylogenetic perspective. Annals of the New York Academy of Sciences, 1008(1), 31–47. https://doi.org/10.1196/annals.1301.004
- Porges, S. W. (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. https://doi.org/10.1016/j.biopsycho.2006.06.009
- Porges, S. W. (2011). The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication, and self-regulation. W W Norton & Co.
- Powers, A., Mekawi, Y., Fickenwirth, M., Nugent, N. R., Dixon, H. D., Minton, S., Kim, Y. J., Gluck, R., Carter, S., Fani, N., Schwartz, A. C., Bradley, B., Umpierrez, G. E., Pace, T. W. W., Jovanovic, T., Michopoulos, V., & Gillespie, C. F. (2021, Oct). Emotion dysregulation and dissociation contribute to decreased heart rate variability to an acute psychosocial stressor in trauma-exposed Black women. Journal of Psychiatric Research, 142, 125–131. https://doi.org/10.1016/j.jpsychires.2021.07.032
- Rabellino, D., Densmore, M., Theberge, J., McKinnon, M. C., & Lanius, R. A. (2018). The cerebellum after trauma: Resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Human Brain Mapping, 39(8), 3354–3374. https://doi.org/10.1002/hbm.24081
- Rafiq, S., Campodonico, C., & Varese, F. (2018). The relationship between childhood adversities and dissociation in severe mental illness: A meta-analytic review. Acta Psychiatrica Scandinavica, 138(6), 509–525. https://doi.org/10.1111/acps.12969
- Rhudy, J. L., Davis, J. L., Williams, A. E., McCabe, K. M., & Byrd, P. M. (2008). Physiological–emotional reactivity to nightmare-related imagery in trauma-exposed persons with chronic nightmares. Behavioral Sleep Medicine, 6(3), 158–177. https://doi.org/10.1080/15402000802162539
- Roelofs, K. (2017). Freeze for action: Neurobiological mechanisms in animal and human freezing. Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1718), 1–10. https://doi.org/10.1098/rstb.2016.0206
- Roydeva, M. I., & Reinders, A. A. T. S. (2021). Biomarkers of pathological dissociation: A systematic review. Neuroscience & Biobehavioral Reviews, 123, 120–202. https://doi.org/10.1016/j.neubiorev.2020.11.019
- Sack, M., Cillien, M., & Hopper, J. W. (2012). Acute dissociation and cardiac reactivity to script-driven imagery in trauma-related disorders. European Journal of Psychotraumatology, 3(1), 1–10. https://doi.org/10.3402/ejpt.v3i0.17419
- Schalinski, I., Elbert, T. R., & Schauer, M. (2013). Cardiac defense in response to imminent threat in women with multiple trauma and severe PTSD. Psychophysiology, 50(7), 691–700. https://doi.org/10.1111/psyp.12051
- Schalinski, I., Schauer, M., & Elbert, T. (2015). The shutdown dissociation scale (shut-d). European Journal of Psychotraumatology, 6(1), 1–13. https://doi.org/10.3402/ejpt.v6.25652
- Schauer, M., & Elbert, T. (2010). Dissociation following traumatic stress. Zeitschrift für Psychologie/Journal of Psychology, 218(2), 109–127. https://doi.org/10.1027/0044-3409/a000018
- Schäflein, E., Sattel, H., Schmidt, U., & Sack, M. (2018). The enemy in the mirror: Self-perception-induced stress results in dissociation of psychological and physiological responses in patients with dissociative disorder. European Journal of Psychotraumatology, 9(sup3), 1–16. https://doi.org/10.1080/20008198.2018.1472991
- Schmahl, C. G., Elzinga, B. M., Ebner, U. W., Simms, T., Sanislow, C., Vermetten, E., McGlashan, T. H., & Douglas Bremner, J. (2004). Psychophysiological reactivity to traumatic and abandonment scripts in borderline personality and posttraumatic stress disorders: A preliminary report. Psychiatry Research, 126(1), 33–42. https://doi.org/10.1016/j.psychres.2004.01.005
- Schneider, M., & Schwerdtfeger, A. (2020, Sep). Autonomic dysfunction in posttraumatic stress disorder indexed by heart rate variability: A meta-analysis. Psychological Medicine, 50(12), 1937–1948. https://doi.org/10.1017/S003329172000207X
- Seligowski, A. V., Lebois, L. A. M., Hill, S. B., Kahhale, I., Wolff, J. D., Jovanovic, T., Winternitz, S. R., Kaufman, M. L., & Ressler, K. J. (2019). Autonomic responses to fear conditioning among women with PTSD and dissociation. Depression and Anxiety, 36(7), 625–634. https://doi.org/10.1002/da.22903
- Siegel, D. J. (1999). The developing mind: Toward a neurobiology of interpersonal experience. Guilford Press.
