ABSTRACT
Background: Exposure to adversity in utero is thought to increase susceptibility to develop posttraumatic stress disorder (PTSD) following later life trauma, due to neurobiological programming effects during critical developmental periods. It remains unknown whether effects of prenatal adversity on PTSD susceptibility are modulated by genetic variations in neurobiological pathways implicated in PTSD susceptibility.
Objective: We investigated whether genetic variation in the glucocorticoid receptor (GR) modulated effects of prenatal famine exposure on late adulthood PTSD symptom severity after trauma exposure in childhood and mid-to-late adulthood.
Method: We included N = 439 term-born singleton adults (mean age: 72 years, 54.2% women) from the Dutch Famine Birth Cohort, born around the time of the Dutch Famine of 1944/1945, divided into exposure and control groups based on timing of the famine during gestation. Participants filled out self-report questionnaires on childhood (Childhood Trauma Questionnaire) and mid-to-late adulthood (Life Events Checklist for DSM-5) trauma, and current PTSD symptom severity (PTSD Checklist for DSM-5). GR haplotypes were determined from four functional GR single nucleotide polymorphisms (ER22/23EK, N363S, BclI and exon 9β) in previously collected DNA. Linear regression analyses were performed to investigate associations of GR haplotype and prenatal famine exposure in conjunction with later life trauma on PTSD symptom severity.
Results: We observed a significant three-way interaction between the GR Bcll haplotype, famine exposure during early gestation, and adulthood trauma exposure on PTSD symptom severity in late adulthood. Only participants exposed to famine during early gestation without the GR Bcll haplotype showed a significantly stronger positive association between adulthood trauma and PTSD symptom severity than non-exposed participants, indicating increased PTSD susceptibility.
Conclusions: Our results illustrate the importance of integrated approaches considering genetics and environmental contexts throughout various life periods, including the rarely investigated prenatal environment, to elucidate how PTSD susceptibility evolves throughout life.
HIGHLIGHTS
Adversity during pregnancy is thought to increase offspring’s PTSD risk following later life trauma, but exact neurobiological mechanisms underlying this process remain unknown.
We found that effects of prenatal famine exposure on PTSD symptom severity were influenced by genetic variation in the glucocorticoid receptor, which signals effects of the stress hormone cortisol.
Integrated approaches considering genetics and environmental contexts throughout both early and later life are important to understand how PTSD risk evolves throughout life.
Antecedentes: Se cree que la exposición a la adversidad in útero aumenta la susceptibilidad a desarrollar Trastorno de Estrés Postraumático (TEPT) después de experimentar un trauma en etapas tardías de la vida, ello debido a los efectos de la programación neurobiológica durante los períodos críticos del desarrollo. Continúa siendo desconocido si los efectos de la adversidad prenatal en la susceptibilidad al TEPT están modulados por variaciones genéticas en las vías neurobiológicas implicadas en la susceptibilidad al TEPT.
Objetivo: Investigamos si la variación genética en el receptor de glucocorticoides (GR, por sus siglas en inglés) moduló los efectos de la exposición prenatal a la hambruna en la severidad de los síntomas del TEPT en la adultez tardía después de la exposición al trauma en la infancia y en la edad adulta media o tardía.
Método: Incluimos adultos nacidos como hijos únicos nacidos a término, N = 439 adultos (edad media: 72 años, 54,2 % mujeres), de la Cohorte de nacimientos de la hambruna holandesa durante los años de 1944/1945, divididos en grupos de exposición y de control, basados en el tiempo de gestación ocurrido durante la temporada de hambruna. Los participantes completaron cuestionarios de auto-reporte sobre el trauma durante la infancia (Cuestionario de Trauma Infantil), la adultez media a tardía (Lista de verificación de eventos de vida para el DSM-5), y la gravedad actual de los síntomas del TEPT (Lista de verificación del TEPT para el DSM-5). Los haplotipos del GR se determinaron a partir de cuatro polimorfismos funcionales de un solo nucleótido del GR (ER22/23EK, N363S, BclI y exón 9β) en ADN recolectado previamente. Se realizaron análisis de regresión lineal para investigar las asociaciones del haplotipo GR y la exposición prenatal a la hambruna en conjunto con el trauma en etapas más tardías de la vida en la gravedad de los síntomas del TEPT.
Resultados: Observamos una interacción significativa de tres vías entre el haplotipo GR Bcll, la exposición a la hambruna durante la gestación temprana y la exposición al trauma en la adultez sobre la gravedad de los síntomas del TEPT en la adultez tardía. Solo los participantes expuestos a la hambruna durante la gestación temprana sin el haplotipo GR Bcll mostraron una asociación positiva significativamente más fuerte entre el trauma durante la adultez y la gravedad de los síntomas del TEPT en comparación con los participantes no expuestos, lo que indica una mayor susceptibilidad al TEPT.
Conclusiones: Nuestros resultados ilustran la importancia de contar con enfoques integrados que consideran la genética y los contextos ambientales a lo largo de varios períodos de la vida, incluido el entorno prenatal, el cual es rara vez investigado, para dilucidar cómo evoluciona la susceptibilidad al TEPT a lo largo de la vida.
背景:由于关键发育时期的神经生物学编程效应,在子宫内逆境暴露被认为会增加晚年生活创伤后发生创伤后应激障碍 (PTSD) 的易感性。目前尚不清楚产前逆境对 PTSD 易感性的影响是否受到PTSD 易感性相关神经生物学通路遗传变异的调节。
目的:我们考查了糖皮质激素受体 (GR) 遗传变异是否调节了产前饥荒暴露对童年期和成年中晚期创伤暴露后成年晚期 PTSD 症状严重程度的影响。
方法:我们纳入了荷兰饥荒出生队列足月出生的 439 名单生子成年人(平均年龄:72 岁,54.2% 为女性),他们出生于 1944/1945 年荷兰饥荒前后,根据怀孕期间经历饥荒的时间分为暴露组和对照组。参与者填写了关于童年期创伤(童年创伤问卷)和成年中晚期创伤(DSM-5 的生活事件清单)以及当前 PTSD 症状严重程度(DSM-5 PTSD 清单)的自我报告问卷。GR 单倍型根据先前收集 DNA 中四种功能性 GR 单核苷酸多态性(ER22/23EK、N363S、BclI 和外显子 9β)确定。进行线性回归分析以考查 GR 单倍型和产前饥荒暴露与晚年生活创伤对 PTSD 症状严重程度的关联。
结果:我们观察到 GR Bcll 单倍型、妊娠早期饥荒暴露和成年创伤暴露对成年后期 PTSD 症状严重程度的显著三重交互作用。 只有无 GR Bcll 单倍型的情况下,妊娠早期饥荒暴露参与者的成年期创伤和 PTSD 症状严重程度之间的正相关性才表现出比无暴露参与者显著更强,表明 PTSD 易感性增加。
结论:我们的结果说明了综合方法的重要性,该方法考虑了贯穿不同生命时期的基因和环境背景,包括很少研究的产前环境,以阐明 PTSD 易感性如何在整个生命过程中演变。
1. Introduction
Posttraumatic stress disorder (PTSD) is a common adverse consequence of traumatic events, with a worldwide conditional risk of 4% following traumatic events (Kessler et al., Citation2017). The fact that most trauma-exposed individuals do not develop PTSD indicates individual differences in susceptibility to PTSD development. Early life trauma is considered an important risk factor for PTSD development upon later trauma exposure during adulthood (Nishith et al., Citation2000; Schumm et al., Citation2006). This is thought to be influenced by permanent changes in neurobiological stress systems in response to early life trauma that promote survival of harsh circumstances in the near future, but thereby also result in increased susceptibility to (mental) health problems later in life (Boyce, Citation2016). The effects of early life adversity that occurs before birth, such as in utero exposure to undernutrition, maternal stress and maternal psychological problems, addictive substances or toxins, are potentially even larger because it coincides with critical periods of organogenesis and brain formation (Boyce, Citation2016). Because of the large and long lasting effects of prenatal adversity as well as its high prevalence throughout the world (Slopen et al., Citation2015), it is essential to investigate the extent of its adverse consequences, also in the context of PTSD.
