ABSTRACT
Background:
Advanced neuroscientific insights surrounding post-traumatic stress disorder (PTSD) and its associated symptomatology should beget psychotherapeutic treatments that integrate these insights into practice. Deep Brain Reorienting (DBR) is a neuroscientifically-guided psychotherapeutic intervention that targets the brainstem-level neurophysiological sequence that transpired during a traumatic event. Given that contemporary treatments have non-response rates of up to 50% and high drop-out rates of >18%, DBR is investigated as a putative candidate for effective treatment of some individuals with PTSD.
Objective:
To conduct an interim evaluation of the effectiveness of an eight-session clinical trial of videoconference-based DBR versus waitlist (WL) control for individuals with PTSD.
Method:
Fifty-four individuals with PTSD were randomly assigned to DBR (N = 29) or WL (N = 25). At baseline, post-treatment, and three-month follow-up, participants’ PTSD symptom severity was assessed using the Clinician Administered PTSD Scale (CAPS-5). This is an interim analysis of a clinical trial registered with the U. S. National Institute of Health (NCT04317820).
Results:
Significant between-group differences in CAPS-total and all subscale scores (re-experiencing, avoidance, negative alterations in cognitions/mood, alterations in arousal/reactivity) were found at post-treatment (CAPS-total: Cohen’s d = 1.17) and 3-month-follow-up (3MFU) (CAPS-total: Cohen’s d = 1.18). Significant decreases in CAPS-total and all subscale scores were observed within the DBR group pre – to post-treatment (36.6% CAPS-total reduction) and pre-treatment to 3MFU (48.6% CAPS-total reduction), whereas no significant decreases occurred in the WL group. After DBR, 48.3% at post-treatment and 52.0% at 3MFU no longer met PTSD criteria. Attrition was minimal with one participant not completing treatment; eight participants were lost to 3MFU.
Conclusions:
These findings provide emerging evidence for the effectiveness of DBR as a well-tolerated treatment that is based on theoretical advances highlighting alterations to subcortical mechanisms in PTSD and associated symptomatology. Additional research utilizing larger sample sizes, neuroimaging data, and comparisons or adjacencies with other psychotherapeutic approaches is warranted.
Trial registration: ClinicalTrials.gov identifier: NCT04317820..
HIGHLIGHTS
First study to evaluate the effects of Deep Brain Reorienting (DBR) therapy on PTSD symptoms.
Eight internet-based DBR sessions resulted in significant decreases in PTSD symptoms post-treatment and at 3-month follow-up in comparison to a waitlist group.
Large effect sizes and a low drop-out rate suggest that DBR may be an effective, well-tolerated neuroscientifically guided treatment for PTSD.
Antecedentes: Los conocimientos neurocientíficos avanzados en torno al trastorno de estrés postraumático (TEPT) y su sintomatología asociada deberían dar lugar a tratamientos psicoterapéuticos que integren estos conocimientos en la práctica. La Reorientación Cerebral Profunda ("DBR", por sus siglas en inglés) es una intervención psicoterapéutica guiada neurocientíficamente que se dirige a la secuencia neurofisiológica a nivel del tronco encefálico que tuvo lugar durante un acontecimiento traumático. Dado que los tratamientos contemporáneos tienen tasas de no respuesta de hasta el 50% y altas tasas de abandono de >18%, la DBR se investiga como un candidato putativo para el tratamiento eficaz de algunos individuos con TEPT.
Objetivo: Llevar a cabo una evaluación preliminar de la eficacia de un ensayo clínico de ocho sesiones de DBR basado en videoconferencia frente al control de lista de espera (‘WL’, por sus siglas en inglés) para individuos con TEPT.