- Simeon, D., Yehuda, R., Knutelska, M., & Schmeidler, J. (2008). Dissociation versus posttraumatic stress: Cortisol and physiological correlates in adults highly exposed to the World Trade Center attack on 9/11. Psychiatry Research, 161(3), 325–329. https://doi.org/10.1016/j.psychres.2008.04.021
- Sledjeski, E. M., & Delahanty, D. L. (2012). Prior peritraumatic dissociative experiences affect autonomic reactivity during trauma recall. Journal of Trauma & Dissociation, 13(1), 32–50. https://doi.org/10.1080/15299732.2011.608628
- Spiegel, D., Loewenstein, R. J., Lewis-Fernandez, R., Sar, V., Simeon, D., Vermetten, E., Cardena, E., Brown, R. J., & Dell, P. F. (2011, Dec 21). Dissociative disorders in DSM-5. Depression and Anxiety, 28(12), E17–E45. https://doi.org/10.1002/da.20923
- Stein, D. J., Koenen, K. C., Friedman, M. J., Hill, E., McLaughlin, K. A., Petukhova, M., Ruscio, A. M., Shahly, V., Spiegel, D., Borges, G., Bunting, B., Caldas-de-Almeida, J. M., de Girolamo, G., Demyttenaere, K., Florescu, S., Haro, J. M., Karam, E. G., Kovess-Masfety, V., Lee, S., … Kessler, R. C. (2013). Dissociation in posttraumatic stress disorder: Evidence from the world mental health surveys. Biological Psychiatry, 73(4), 302–312. https://doi.org/10.1016/j.biopsych.2012.08.022
- Stewart, L. A., Clarke, M., Rovers, M., Riley, R. D., Simmonds, M., Stewart, G., Tierney, J. F., & Group, P.-I. D. (2015, Apr 28). Preferred reporting items for systematic review and meta-analyses of individual participant data: The PRISMA-IPD statement. JAMA, 313(16), 1657–1665. https://doi.org/10.1001/jama.2015.3656
- Stiglmayr, C., Braakmann, D., Haaf, B., Stieglitz, R. D., & Bohus, M. (2003, Jul). Development and characteristics of dissociation-tension-scale acute (DSS-Akute). PPmP - Psychotherapie · Psychosomatik · Medizinische Psychologie, 53(7), 287–294. https://doi.org/10.1055/s-2003-40495 (Entwicklung und psychometrische Charakteristika der Dissoziations-Spannungs-Skala akut (DSS-akut).)
- Stiglmayr, C., Schimke, P., Wagner, T., Braakmann, D., Schweiger, U., Sipos, V., Fydrich, T., Schmahl, C., Ebner-Priemer, U., Kleindienst, N., Bischkopf, J., Auckenthaler, A., & Kienast, T. (2010, May). Development and psychometric characteristics of the dissociation tension scale. Journal of Personality Assessment, 92(3), 269–277. https://doi.org/10.1080/00223891003670232
- Stiglmayr, C., Schmahl, C., Bremner, J. D., Bohus, M., & Ebner-Priemer, U. (2009). Development and psychometric characteristics of the DSS-4 as a short instrument to assess dissociative experience during neuropsychological experiments. Psychopathology, 42(6), 370–374. https://doi.org/10.1159/000236908
- Strigo, I. A., Simmons, A. N., Matthews, S. C., Grimes, E. M., Allard, C. B., Reinhardt, L. E., Paulus, M. P., & Stein, M. B. (2010). Neural correlates of altered pain response in women with posttraumatic stress disorder from intimate partner violence. Biological Psychiatry, 68(5), 442–450. https://doi.org/10.1016/j.biopsych.2010.03.034
- Terpou, B. A., Harricharan, S., McKinnon, M. C., Frewen, P., Jetly, R., & Lanius, R. A. (2019). The effects of trauma on brain and body: A unifying role for the midbrain periaqueductal gray. Journal of Neuroscience Research, 97(9), 1110–1140. https://doi.org/10.1002/jnr.24447
- Thornton, A., & Lee, P. (2000). Publication bias in meta-analysis its causes and consequences. Journal of Clinical Epidemiology, 53(2), 207–216. https://doi.org/10.1016/s0895-4356(99)00161-4
- van der Kolk, B. A. (1988). The trauma spectrum: The interaction of biological and social events in the genesis of the trauma response. Journal of Traumatic Stress, 1(3), 273–290. https://doi.org/10.1002/jts.2490010302
- Vermes, J. S., Ayres, R., Goes, A. S., Real, N. D., Araujo, A. C., Schiller, D., Neto, F. L., & Corchs, F. (2020, Nov 1). Targeting the reconsolidation of traumatic memories with a brief 2-session imaginal exposure intervention in post-traumatic stress disorder. Journal of Affective Disorders, 276, 487–494. https://doi.org/10.1016/j.jad.2020.06.052
- Volchan, E., Rocha-Rego, V., Bastos, A. F., Oliveira, J. M., Franklin, C., Gleiser, S., Berger, W., Souza, G. G. L., Oliveira, L., David, I. A., Erthal, F. S., Pereira, M. G., & Figueira, I. (2017). Immobility reactions under threat: A contribution to human defensive cascade and PTSD. Neuroscience & Biobehavioral Reviews, 76(Pt A), 29–38. https://doi.org/10.1016/j.neubiorev.2017.01.025
- Volchan, E., Souza, G. G., Franklin, C. M., Norte, C. E., Rocha-Rego, V., Oliveira, J. M., David, I. A., Mendlowicz, M. V., Coutinho, E. S., Fiszman, A., Berger, W., Marques-Portella, C., & Figueira, I. (2011). Is there tonic immobility in humans? Biological evidence from victims of traumatic stress. Biological Psychology, 88(1), 13–19. https://doi.org/10.1016/j.biopsycho.2011.06.002
- Vonderlin, R., Kleindienst, N., Alpers, G. W., Bohus, M., Lyssenko, L., & Schmahl, C. (2018). Dissociation in victims of childhood abuse or neglect: A meta-analytic review. Psychological Medicine, 48(15), 2467–2476. https://doi.org/10.1017/S0033291718000740