Prenatal adversity is associated with increased risk for a broad range of negative long-term consequences in the offspring, including physical and mental health problems (Lumey et al., Citation2011; Van den Bergh et al., Citation2020). Prenatal adversity has been associated with long-term changes in glucocorticoid (GC) levels and in the signalling of glucocorticoid effects throughout the body (Harris & Seckl, Citation2011; Reynolds et al., Citation2013; Seckl & Meaney, Citation2006). Glucocorticoids (cortisol in humans) are the end product of the hypothalamic–pituitary–adrenal (HPA) axis, which is activated upon (traumatic) stress, and play a pivotal role in stress reactivity and recovery. Increasing evidence from prospective studies in trauma-exposed adults indicates that individual variability in GC signalling during and acutely following trauma is associated with PTSD susceptibility. Such associations have been observed for circulating cortisol levels, but also for methylation status, gene and protein expression for various molecular markers more downstream in the GC signalling pathway (Carmi et al., Citation2023; Steudte-Schmiedgen et al., Citation2016; Van Zuiden et al., Citation2011; Van Zuiden et al., Citation2012). However, prenatal adversity itself has only been scarcely studied in relation to PTSD susceptibility. One previous study showed that self-reported major symptoms of prenatal maternal genital infection were associated with increased risk for PTSD in early adulthood, and these effects were more apparent when only analysing trauma-exposed individuals (Betts et al., Citation2015). This suggests that prenatal adversity increases PTSD susceptibility after trauma exposure in later life. In the Dutch famine birth cohort, also investigated in the current study, we recently observed that men exposed to famine during early gestation more commonly reported a broad range of mild psychological symptoms (including PTSD, psychotic, depression, and anxiety symptoms) in late adulthood compared to unexposed men (Hilberdink et al., Citation2022). It was, however, not investigated whether prenatal exposure to famine influenced the effect of trauma exposure in later life on PTSD susceptibility.
Accumulating evidence indicates that differences in susceptibility to adverse mental health outcomes after childhood adversity are dependent on genotypic variation. For example, it has been observed that the effects of childhood adversity on trauma-induced mental health problems throughout adulthood are moderated by single nucleotide polymorphisms (SNPs) in genes regulating GC signalling throughout the body (Binder et al., Citation2008). The effects of GCs are signalled via two types of receptors, initiating subsequent genomic and non-genomic stress responses. Of these two receptor types, the glucocorticoid receptor (GR) is primarily activated under stressful conditions when GC levels are high (De Kloet, Citation2005; Oakley & Cidlowski, Citation2013). Upon stress-induced cortisol release, a negative GC feedback loop via GRs in the hypothalamus and anterior pituitary eventually results in HPA axis inhibition and thereby recovery from stress (Oakley & Cidlowski, Citation2013). Several common functional SNPs in the GR gene NR3C1 have been identified, which directly influence GR function in the direction of either hyposensitivity (i.e. SNPs GR9β (Kumsta et al., Citation2009) and ER22/23 EK (Manenschijn et al., Citation2009; Quax et al., Citation2013)) or hypersensitivity (i.e. SNPs BclI (DeRijk, Citation2009; Van Rossum et al., Citation2003) and N363S (Manenschijn et al., Citation2009)) to the effects of GCs. Several studies have observed that these SNPs are associated with PTSD susceptibility following childhood and adulthood trauma, with repeated but not fully consistent findings of increased PTSD susceptibility in carriers of the BclI SNP (Castro-Vale et al., Citation2021; Hauer et al., Citation2011; Lian et al., Citation2014). This fits with findings that pre-trauma high peripheral GR numbers and higher signalling of GC effects by GRs predicted increased susceptibility to develop PTSD symptoms following trauma exposure in adulthood (Van Zuiden et al., Citation2011; Van Zuiden et al., Citation2012; van Zuiden et al., Citation2012). Moreover, pre-trauma GR numbers were increased in adult carriers of a BclI SNP haplotype who were previously exposed to childhood trauma compared to non-carriers or non-childhood trauma exposed participants (Van Zuiden et al., Citation2012). As of yet, it remains unknown whether the effects of prenatal adversity on PTSD susceptibility are also conditionally dependent on genotypic variation in GR function.
In the current study we investigated whether associations between childhood trauma or mid-to-late adulthood trauma and PTSD symptom severity in late adulthood were moderated by prenatal exposure to famine. Furthermore, we investigated whether variation in the GR gene moderated the association between prenatal exposure to famine, childhood or adulthood trauma and PTSD symptom severity in late adulthood. We investigated these associations in members of the Dutch famine birth cohort, a historical birth cohort of individuals born around the time of the Dutch famine of 1944-1945, who have been followed over the past decades with the aim to investigate long-term effects of prenatal famine exposure on adult health (Bleker et al., Citation2021).
2. Methods
2.1. Participants and procedures
Participants were members of the Dutch famine birth cohort. This cohort consists of N = 2414 individuals who were born alive as term singletons in the Wilhelmina Gasthuis (WG) hospital in Amsterdam between 1 November 1943 and 28 February 1947. The selection procedures and follow-up of the cohort have been described in detail elsewhere. Six waves of data collection occurred (Bleker et al., Citation2021). In the current study, we used DNA collected from fasting blood samples in wave II (2002-2004), approximately 60 years after the famine. We additionally used questionnaire data collected in wave V (2018-2019), approximately 75 years after the famine. During wave V, eligible cohort members (N = 1207) were invited by mail to participate in a paper-and-pencil survey, which also included questionnaires on childhood trauma, adulthood trauma and PTSD symptom severity further described below. Additionally, participants provided demographic information including marital status and highest completed education. Eventually N = 595 (49.3%) cohort members provided written informed consent to participate (Hilberdink et al., Citation2022). In the current study, we ultimately included N = 439 (73.8%) participants, for whom DNA samples and questionnaire data on PTSD symptom severity were available. The Medical Ethics Committee of the Academic Medical Centre, Amsterdam, the Netherlands, approved the study for wave II and concluded that a full review and official approval for wave V was not required according to Dutch law for medical research. The study was carried out in accordance with the Declaration of Helsinki. Participants provided written informed consent for each data collection wave.
2.2. Measures
2.2.1. Exposure to famine
Exposure to famine during gestation was defined as an average maternal daily calorie intake of less than 1000 calories during any 13-week period of gestation, as based on the official daily rations of the general population (Ravelli et al., Citation1998). As such, children born in Amsterdam between 7 January 1945 and 8 December 1945, were considered to be exposed to famine during prenatal life. In line with all previous publications on the cohort, three 16-week periods were distinguished; children who were mainly exposed during late gestation (born between 7 January and 28 April 1945), mid gestation (born between 29 April and 18 August 1945) or early gestation (born between 19 August and 8 December 1945). A sample of the individuals born within 1 year before the famine or conceived up to 1 year after the end of the famine were considered unexposed to famine and were eligible as controls for comparisons (born before the famine between 1 November 1943 and 7 January 1945 or conceived after the famine and born between 9 December 1945 and 28 February 1947).