Método: Cincuenta y cuatro individuos con TEPT fueron asignados aleatoriamente a DBR (N = 29) o WL (N = 25). Al inicio, después del tratamiento y a los tres meses de seguimiento, se evaluó la gravedad de los síntomas de TEPT de los participantes mediante la Escala de TEPT administrada por el clínico (CAPS-5). Se trata de un análisis preliminar de un ensayo clínico registrado en el Instituto Nacional de Salud de EE.UU. (NCT04317820).
Resultados: Se encontraron diferencias significativas entre los grupos en las puntuaciones totales de la CAPS y de todas las subescalas (reexperimentación, evitación, alteraciones negativas en las cogniciones/el estado de ánimo, alteraciones en la excitación/reactividad) después del tratamiento (CAPS-total: d de Cohen = 1,17) y a los 3 meses de seguimiento (3MFU) (CAPS-total: d de Cohen = 1,18). Se observaron disminuciones significativas en las puntuaciones de CAPS-total y de todas las subescalas en el grupo de DBR antes y después del tratamiento (36,6% de reducción de CAPS-total) y antes del tratamiento hasta 3MFU (48,6% de reducción de CAPS-total), mientras que no se produjeron disminuciones significativas en el grupo de WL. Después de la DBR, el 48,3% en el postratamiento y el 52,0% en el 3MFU dejaron de cumplir los criterios de TEPT. La deserción fue mínima, con un participante que no completó el tratamiento; ocho participantes se perdieron en 3MFU.
Conclusiones: Estos hallazgos proporcionan evidencia emergente de la eficacia de la DBR como un tratamiento bien tolerado que se basa en avances teóricos que destacan alteraciones de los mecanismos subcorticales en el TEPT y la sintomatología asociada. Se justifica la realización de investigaciones adicionales que utilicen muestras de mayor tamaño, datos de neuroimagen y comparaciones o adyacencias con otros enfoques psicoterapéuticos.
背景:关于创伤后应激障碍(PTSD)及其相关症状的先进神经科学见解应该催生将这些见解融入实践的心理治疗方法。深度脑重定向(DBR)是一种神经科学指导的针对创伤事件期间发生的脑干水平神经生理序列的心理治疗干预措施。 鉴于当代治疗的无反应率高达 50% 且退出率高达超过18%,DBR 被认为是有效治疗某些 PTSD 患者的假定候选方法。
目的:对针对 PTSD 患者进行的基于视频会议的 DBR 组 与候补名单 (WL) 控制组的八期临床试验的有效性进行中期评估。
方法:54 名 PTSD 患者被随机分配至 DBR 组(N = 29)或 WL组( N = 25)。在基线、治疗后和三个月的随访中,使用临床医生管理 PTSD 量表 (CAPS-5) 评估参与者的 PTSD 症状严重程度。 这是对在美国国立卫生研究院注册的临床试验 (NCT04317820) 的中期分析。
结果:治疗后发现 CAPS 总分和所有子量表评分(再体验、回避、认知/情绪的负性改变、唤起/反应性改变)(CAPS 总分:Cohen's d = 1.17)和 3 个月随访 (3MFU)(CAPS 总分:Cohen d = 1.18)存在显著组间差异。观察到DBR 组的 CAPS 总分和所有子量表分数在治疗前到治疗后(CAPS 总分减少 36.6%)和治疗前到3MFU(CAPS 总减少 48.6%)显著下降,但WL组没有显著下降。 DBR 后,治疗后 48.3% 和 3MFU 时 52.0% 不再符合 PTSD 标准。人员流失率极低,仅一名参与者没有完成治疗;8 名参与者在 3MFU 流失。
结论:这些发现为 DBR 作为一种耐受性良好、基于强调 PTSD 皮层下机制改变和相关症状学理论进展的治疗方法的有效性提供了新的证据。需要利用更大的样本量、神经影像数据以及与其他心理治疗方法的比较或邻接进行更多研究。
1. Introduction
Post-traumatic stress disorder (PTSD) is an incapacitating psychiatric condition that develops in the wake of perceived threat to one’s life or integrity (American Psychiatric Association, Citation2013). The core symptomatology of PTSD includes intrusive re-experiencing of traumatic events, avoidance of external or internal (thoughts, feelings) trauma-related stimuli, alterations in cognition and mood, and changes to arousal modulation. Recent scientific developments have