2.2.2. Childhood trauma
Childhood trauma (CT) was assessed with the short form of the Dutch Childhood Trauma Questionnaire (CTQ-SF) (Bernstein et al., Citation2003; Van Schie et al., Citation2017) This self-report questionnaire assesses traumatic maltreatment in childhood across five dimensions: physical, sexual and emotional abuse as well as physical and emotional neglect. It additionally contains the Minimisation-Denial (MD) subscale which assesses reporter bias (Thombs et al., Citation2009). Participants rated each item on a scale ranging from 1-5, ‘Never True’ to ‘Very Often True’. Two items from physical and five from emotional neglect were reverse coded before total and subscale scores were calculated. Due to translation inconsistencies, one item of the original questionnaire relating to sexual molestation was removed from the Dutch CTQ, resulting in a total of 27 items (4 for sexual abuse, 3 for MD and 5 for all other subscales) (Van Schie et al., Citation2017). The total CTQ score (range 24-120) was determined by summing all item scores, except for the items of the MD subscale. Higher total CTQ scores indicated more severe exposure to trauma during childhood. The MD subscale score was obtained by recoding the scores on the 3 corresponding CTQ items (1–4 = 0 and 5 = 1), resulting in a value ranging from 0-3, with higher scores indicating higher probability of under-reporting CT.
2.2.3. Adulthood trauma
Exposure to potential traumatic events (PTEs) in mid-to-late adulthood was assessed with a self-report version of the Dutch Life Events Checklist (LEC-5) for the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) (Boeschoten et al., Citation2018). This questionnaire enquires about 17 different PTEs that could be either directly experienced, witnessed, encountered during work or happened to a close friend or family member. Participants were specifically asked about events in the past 15 years, since the completion of wave IV. A total score (range 0-17) was determined by summing the types of PTEs endorsed. Participants with more than 2 missing items were removed from the analyses (n = 5).
2.2.4. PTSD symptom severity
The Dutch version of the PTSD checklist for the DSM-5 (PCL-5) was used to assess PTSD symptom severity in the past month (Bovin et al., Citation2016; Van Praag et al., Citation2020). This self-report questionnaire consists of 20 items, each measuring the severity of one of the DSM-5 symptom clusters of PTSD (intrusions, avoidance, negative alterations in cognition and mood, and alterations in arousal and reactivity) on a scale from 0-4, ranging from ‘Not at all’ to ‘Extremely’. A total score (range 0-80) was determined by adding up all item scores, with higher scores indicating higher overall PTSD symptom severity. Probable PTSD diagnostic status was also determined, using a total score cut-off of 31 (Van Praag et al., Citation2020). In case of one missing item, this missing data was imputed with the participant’s grand mean (n = 6). Participants with more than one missing item were removed from the analyses (n = 11).
2.3. Genotyping and haplotype identification
Venipunctures were performed after overnight fasting. Subsequently, genomic DNA material was extracted from the blood samples using standard procedures and stored at −80◦C until genotyping (De Rooij et al., Citation2006). Genotyping of four GR SNPs (ER22/23EK (rs6189/rs6190), N363S (rs6195), BclI (rs41423247) and exon 9β (rs6198)) was conducted with TaqMan allelic discrimination assays. Applied Biosystems (Foster City, CA) designed and optimised these assays. Two ng of genomic DNA was used in the assay, which was performed on the Taqman Prism 7900HT platform according to the manufacturer’s instructions. Cluster separation was first tested by running assays on 90 non-study related blood bank samples. For analyses of the study samples, batch assignment was randomised and laboratory staff was blinded for prenatal exposure. Independent end-point readings from this platform were used to analyse genotyping results, which were replicated within a randomly selected 5% of samples (Fang et al., Citation2005).
We investigated the effects of GR haplotypes instead of individual SNPs, which increases the power to detect associations between genetic variants and complex diseases (Morris & Kaplan, Citation2002), such as PTSD. Haplotypes are sets of polymorphisms that are inherited together and thus capture the combined effects of the present SNPs within a specific gene within an individual. The R-package Haplo.stats 1.8.7 (Sinnwell et al., Citation2022) was used to determine the haplotypes present in the current study sample. This package uses an expectation-maximization algorithm to assign each participant to a haplotype pair and to obtain maximum likelihood estimations of haplotype frequencies across both alleles per participant. For N = 30 participants (6.8%), information on at least one SNP was missing, resulting in posterior haplotype probabilities <1 for these participants. As subsequent sensitivity analyses without these N = 30 participants indicated robustness of our results, we decided to retain these participants in the analyses. Haploview (Barrett et al., Citation2005) was used to test potential deviation from Hardy-Weinberg equilibrium (HWE) to avoid drawing false associations between genotype and phenotype.
2.4. Statistical analyses
All analyses were performed in R (v4.0.5) (R core team, 2021). Potential differences between participants in the four exposure groups (unexposed, exposed in early, mid or late gestation) were tested using Kruskal–Wallis tests for continuous variables and Fisher’s exact tests for categorical variables. PCL-5 total scores and CTQ total scores were log-transformed due to heteroscedasticity. We performed linear regression analyses to investigate the associations of GR haplotype and prenatal famine in conjunction with later life trauma on PTSD symptom severity (PCL-5 total score). Each model included three main effects for prenatal famine (dichotomous dummy variables for early, mid and late exposure groups resulting in the unexposed control group as the reference), a main effect for GR SNP haplotype carrier status (dichotomous dummy variable, coded as carriers (homozygous (two copies of the investigated haplotype present within a participants’ haplotype pair) or heterozygous (one copy present within the haplotype pair)) versus non-carriers (no copies present within the haplotype pair)) and a main effect for later life trauma (containing either childhood trauma: continuous CTQ total score; or adulthood trauma: continuous LEC total score). Additionally, the regression analyses included 2-way interactions for prenatal famine*later life trauma; prenatal famine*haplotype; and later life trauma*haplotype; and a three-way interaction for prenatal famine*later life trauma*haplotype. Separate models were applied for each haplotype as well as for childhood and adulthood trauma. Sex and marital status were included as covariates in all models. Marital status was dummy coded with single or widower compared to long-term relationship as reference group, with N = 4 (0.9%) participants excluded due to missing data. MD scores were included as an additional covariate in the childhood trauma regression models to correct for potential minimisation of childhood trauma. Participants with missing data on either CTQ total score (n = 6, 1.4%), CTQ MD score (n = 4, 0.9%) or LEC-5 score (n = 5, 1.1%) were removed from the respective analyses in a pairwise manner (see and for specification of all effects included in the regression models).
As previous studies observed sex-specificity in both the effects of prenatal adversity (Betts et al., Citation2015; De Rooij et al., Citation2011) and genetic variation in GR (Lindholm et al., Citation2020; Sarubin et al., Citation2017) on psychological symptom severity in adulthood, we additionally explored sex-specificity of the observed effects by performing exploratory analyses for men and women separately for the models with significant haplotype interaction effects. For each model, robust bootstrapped linear regression models with bias corrected and accelerated (BCA) confidence intervals, based on 5000 samples were used. A Bonferroni-corrected confidence interval of 97.5% was used to correct for multiple comparisons with regard to later life trauma type (adulthood and childhood trauma) in the primary analyses. This confidence interval was also used in the exploratory analyses. As the haplotypes consisted of SNPs within the same gene and are therefore not independent, no multiple testing correction was applied to account for the separate haplotype models (Castro-Vale et al., Citation2021).