advanced our understanding of the underlying neurobiological mechanisms disrupted in PTSD, including identification of altered functional connectivity not only of cortical and subcortical regions but also across intrinsic connectivity networks (Akiki et al., Citation2017; Bao et al., Citation2021; Harricharan et al., Citation2017; Lanius et al., Citation2010; Sripada et al., Citation2012; Terpou et al., Citation2020; for reviews, see Kearney & Lanius, Citation2022; Lanius et al., Citation2018). Despite such knowledge, the integration of neuroscientific findings into psychotherapeutic practice remains largely nascent. Although gold-standard treatment approaches appear to attenuate PTSD-related symptoms (Cusack et al., Citation2016; McLean et al., Citation2022; Mendes et al., Citation2008; Watts et al., Citation2013), dropout rates associated with these treatments approximate one in every five persons (Imel et al., Citation2013; Lewis et al., Citation2020) with up to half of individuals deemed treatment nonresponsive (Bradley et al., Citation2005; Imel et al., Citation2013; Kar, Citation2011; Michelson et al., Citation1998; Pitman et al., Citation1991; Schottenbauer et al., Citation2008; van Minnen et al., Citation2002). Accordingly, there is an urgent need for novel, neuroscientifically guided treatments that address potential barriers to healing in trauma-related conditions such as PTSD.
1.1. Deep Brain Reorienting
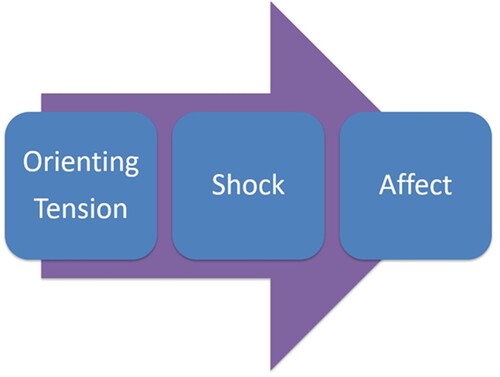
Deep Brain Reorienting (DBR) is a psychotherapeutic approach that targets the ordered neurophysiological sequence that is thought to have occurred in response to a traumatic event and which persists when triggered (Corrigan & Christie-Sands, Citation2020). This well-replicated sequence includes (1) orienting tension; (2) shock; and (3) affective responses (), processes mediated by the superior colliculus (SC), locus coeruleus (LC), and periaqueductal grey (PAG), respectively. These subcortical regions of the midbrain and brainstem appear to come online in rapid yet predictable succession upon encountering salient stimuli, providing a quick preparatory sequence oriented to protective, survival-oriented action (Lanius et al., Citation2017; Citation2018; Liddell et al., Citation2005; Rabellino et al., Citation2016; Terpou et al., Citation2019). In particular, the SC’s role in orienting the head and neck toward or away from a stimulus (Gandhi & Katnani, Citation2011; May, Citation2006; Meredith et al., Citation1992; Sparks, Citation1988) is emphasized in DBR, given that this basic neurophysiological response ensues before affective sequalae and cognitive appraisal, providing an early target for rapid therapeutic intervention.
Figure 1. Deep Brain Reorienting sequence. The orienting response elicits tension in the forehead, around the eyes, or in the back of the neck in preparation for turning toward or away from salient stimuli. Shock may consequentially arise as the high-energy impact experienced in the body before any affective response. Affect, such as fear, rage, or grief, ensues after the initial orienting and shock responses.