3. Results
3.1. Participant characteristics
All participants were in their late adulthood (age range: 71–74 years) when filling out the questionnaires on trauma exposure and PTSD symptom severity. No significant differences in sex, educational level, marital status, nor in reported childhood and adulthood trauma exposure were observed between participants exposed to famine in early, mid or late gestation and non-exposed control groups. Furthermore, there were no significant group differences in PTSD symptom severity or probable PTSD prevalence ().
Table 1. Characteristics of participant groups according to timing of exposure to prenatal famine.
3.2. Identified GR haplotypes
All SNPs conformed to HWE (all p-values > 0.6). Five haplotypes were found, with the most frequent haplotype (41.9%) consisting of the common major alleles of the four SNPs as reported in the general population (). Other identified haplotypes were: the BclI haplotype, containing the minor allele of the BclI SNP and the major alleles for all other three SNPs (frequency: 35.9%); the exon 9β haplotype, containing the minor allele of the exon 9β SNP and the major alleles for all other three SNPs (frequency: 15.2%), the N363S haplotype, containing the minor allele of the N363S SNP and the major alleles for all other three SNPs (frequency: 3.8%), and finally the ER22/23K + exon 9β haplotype, containing the minor alleles for these two SNPs and the major alleles for the two remaining SNPs (frequency: 3.2%).
Figure 1. Overview of the five identified glucocorticoid receptor haplotypes based on the four investigated common single nucleotide polymorphisms (SNPs) within this gene, and their respective frequencies within the cohort (based on two estimated haplotypes per participant).
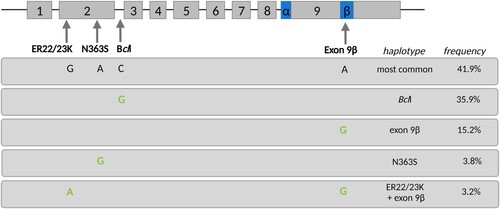
Haplotype frequencies for these five haplotypes did not differ significantly between those exposed to famine in early, mid or late gestation and those unexposed (Fisher’s exact test, p = .873, ). Additionally, across exposure groups, haplotype frequencies did not significantly differ between those with and without probable PTSD (Fisher’s exact test, p = .942, ).
Table 2. Frequencies of haplotype carriers within participant groups according to occurrence and timing of exposure to prenatal famine and according to probable PTSD diagnostic status.
Subsequent analyses on the associations between haplotype carrier status, famine, later life trauma and PTSD symptom severity could not be conducted for the two least frequent haplotypes, N363S and ER22/23K + exon 9β, as the number of participants who were haplotype carriers within the exposure groups was too small to yield reliable results (). Thus, we performed linear regression models for the most common haplotype, the BclI and the exon 9β haplotypes only.
3.3. Effects of GR haplotype, prenatal famine and childhood trauma on PTSD symptom severity
We investigated the effects of haplotype carrier status, prenatal famine exposure and childhood trauma, and their interactions on PTSD symptom severity for each of the 3 haplotypes (most common haplotype; Bcll; and exon 9β) separately (see ). In the model regarding the BclI haplotype, we found a significant main effect of CTQ total score on PCL-5 total score (B = 1.21, SE = 0.32, 97.5% CI [0.49–1.93]). A similar main effect for CTQ total score was found in the model regarding the exon 9β haplotype (B = 1.18, SE = 0.25, 97.5% CI [0.62–1.73]). We did not find a significant main effect of CTQ total score in the model regarding the most common haplotype. No significant main effects of prenatal famine exposure or haplotype carrier status, nor any significant interaction effects were observed within any of the haplotype models.
Table 3. Results of linear regression analyses including the effects of GR haplotype carrier status, prenatal famine exposure and childhood trauma on PTSD symptom severity in late adulthood, with separate models for the most common haplotype, the BclI haplotype and the Exon 9β haplotype.
3.4. Effects of GR haplotype, prenatal famine and adulthood trauma on PTSD symptom severity
We investigated the effects of haplotype carrier status, prenatal famine exposure and adulthood trauma, and their interactions on PTSD symptom severity for each of the 3 haplotypes (most common haplotype; Bcll; and exon 9β) separately (see ). For the model regarding the BclI haplotype, we found a significant three-way interaction between LEC total score, exposure to famine in early gestation and BclI haplotype carrier status (B = −1.01, SE = 0.28, 97.5% CI [−1.77 – −0.18]; ), a significant two-way interaction between LEC total score and exposure to famine in early gestation (B = 0.58, SE = 0.20, 97.5% CI [0.17–1.01]), and an additional significant two-way interaction between BclI haplotype carrier status and exposure to famine in early gestation (B = 1.48, SE = 0.51, 97.5% CI [0.05–2.72]). We also found a significant main effect of LEC total score in all haplotype models (most common haplotype; B = 0.22, SE = 0.08, 97.5% CI [0.02–0.44], BclI; B = 0.16, SE = 0.07, 97.5% CI [0.01–0.30] and exon 9β; B = 0.19, SE = 0.06, 97.5% CI [0.06–0.33]). The association between LEC score, i.e. the amount of adulthood trauma, and PCL-5 total score, i.e. PTSD symptom severity, was positive for participants exposed to famine in early gestation without the BclI haplotype, just as it was for the other exposure and control groups irrespective of their haplotype carrier status (, ). However, this positive association was more pronounced in participants exposed to famine in early gestation than in participants who were both non-carrier of the BclI haplotype and not exposed to famine during gestation (, left panel). In contrast, participants carrying the BclI haplotype and exposed to famine in early gestation showed a negative association between LEC total score and PCL-5 total score (, right panel). Regarding the most common and exon 9β haplotype models, there were no significant main or interaction effects of haplotype carrier status nor of prenatal famine exposure on PTSD symptom severity.
Figure 2. Visualisation of PTSD symptom severity scores as a function of BclI haplotype carrier status, prenatal famine exposure and adulthood trauma. The association between the number of trauma types experienced during adulthood (LEC total score) and PTSD symptom severity (log-transformed PCL-5 total scores) is depicted for each prenatal exposure group and controls separately, divided into non-carriers (left panel) and BclI haplotype carriers (homozygous or heterozygous, right panel).
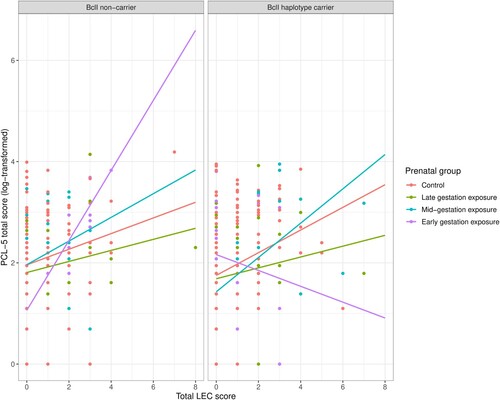
Table 4. Results of linear regression analyses including the effects of GR haplotype carrier status, prenatal famine exposure and adulthood trauma on PTSD symptom severity in late adulthood, with separate models for the most common haplotype, the BclI haplotype and the Exon 9β haplotype.
3.5. Potential sex-specific effects
Exploratory analyses were performed with separate models for men and women to investigate potential sex-specific effects for the model containing the significant three-way interaction between BclI haplotype carrier status, prenatal exposure in early gestation, and adulthood trauma on PTSD symptom severity. In both the models for men and women, the directionality of the effects was the same as in the model including men and women combined. Although the 97.5% confidence intervals overlapped between men and women, the three-way interaction between Bcll haplotype carrier status, exposure in early gestation, and LEC total score was only significant in men (B = −1.44, SE = 0.44, CI [−2.60 – −0.01]) and not in women (B = −0.64, SE = 0.41, CI [−1.92–0.39]; ). None of the other effects concerning haplotype carrier status, prenatal famine exposure, and LEC total scores were significant.