Although the notion of a natural healing process achieved through guided bodily awareness is not unique relative to other somatic-based psychotherapy approaches for trauma-related conditions (Levine, Citation2010; Ogden et al., Citation2006; Warner et al., Citation2014), DBR is distinctive in several ways. First, DBR goes beyond eliciting simple mindfulness of bodily responses through its capacity to harness knowledge of midbrain neuroanatomy to direct body awareness by means of a neurobiologically-sound methodological sequence. In DBR, this series of responses is slowed down, tracked, and attuned to by a trained therapist, creating a necessary gap between stimulus and response thus allowing critical time for the embodied memory to become dynamic and open to change (Kurtz, Citation1990). DBR also attends uniquely to pre-affective shock, a form of emotional overwhelm thought to be akin to the shock that arises from overwhelming physical pain or homeostatic disruption (Gantt, Citation1944; Hoch, Citation1943; Moleen, Citation1930). Finally, DBR emphasizes the orienting response associated with the individual’s initial reaction to traumatic stimuli. Its focused attention on orienting tension, which can be elicited by internalized representations of traumatic events through the SC’s receipt of cortical input (Pavlou & Casey, Citation2010), is thought to allow for affect to be processed in a more regulated manner than in traditional therapies. Here, affect regulation can be facilitated through the orienting tension, allowing the individual to not only become mindful of their body but also to maintain an embodied state throughout the session. Without attention to orienting tension, the individual may instead become easily overwhelmed by affect and dissociate, a response which has been associated with less optimal treatment outcomes (Kleindienst et al., Citation2011; Price et al., Citation2014; Spitzer et al., Citation2007; but also see Halvorsen et al., Citation2014; Zoet et al., Citation2018).
1.2. Psychotherapeutic targets of the neurobiology of the O-T-(S)-A sequence
As noted, DBR directly targets the typical sequence of neurobiologically-driven response associated with exposure to traumatic stimuli. Therapeutically, this process unfolds as:
1.2.1. O: orienting
Orienting to the present Before processing is started by the turning of attention to the activating stimulus (a salient moment within the traumatic event), the individual is guided towards being grounded in the present moment, in the body, and in the awareness of how the body is positioned in relation to all that surrounds it. This perceptual awareness of the body in physical space is thought to be mediated by the superior colliculus (SC; Merker, Citation2013).
Orienting to the traumatic event Following orientation to present experience, the individual is guided to bring the previously identified activating stimulus to mind, specifically a remembrance of a salient moment within the traumatic event. Here, the SC’s dense connectivity with the cortex allows for internal representations or remembrances of the original stimulus to provoke the initial orienting response that was present during the traumatic event (Lanius et al., Citation2018). Animal research has shown that whereas stimulation of the deep layers of the SC provokes a defensive ‘orienting away’ response, stimulation of the rostral SC elicits an ‘orienting toward’ or approach response (Comoli et al., Citation2012; Dean et al., Citation1989; DesJardin et al., Citation2013; Sahibzada et al., Citation1986). Notably, this targeting of neurobiological orienting responses as mediated by the SC is intended, in part, to address the residues of early attachment disruption, where orienting toward a caregiver resulted in negatively valenced or conflicted responses (Corrigan & Christie-Sands, Citation2020). Given that patterns of insecure or disorganized attachment in early childhood coincide with increased prevalence and severity of trauma-related conditions (Besser & Neria, Citation2012; Mikulincer et al., Citation2015; Roche et al., Citation1999; Woodhouse et al., Citation2015), DBR may be particularly poised to address early attachment-related trauma.
1.2.2. T: tension
Following this orienting response, fleeting tension (T) around the eyes, in the forehead, or in the back of the neck emerges in preparation for a motoric response of the head, neck and upper body, processes initiated by the deep layers of the SC (Corneil et al., Citation2002, Citation2008; Wallace et al., Citation1996). The therapist asks the individual to notice this tension and guides them in becoming aware of it. Critically, the SC has strong connections with the reticular formation (Cauzzo et al., Citation2022; Yasui et al., Citation1994), a region spanning the brainstem and midbrain that manages arousal and motor responses, as well as with the ventromedial tegmentum, which modulates musculoskeletal tension (Graham, Citation1977; Yasui et al., Citation1994). This orienting tension is the point of focus at the start of processing as it facilitates embodiment and provides a necessary anchor against emotional overwhelm.
1.2.3. S: shock
During a traumatic experience, an individual may experience a sudden and overwhelming mismatch between expectation and environmental or relational response and experience an emotional shock not dissimilar to how gross injury to the body produces surgical shock (Gantt, Citation1944; Hoch, Citation1943; Moleen, Citation1930). This high-energy emotional impact can be deeply disorienting and is hypothesized to be elicited by excessive activation of the innate alarm system (IAS), a preconscious defense system wired to rapidly detect environmental or looming threat (Lanius et al., Citation2017; Li et al., Citation2018; Liddell et al., Citation2005; Tamietto & de Gelder, Citation2010). The IAS is comprised of the SC, LC, PAG, and amygdala and is excessively activated in response to subliminal threat presentation in individuals with PTSD (Rabellino et al., Citation2015, Citation2016; Steuwe et al., Citation2014; Terpou et al., Citation2019). The LC plays a central role in heightening this physiological activation and musculoskeletal tension through its connections to noradrenergic pathways (Breton-Provencher et al., Citation2021; Sara, Citation2009), the spinal cord (Fung et al., Citation1991; Proudfit & Clark, Citation1991) and autonomic nuclei (Samuels & Szabadi, Citation2008; Sara & Bouret, Citation2012) while remaining hitherto devoid of visceral affective colouring. Space and time are provided for the shock response, which anecdotally has manifested as a bracing tension in the shoulders or upper torso, a pulling sensation behind the eyes, a surge of energy through the body, or a sense of hollowing or emptying, to be fully expressed and attuned to by the therapist. Crucially, the therapist ensures that the participant remains grounded through awareness of the orienting tension, which serves as an anchor to return to if the shock response becomes too uncomfortable or distressing.
1.2.4. A: affect
With support from the therapist, the individual next identifies the initial affect linked with the sequence, such as fear, rage, grief, or shame. Downstream of initial orienting tension and LC activation, affective responses at this stage of processing are thought to be mediated by the PAG. This midbrain region integrates incoming sensorimotor information from the SC with affective information from limbic cortical and subcortical structures to evaluate an event’s emotional significance and effect the appropriate motoric defense or approach response (Kozlowska et al., Citation2015; Lanius et al., Citation2018; Li et al., Citation2018; Mobbs et al., Citation2007; Schauer & Elbert, Citation2015). Notably, visceral, respiratory, and raw affective components of the sequence surface from the PAG (Bandler & Keay, Citation1996; Panksepp, Citation1998), processes that remain modulated when an individual is anchored by the orienting tension. However, without adequate attention to the orienting tension and to processing of any shock, overactivation of the PAG may result in a flooding of affect and excessive fight/flight responses via the dorsal/lateral PAG or shutdown/passive defensive responses via the ventrolateral PAG (Jansen et al., Citation1998; Keay & Bandler, Citation2002). Thus, in DBR, the therapist ensures a slow progression through each component of the sequence prior to affect and, to prevent overwhelm, encourages the individual to remain aware of the orienting tension while also experiencing the emotion(s) that eventually surface(s).