4. Discussion
In the current study we investigated whether prenatal famine exposure and genetic variation in the GR moderated the associations between childhood trauma or mid-to-late adulthood trauma and PTSD symptom severity in late adulthood. We observed that famine exposure in early gestation and the GR BclI haplotype together moderated the association between adulthood trauma and PTSD symptom severity, but not the association between childhood trauma and PTSD symptom severity. Participants exposed to famine during early gestation, but only those not carrying the GR Bcll haplotype, showed a significantly stronger association between adulthood trauma and PTSD symptom severity compared to non-exposed controls, indicating increased PTSD susceptibility following later life trauma. This increased susceptibility was not observed for participants who were exposed to famine during mid or late gestation. It was previously observed that prenatal exposure to maternal major infection increased PTSD susceptibility upon lifetime trauma exposure in 21 year old adults (Betts et al., Citation2015). Just as for this previous study, our exploratory analyses tentatively indicate that the observed effects were more pronounced in men exposed to prenatal adversity than in women exposed to prenatal adversity. Our findings also extend the previous findings on the impact of prenatal adversity on PTSD susceptibility in three ways. First, we investigated potential differential effects based on gestational timing of exposure within pregnancy, and observed that the effects were specific to exposure during early gestation. Secondly, our findings indicate that the influence of prenatal adversity on PTSD susceptibility following later life trauma extends well beyond the timeframe of early adulthood in the previous study by Betts et al. (Citation2015), as we inquired on adulthood trauma exposure occurring within mid-to-late adulthood. Thirdly, we observed that the effect of prenatal adversity on PTSD susceptibility after later life trauma was dependent on common genetic variation. Significant moderation effects were observed for the GR Bcll haplotype, containing the minor allele of the functional BclI SNP, a C to G nucleotide substitution, 646 base pairs downstream from exon 2 on the NR3C1 gene, associated with GR hypersensitivity to GCs (Van Rossum et al., Citation2003). Several previous studies in adults demonstrated that the presence of the Bcll SNP was associated with increased PTSD symptom development following adulthood trauma (Hauer et al., Citation2011; Lian et al., Citation2014). Furthermore, both the Bcll SNP and haplotype have been associated with increased presence of GR-related phenotypes of PTSD susceptibility in adults (e.g. higher peripheral GR number, higher peri-stress cortisol levels, lower acute post-trauma cortisol), either as main effect or in interaction with childhood trauma (Bachmann et al., Citation2005; Hauer et al., Citation2011; Li-Tempel et al., Citation2016; Van Zuiden et al., Citation2012). However, our findings contrasted these previous findings, which indicated increased PTSD susceptibly in adulthood for Bcll carriers. Instead, we observed that participants exposed to famine during early gestation with the GR Bcll haplotype seemed to be protected from the adverse effects on PTSD susceptibility following adulthood trauma. Previously, it was observed that the GR Bcll SNP moderated the effect of prenatal maternal psychological symptoms measured during week 20 of pregnancy on emotional and behavioural problems in their three year old children in a similar manner as in our study: children of mothers with psychological problems during pregnancy carrying the G allele of the Bcll SNP had fewer emotional and behavioural problems than children of mothers with psychological problems during pregnancy without the SNP (Velders et al., Citation2012). Additionally, within the Dutch Famine Birth Cohort, previous studies have shown gene-early environment interactions suggesting that the direction of protective or harmful effects of various SNP alleles on physical health conditions turned around depending on prenatal famine exposure (Botden et al., Citation2012; De Rooij et al., Citation2006; van Hoek et al., Citation2009). For instance, minor alleles of two SIRT1 SNPs, which in the general population increase the risk for type 2 diabetes, were associated with a lower prevalence of diabetes in individuals who had been exposed to famine prenatally (Botden et al., Citation2012).
In the absence of reported mid-to-late adulthood trauma, participants exposed during early gestation with the Bcll haplotype had higher mean PTSD symptoms than those exposed during early gestation without the Bcll haplotype. Although we did not observe significant moderation effects of prenatal famine exposure nor of the Bcll haplotype on the association between childhood trauma and PTSD symptom severity, we cannot exclude that these higher PTSD symptoms resulted from exposure to traumatic events occurring between the time periods of childhood and mid-to-late adulthood investigated within this study. Additionally, we cannot exclude that our observed higher PTSD symptoms result from higher scores on those questionnaire items associated with other psychological problems, such as depression and anxiety, especially as the BclI SNP was previously found to be associated with higher state anxiety (Lindholm et al., Citation2020) and increased risk for depression (van Rossum et al., Citation2006) in adulthood.
Our observed three-way interaction between GR haplotype, prenatal famine exposure and adulthood trauma on PTSD symptom severity seems to fit with the previously proposed three-hit model of vulnerability and resilience for stress-related mental health disorders following early life adversity (Daskalakis et al., Citation2013). This model posits that the interaction between genetic predisposition (Hit 1) and early life environmental factors (Hit 2) gives rise to particular programmed neurobiological phenotypes. These programmed phenotypes may promote either vulnerability or resilience to psychological problems in later life depending on the circumstances in the environment during later life (Hit 3).
As our investigations are purely associative, the exact neurobiological mechanisms underlying our observed interactions between the GR Bcll haplotype, famine exposure during early gestation and adulthood trauma exposure cannot be inferred from the current study. Based on the existing literature however, we hypothesise that the Bcll haplotype may have influenced famine exposure-related in utero alterations in HPA axis function and GC signalling by GRs. The Dutch famine undoubtedly induced physical as well as psychological stress in the exposed pregnant women, likely resulting in extensive prolonged HPA axis activation and high GC levels. Placental 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) partially protects the foetus from high maternal GC levels. However this protection is not complete, and might not even be present yet, during the earliest phases of pregnancy when the placenta is still being formed. After the placenta starts to function, placental 11β-HSD2 expression increases, before it peaks in mid-pregnancy, after which it decreases again through late gestation (Howland et al., Citation2017). Thus, it is conceivable that during early pregnancy, famine exposure particularly resulted in high in utero GC levels compared to later gestation, resulting in compensatory alterations in the developing foetal HPA axis and central GRs modulated by functional GR SNPs. Such prenatal adversity-induced effects on HPA axis function and GC signalling by GRs in offspring were previously found to persist well into adulthood (Harris & Seckl, Citation2011; Howland et al., Citation2017; Reynolds et al., Citation2013; Seckl & Meaney, Citation2006), and may be further influenced by the presumed persistent functional effects of the carried GR haplotypes on GC signalling throughout life in general and during trauma exposure in particular.
These hypothesised effects may be (partially) mediated by epigenetic modifications, as were previously observed upon prenatal famine exposure (Tobi et al., Citation2015). Of relevance for the biological pathway investigated in this study, such modifications may also have occurred at the level of NR3C1 promotor methylation, which directly influences GR gene expression (Motavalli et al., Citation2021). A recent study observed that low NR3C1-1F promotor methylation level within 6 h post-trauma was associated with increased risk for PTSD diagnosis and higher symptom severity in adults at 13-months post-trauma (Carmi et al., Citation2023). Converging evidence indicates that prenatal adversity may alter GR promotor methylation (Chalfun et al., Citation2022), although it is not yet clear how stable these epigenetic changes are throughout life. While it remains unknown whether GR genotype directly moderates the impact of prenatal adversity on adulthood NR3C1 promotor methylation, it is known that methylation has a strong genetic component. Of specific relevance for our observed apparently protective effect of the Bcll haplotype in early exposed individuals upon adulthood trauma, specifically the Bcll SNP was previously found to be associated with methylation status in several promotor regions of NR3C1 in healthy adults, while other investigated common GR SNPs were not associated with methylation (Li-Tempel et al., Citation2016).