1.3. Study objective and hypotheses
Despite the development of detailed theory surrounding the mechanisms of action in DBR, research has yet to be conducted to assess its effectiveness in treating trauma-related symptomatology in any psychiatric condition, including PTSD. Here, we conducted an interim analysis of behavioural data gained through the first randomized clinical trial designed to assess the effectiveness of DBR therapy for individuals with PTSD, including those with childhood traumatization given its high correspondence with the later development of trauma-related conditions (Mikulincer et al., Citation2015; Ogle et al., Citation2015; Woodhouse et al., Citation2015). DBR sessions were internet-based via videoconferencing, an efficacious alternative to in-person psychotherapy for PTSD that accommodated COVID-19 restrictions (Bongaerts et al., Citation2022; Greenwood et al., Citation2022; Poletti et al., Citation2020). This interim investigation was conducted on PTSD severity scores pre – and post-DBR treatment for participants who have surpassed the three-month follow-up timepoint at the time of analysis, which was three years since the onset of the clinical trial. We tested the hypotheses that those receiving eight sessions of DBR (N = 29) would experience significantly greater PTSD symptom reduction post-treatment as compared to those in a waitlist condition (WL; N = 25) and that these clinical improvements would be durable three months after protocol completion.
2. Methods
1. Trial Design.
This parallel two-group randomized clinical trial was approved by the Health Sciences Research Ethics Board at Western University (#114501) and registered with the U. S. National Institute of Health (NIH) (ClinicalTrials.gov Identifier: NCT04317820) prior to participant recruitment. The trial compared delivery of eight weekly internet-based DBR sessions in parallel with a WL condition of the same duration. Before randomization, participants agreed to the possibility of being placed in a WL control condition, which required that they would not receive any psychotherapeutic or novel pharmaceutical interventions during the waitlist period but would be offered videoconference-based DBR treatment of equivalent duration after the waitlist period ended.
The treatment protocol was developed in collaboration with Dr. Frank Corrigan, the author who developed DBR through extensive theoretical and neuroscientific understanding of the subcortical brain processes involved in trauma responding, which aligned with clinical experience. Dr. Corrigan provided training and regular supervision to treating therapists throughout the study, and all DBR sessions were audio recorded and reviewed with Dr. Corrigan using a fidelity checklist (for details see Supplemental material). Participants were recruited through referrals from physicians and mental health professionals, advertisements in clinics or community programmes for traumatic stress, and advertisements within the London, Ontario community. For those interested, an initial telephone screening was conducted by the clinical coordinator, and full assessments were completed virtually via WebEx (Cisco Systems, Inc.) videoconferencing by clinically trained members of the research team blinded to treatment arm.
After all pre-treatment assessment measures were completed and the participant was deemed eligible, randomization into the active treatment (DBR) or waitlist (WL) condition was carried out by a coin flip. All DBR sessions took place over WebEx videoconferencing due to the preventative measures and restrictions employed throughout the COVID-19 pandemic. When COVID-19 restriction levels allowed, participants came to Robarts Research Center at Western University for a 7 T fMRI scan before DBR treatment commenced and after treatment completion; these data will be subsequently analyzed once sufficient power for neuroimaging analysis is reached. Participants provided verbal consent and were financially compensated for their time and participation in the clinical assessments (25 CAD each) and fMRI scans (50 CAD each). All clinical assessments were conducted separately from treatment sessions by the same blinded clinical assessor who conducted the initial assessment, absolving the need to assess interrater reliability.
2. Eligibility and Screening.
Participants were recruited beginning in March 2020, with recruitment efforts ongoing at the time of analysis. To be eligible for participation, individuals had to be between the ages of 18–65, be English speaking, and meet diagnostic criteria for PTSD according to the Diagnostic and Statistical Manual of Mental Disorders – fifth edition (DSM-5; American Psychiatric Association, Citation2013). Participants who were currently taking psychotropic medication were required to be on a stable dosage prior to onset of treatment and were asked to adhere to this medication regime wherever possible until completion of the 3-month follow-up assessment.