The Dutch famine birth cohort is unique in terms of its exposure to prenatal adversity confined to specific gestational periods. Together with the long-term follow up of individuals now more than seven decades following their prenatal exposure, this provides a rare opportunity to investigate the current research questions. However, the sample size was small, especially for the genetic analyses and even more so for the exploratory sex-specific analyses. Moreover, our participants were commonly of Western-European descend, with their birth mothers all residing in inner city Amsterdam around the time of the famine. Thus, the genetic structure of our participant sample is likely rather homogenous. Also the influence of shared environmental factors within our study sample cannot be fully excluded – even despite the observation of specific effects for the early gestation group.
Another limitation resulting from our small sample size was the combined analysis of homozygote and heterozygote haplotype carriers, as differences in associations between the investigated GR SNPs and susceptibility to develop mental health problems were previously observed between individuals with one versus two copies of the investigated haplotypes (Bachmann et al., Citation2005; Bet et al., Citation2009; Velders et al., Citation2012). Furthermore, we specifically investigated selected common SNPs with functional consequences within a probable neurobiological pathway underlying PTSD susceptibility. Although our study provides first evidence that such SNPs may interact with environmental factors throughout multiple periods in life to contribute to PTSD susceptibility, there are inherent limitations and pitfalls to using SNP based analyses to predict complex traits and disorders, such as PTSD (Wray et al., Citation2013). Additionally, given the small sample size we opted not to include additional covariates beyond age, sex, marital status, and probable underreporting of childhood trauma. Thus, potential confounding effects of co-morbid psychiatric symptoms as well as the post-gestational exposome (other than child and adulthood trauma) were not investigated. Therefore, the current results should be interpreted with caution and should ideally be validated in a larger and more heterogeneous population, although this will likely be difficult to achieve as populations with equal long-term follow-up as our study remain scarce.
Furthermore, childhood trauma and adulthood trauma were retrospectively assessed. Although our childhood trauma-related analyses were corrected for potential underreporting (Thombs et al., Citation2009), we cannot exclude that other types of reporting bias may have occurred, especially when considering the sample consisted of aging individuals. Furthermore, we only investigated linear (interaction) effects of childhood or adulthood trauma exposure on PTSD symptom severity, while the described three-hit model also poses potential non-linear dose-dependent effects of the amount of trauma exposure on susceptibility to (traumatic) stress-related mental health problems (Daskalakis et al., Citation2013).
In conclusion, we observed that the association between famine exposure in early gestation and adulthood PTSD symptom severity was only detected when considered in interaction with genetic variation in a potential neurobiological pathway underlying PTSD susceptibility upon adulthood trauma exposure. Although our results remain to be validated, they illustrate the necessity of an integrated approach that considers genetics and the environmental context throughout various life periods, including the rarely investigated prenatal environment, when aiming to elucidate how PTSD susceptibility evolves throughout life.
Supplemental Material
Download MS Word (18.8 KB)Acknowledgements
The authors thank all participants for their time and involvement, and are grateful for the Dutch famine birth cohort team for providing data. MvZ and SdR conceived and designed the current study. CEH performed data collection, supervised by MvZ and SdR. KDG performed data analysis and interpretation of data under supervision of MvZ. KDG and MvZ wrote the original draft of the manuscript, which was critically revised by all other authors for important intellectual content. All authors have read and approved the final manuscript.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, MVZ. The data are not publicly available as they contain information that could compromise participant privacy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bachmann, A. W., Sedgley, T. L., Jackson, R. V., Gibson, J. N., Young, R. M., & Torpy, D. J. (2005). Glucocorticoid receptor polymorphisms and post-traumatic stress disorder. Psychoneuroendocrinology, 30(3), 297–306. https://doi.org/10.1016/j.psyneuen.2004.08.006
- Barrett, J. C., Fry, B., Maller, J., & Daly, M. J. (2005). Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics (Oxford, England), 21(2), 263–265. https://doi.org/10.1093/bioinformatics/bth457
- Bernstein, D. P., Stein, J. A., Newcomb, M. D., Walker, E., Pogge, D., Ahluvalia, T., Stokes, J., Handelsman, L., Medrano, M., Desmond, D., & Zule, W. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27(2), 169–190. https://doi.org/10.1016/S0145-2134(02)00541-0
- Bet, P. M., Penninx, B. W., Bochdanovits, Z., Uitterlinden, A. G., Beekman, A. T., van Schoor, N. M., Deeg, D. J., & Hoogendijk, W. J. (2009). Glucocorticoid receptor gene polymorphisms and childhood adversity are associated with depression: New evidence for a gene–environment interaction. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 150B(5), 660–669. https://doi.org/10.1002/ajmg.b.30886
- Betts, K. S., Salom, C. L., Williams, G. M., Najman, J. M., & Alati, R. (2015). Associations between self-reported symptoms of prenatal maternal infection and post-traumatic stress disorder in offspring: Evidence from a prospective birth cohort study. Journal of Affective Disorders, 175, 241–247. https://doi.org/10.1016/j.jad.2015.01.011
- Binder, E. B., Bradley, R. G., Liu, W., Epstein, M. P., Deveau, T. C., Mercer, K. B., Tang, Y., Gillespie, C. F., Heim, C. M., Nemeroff, C. B., & Schwartz, A. C. (2008). Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama, 299(11), 1291–1305. https://doi.org/10.1001/jama.299.11.1291
- Bleker, L. S., de Rooij, S. R., Painter, R. C., Ravelli, A. C., & Roseboom, T. J. (2021). Cohort profile: The Dutch famine birth cohort (DFBC)—a prospective birth cohort study in The Netherlands. BMJ Open, 11(3), e042078. https://doi.org/10.1136/bmjopen-2020-042078
- Boeschoten, M. A., Van der Aa, N., Bakker, A., Ter Heide, F. J. J., Hoofwijk, M. C., Jongedijk, R. A., Van Minnen, A., Elzinga, B. M., & Olff, M. (2018). Development and evaluation of the Dutch clinician-administered PTSD scale for DSM-5 (CAPS-5). European Journal of Psychotraumatology, 9(1), 1546085. https://doi.org/10.1080/20008198.2018.1546085
- Botden, I. P., Zillikens, M. C., De Rooij, S. R., Langendonk, J. G., Danser, A. J., Sijbrands, E. J., & Roseboom, T. J. (2012). Variants in the SIRT1 gene may affect diabetes risk in interaction with prenatal exposure to famine. Diabetes Care, 35(2), 424–426. https://doi.org/10.2337/dc11-1203
- Bovin, M. J., Marx, B. P., Weathers, F. W., Gallagher, M. W., Rodriguez, P., Schnurr, P. P., & Keane, T. M. (2016). Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders–fifth edition (PCL-5) in veterans. Psychological Assessment, 28(11), 1379. https://doi.org/10.1037/pas0000254
- Boyce, W. T. (2016). Differential susceptibility of the developing brain to contextual adversity and stress. Neuropsychopharmacology, 41(1), 142–162. https://doi.org/10.1038/npp.2015.294
- Carmi, L., Zohar, J., Juven-Wetzler, A., Desarnaud, F., Makotkine, L., Bierer, L.M., Cohen, H. and Yehuda, R. (2023). Promoter methylation of the glucocorticoid receptor following trauma may be associated with subsequent development of PTSD. The World Journal of Biological Psychiatry, 1–9. https://doi.org/10.1080/15622975.2023.2177342
- Castro-Vale, I., Durães, C., van Rossum, E. F., Staufenbiel, S. M., Severo, M., Lemos, M. C., & Carvalho, D. (2021). The glucocorticoid receptor gene (NR3C1) 9β SNP is associated with posttraumatic stress disorder. Paper Presented at the Healthcare.
- Chalfun, G., Reis, M. M., de Oliveira, M. B. G., de Araújo Brasil, A., dos Santos Salú, M., da Cunha, A. J. L. A., Prata-Barbosa, A., & de Magalhães-Barbosa, M. C. (2022). Perinatal stress and methylation of the NR3C1 gene in newborns: Systematic review. Epigenetics, 17(9), 1003–1019. https://doi.org/10.1080/15592294.2021.1980691
- Daskalakis, N. P., Bagot, R. C., Parker, K. J., Vinkers, C. H., & de Kloet, E. R. (2013). The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology, 38(9), 1858–1873. https://doi.org/10.1016/j.psyneuen.2013.06.008
- De Kloet, E. (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6(6), 463. https://doi.org/10.1038/nrn1683
- De Rooij, S., Painter, R., Phillips, D., Räikkönen, K., Schene, A., & Roseboom, T. (2011). Self-reported depression and anxiety after prenatal famine exposure: Mediation by cardio-metabolic pathology? Journal of Developmental Origins of Health and Disease, 2(3), 136–143. https://doi.org/10.1017/S2040174411000055
- De Rooij, S. R., Painter, R. C., Phillips, D. I., Osmond, C., Tanck, M. W., Defesche, J. C., Bossuyt, P. M., Michels, R. P., Bleker, O. P., & Roseboom, T. J. (2006). The effects of the Pro12Ala polymorphism of the peroxisome proliferator–activated receptor-γ2 gene on glucose/insulin metabolism interact with prenatal exposure to famine. Diabetes Care, 29(5), 1052–1057. https://doi.org/10.2337/dc05-1993
- DeRijk, R. H. (2009). Single nucleotide polymorphisms related to HPA axis reactivity. Neuroimmunomodulation, 16(5), 340–352. https://doi.org/10.1159/000216192
- Fang, Y., van Meurs, J. B., d’Alesio, A., Jhamai, M., Zhao, H., Rivadeneira, F., Hofman, A., van Leeuwen, J. P., Jehan, F., Pols, H. A., & Uitterlinden, A. G. (2005). Promoter and 3′-untranslated-region haplotypes in the vitamin D receptor gene predispose to osteoporotic fracture: The Rotterdam study. The American Journal of Human Genetics, 77(5), 807–823. https://doi.org/10.1086/497438
- Harris, A., & Seckl, J. (2011). Glucocorticoids, prenatal stress and the programming of disease. Hormones and Behavior, 59(3), 279–289. https://doi.org/10.1016/j.yhbeh.2010.06.007
- Hauer, D., Weis, F., Papassotiropoulos, A., Schmoeckel, M., Beiras-Fernandez, A., Lieke, J., Kaufmann, I., Kirchhoff, F., Vogeser, M., Roozendaal, B., & Briegel, J. (2011). Relationship of a common polymorphism of the glucocorticoid receptor gene to traumatic memories and posttraumatic stress disorder in patients after intensive care therapy. Critical Care Medicine, 39(4), 643–650. https://doi.org/10.1097/CCM.0b013e318206bae6
- Hilberdink, C. E., van Zuiden, M., Olff, M., Roseboom, T., & de Rooij, S. R. (2022). The impact of adversities across the lifespan on psychological symptom profiles in late adulthood: A latent profile analysis. https://osf.io/c6fgd.
- Howland, M. A., Sandman, C. A., & Glynn, L. M. (2017). Developmental origins of the human hypothalamic-pituitary-adrenal axis. Expert Review of Endocrinology & Metabolism, 12(5), 321–339. https://doi.org/10.1080/17446651.2017.1356222
- Kessler, R. C., Aguilar-Gaxiola, S., Alonso, J., Benjet, C., Bromet, E. J., Cardoso, G., Degenhardt, L., de Girolamo, G., Dinolova, R. V., Ferry, F., & Florescu, S. (2017). Trauma and PTSD in the WHO world mental health surveys. European Journal of Psychotraumatology, 8(sup5), 1353383. https://doi.org/10.1080/20008198.2017.1353383
- Kumsta, R., Moser, D., Streit, F., Koper, J. W., Meyer, J., & Wüst, S. (2009). Characterization of a glucocorticoid receptor gene (GR, NR3C1) promoter polymorphism reveals functionality and extends a haplotype with putative clinical relevance. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 150B(4), 476–482. https://doi.org/10.1002/ajmg.b.30837
- Li-Tempel, T., Larra, M. F., Sandt, E., Mériaux, S. B., Schote, A. B., Schächinger, H., Muller, C. P., & Turner, J. D. (2016). The cardiovascular and hypothalamus-pituitary-adrenal axis response to stress is controlled by glucocorticoid receptor sequence variants and promoter methylation. Clinical Epigenetics, 8(1), 12. https://doi.org/10.1186/s13148-016-0180-y
- Lian, Y., Xiao, J., Wang, Q., Ning, L., Guan, S., Ge, H., Li, F., & Liu, J. (2014). The relationship between glucocorticoid receptor polymorphisms, stressful life events, social support, and post-traumatic stress disorder. BMC Psychiatry, 14(1), 1–10. https://doi.org/10.1186/s12888-014-0232-9
- Lindholm, H., Krettek, A., Malm, D., Novembre, G., & Handlin, L. (2020). Genetic risk-factors for anxiety in healthy individuals: Polymorphisms in genes important for the HPA axis. BMC Medical Genetics, 21(1), 1–8. https://doi.org/10.1186/s12881-020-01123-w
- Lumey, L. H., Stein, A. D., & Susser, E. (2011). Prenatal famine and adult health. Annual Review of Public Health, 32(1), 237–262. https://doi.org/10.1146/annurev-publhealth-031210-101230
- Manenschijn, L., Van Den Akker, E. L., Lamberts, S. W., & Van Rossum, E. F. (2009). Clinical features associated with glucocorticoid receptor polymorphisms. Annals of the New York Academy of Sciences, 1179(1), 179–198. https://doi.org/10.1111/j.1749-6632.2009.05013.x
- Morris, R. W., & Kaplan, N. L. (2002). On the advantage of haplotype analysis in the presence of multiple disease susceptibility alleles. Genetic Epidemiology: The Official Publication of the International Genetic Epidemiology Society, 23(3), 221–233. https://doi.org/10.1002/gepi.10200
- Motavalli, R., Majidi, T., Pourlak, T., Abediazar, S., Shoja, M. M., Zununi Vahed, S., & Etemadi, J. (2021). The clinical significance of the glucocorticoid receptors: Genetics and epigenetics. The Journal of Steroid Biochemistry and Molecular Biology, 213, 105952. https://doi.org/10.1016/j.jsbmb.2021.105952
- Nishith, P., Mechanic, M. B., & Resick, P. A. (2000). Prior interpersonal trauma: The contribution to current PTSD symptoms in female rape victims. Journal of Abnormal Psychology, 109(1), 20. https://doi.org/10.1037/0021-843X.109.1.20
- Oakley, R. H., & Cidlowski, J. A. (2013). The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. Journal of Allergy and Clinical Immunology, 132(5), 1033–1044. https://doi.org/10.1016/j.jaci.2013.09.007
- Quax, R. A., Manenschijn, L., Koper, J. W., Hazes, J. M., Lamberts, S. W., Van Rossum, E. F., & Feelders, R. A. (2013). Glucocorticoid sensitivity in health and disease. Nature Reviews Endocrinology, 9(11), 670–686. https://doi.org/10.1038/nrendo.2013.183
- Ravelli, A. C., van der Meulen, J. H., Michels, R., Osmond, C., Barker, D. J., Hales, C. N., & Bleker, O. P. (1998). Glucose tolerance in adults after prenatal exposure to famine. The Lancet, 351(9097), 173–177. https://doi.org/10.1016/S0140-6736(97)07244-9
- Reynolds, R. M., Labad, J., Buss, C., Ghaemmaghami, P., & Räikkönen, K. (2013). Transmitting biological effects of stress in utero: Implications for mother and offspring. Psychoneuroendocrinology, 38(9), 1843–1849. https://doi.org/10.1016/j.psyneuen.2013.05.018
- Sarubin, N., Hilbert, S., Naumann, F., Zill, P., Wimmer, A. M., Nothdurfter, C., Rupprecht, R., Baghai, T. C., Bühner, M., & Schüle, C. (2017). The sex-dependent role of the glucocorticoid receptor in depression: Variations in the NR3C1 gene are associated with major depressive disorder in women but not in men. European Archives of Psychiatry and Clinical Neuroscience, 267(2), 123–133. https://doi.org/10.1007/s00406-016-0722-5
- Schumm, J. A., Briggs-Phillips, M., & Hobfoll, S. E. (2006). Cumulative interpersonal traumas and social support as risk and resiliency factors in predicting PTSD and depression among inner-city women. Journal of Traumatic Stress, 19(6), 825–836. https://doi.org/10.1002/jts.20159
- Seckl, J. R., & Meaney, M. J. (2006). Glucocorticoid “programming” and PTSD risk. Annals of the New York Academy of Sciences, 1071(1), 351–378. https://doi.org/10.1196/annals.1364.027
- Sinnwell, J. P., Schaid, D. J., & Sinnwell, M. J. P. (2022). Package ‘haplo. stats’.
- Slopen, N., Loucks, E. B., Appleton, A. A., Kawachi, I., Kubzansky, L. D., Non, A. L., Buka, S., & Gilman, S. E. (2015). Early origins of inflammation: An examination of prenatal and childhood social adversity in a prospective cohort study. Psychoneuroendocrinology, 51, 403–413. https://doi.org/10.1016/j.psyneuen.2014.10.016
- Steudte-Schmiedgen, S., Kirschbaum, C., Alexander, N., & Stalder, T. (2016). An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neuroscience & Biobehavioral Reviews, 69, 124–135. https://doi.org/10.1016/j.neubiorev.2016.07.015
- Thombs, B. D., Bernstein, D. P., Lobbestael, J., & Arntz, A. (2009). A validation study of the Dutch Childhood Trauma Questionnaire-Short Form: Factor structure, reliability, and known-groups validity.
- Tobi, E. W., Slieker, R. C., Stein, A. D., Suchiman, H. E. D., Slagboom, P. E., Van Zwet, E. W., Heijmans, B. T., & Lumey, L. H. (2015). Early gestation as the critical time-window for changes in the prenatal environment to affect the adult human blood methylome. International Journal of Epidemiology, 44(4), 1211–1223. https://doi.org/10.1093/ije/dyv043
- Van den Bergh, B. R., van den Heuvel, M. I., Lahti, M., Braeken, M., de Rooij, S. R., Entringer, S., Hoyer, D., Roseboom, T., Räikkönen, K., King, S., & Schwab, M. (2020). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience & Biobehavioral Reviews, 117, 26–64. https://doi.org/10.1016/j.neubiorev.2017.07.003
- van Hoek, M., Langendonk, J. G., de Rooij, S. R., Sijbrands, E. J., & Roseboom, T. J. (2009). Genetic variant in the IGF2BP2 gene may interact with fetal malnutrition to affect glucose metabolism. Diabetes, 58(6), 1440–1444. https://doi.org/10.2337/db08-1173
- Van Praag, D. L., Fardzadeh, H. E., Covic, A., Maas, A. I., & von Steinbüchel, N. (2020). Preliminary validation of the Dutch version of the posttraumatic stress disorder checklist for DSM-5 (PCL-5) after traumatic brain injury in a civilian population. PloS one, 15(4), e0231857. https://doi.org/10.1371/journal.pone.0231857
- van Rossum, E. F., Binder, E. B., Majer, M., Koper, J. W., Ising, M., Modell, S., Salyakina, D., Lamberts, S. W., & Holsboer, F. (2006). Polymorphisms of the glucocorticoid receptor gene and major depression. Biological Psychiatry, 59(8), 681–688. https://doi.org/10.1016/j.biopsych.2006.02.007
- Van Rossum, E. F., Koper, J. W., Van Den Beld, A. W., Uitterlinden, A. G., Arp, P., Ester, W., Janssen, J. A., Brinkmann, A. O., De Jong, F. H., Grobbee, D. E., & Pols, H. A. (2003). Identification of the BclI polymorphism in the glucocorticoid receptor gene: Association with sensitivity to glucocorticoids in vivo and body mass index. Clinical Endocrinology, 59(5), 585–592. https://doi.org/10.1046/j.1365-2265.2003.01888.x
- Van Schie, C. C., van Harmelen, A.-L., Hauber, K., Boon, A., Crone, E. A., & Elzinga, B. M. (2017). The neural correlates of childhood maltreatment and the ability to understand mental states of others. European Journal of Psychotraumatology, 8(1), 1272788. https://doi.org/10.1080/20008198.2016.1272788
- Van Zuiden, M., Geuze, E., Willemen, H. L., Vermetten, E., Maas, M., Amarouchi, K., Kavelaars, A., & Heijnen, C. J. (2012). Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: A prospective study. Biological Psychiatry, 71(4), 309–316. https://doi.org/10.1016/j.biopsych.2011.10.026
- Van Zuiden, M., Geuze, E., Willemen, H. L., Vermetten, E., Maas, M., Heijnen, C. J., & Kavelaars, A. (2011). Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. American Journal of Psychiatry, 168(1), 89–96. https://doi.org/10.1176/appi.ajp.2010.10050706
- van Zuiden, M., Heijnen, C. J., Maas, M., Amarouchi, K., Vermetten, E., Geuze, E., & Kavelaars, A. (2012). Glucocorticoid sensitivity of leukocytes predicts PTSD, depressive and fatigue symptoms after military deployment: A prospective study. Psychoneuroendocrinology, 37(11), 1822–1836. https://doi.org/10.1016/j.psyneuen.2012.03.018
- Velders, F.P., Dieleman, G., Cents, R.A., Bakermans-Kranenburg, M.J., Jaddoe, V.W., Hofman, A., Van IJzendoorn, M.H., Verhulst, F.C. and Tiemeier, H., (2012). Variation in the glucocorticoid receptor gene at rs41423247 moderates the effect of prenatal maternal psychological symptoms on child cortisol reactivity and behavior. Neuropsychopharmacology, 37(11), 2541–2549. https://doi.org/10.1038/npp.2012.118
- Wray, N. R., Yang, J., Hayes, B. J., Price, A. L., Goddard, M. E., & Visscher, P. M. (2013). Pitfalls of predicting complex traits from SNPs. Nature Reviews Genetics, 14(7), 507–515. doi:10.1038/nrg3457