Grounds for exclusion from the study included past or current bipolar disorder, past or current psychotic disorder, substance use disorder within the three months prior to study commencement, extensive narcotic use, neurological or developmental disorder, serious untreated medical illness, history of head injury involving loss of consciousness, current participation in counselling more extensive than supportive therapy (i.e. Exposure Therapy, Cognitive–Behavioural Therapy) and/or incompatibility with 7-Tesla MRI safety standards given that this study included an fMRI component. In addition, clinical judgement was used to assess the severity of any identity fragmentation and suicidality; if participants exhibited pronounced instability in identity presentations and/or current suicidality with intent and/or plan, a strict eight-session protocol was deemed unsafe in its inability to address any potential issues that could arise toward the end of treatment. Decision to exclude based on severity of identity disturbances and/or suicidality was a careful and collaborative effort between the clinical coordinator, clinical assessors, and the supervising Principal Investigator, a licensed psychiatrist with many years’ experience in the assessment and treatment of PTSD.
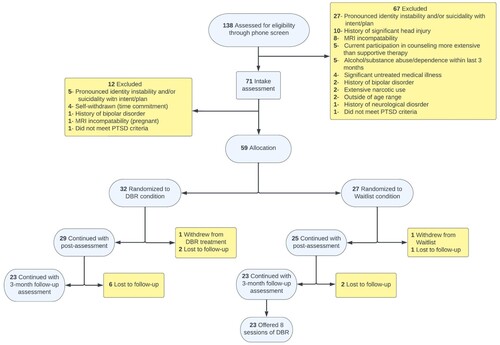
provides further information about study recruitment, randomization, and participant flow following the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Schulz et al., Citation2010). 138 participants were screened and 59 were randomized once determined to meet study eligibility. One participant dropped out of the DBR condition with eight lost to follow-up assessments; one participant withdrew from the waitlist condition with three lost to follow-up assessments.
2.1 Clinical Assessments
2.1.1. Primary outcome measure
PTSD diagnosis and severity scores were determined using the Clinician Administered PTSD Scale – 5th edition (CAPS-5; Weathers et al., Citation2013), a gold standard clinician-administered assessment used to both diagnose PTSD and to obtain a measure of PTSD severity (range of 0 to 80, Cronbach’s α = .84) with higher scores denoting more severe symptoms (Blake et al., Citation1995; Weathers et al., Citation2013). A detailed trauma history was collected during the initial clinical assessment with guidance from the Life Events Checklist for DSM-5 (LEC-5) to establish exposure to a PTSD Criterion A traumatic event(s). The CAPS-5 was pre-determined as the primary outcome measure of this registered trial.
2.1.2. Additional clinical assessments and secondary outcome measures
The Mini-International Neuropsychiatric Interview (M.I.N.I; Sheehan et al., Citation1998) was administered to determine any co-existing psychiatric conditions and to screen for exclusion criteria (e.g. history of bipolar or psychotic disorders). The Childhood Trauma Questionnaire (CTQ; Bernstein & Fink, Citation1998) was also administered during the initial (pre-treatment) assessment to assess levels of early life adversity. The CTQ is comprised of 5 subscales with individuated cut-scores for moderate to severe/extreme childhood adverse experiences, including emotional abuse (score > 12), physical abuse (score > 9), sexual abuse (score > 7), emotional neglect (score > 14), and physical neglect (score > 9). A session form reviewing recent symptoms was verbally administered for both the DBR group (administered by the treating therapist weekly) and WL group (administered by the clinical coordinator during a telephone check-in biweekly). Additional secondary measures of depression, dissociation, moral injury, and experiences of guilt and shame were also administered and will be the focus of additional analyses once the clinical trial is completed in full.
2.2. Intervention
DBR intervention consisted of eight weekly 90-minute sessions provided by a DBR-trained, licensed psychotherapist. Throughout a DBR session, the therapist’s main duty is to attune to the participant while assisting them in attending to bodily sensations, particularly in the head, face, and neck. If any affective responses or sensations feel unmanageable at any point in the sequence, or any signs of impending dissociative symptomatology emerge, a return to the orienting tension is guided by the therapist to avoid emotional overwhelm or dissociation. In very rare instances in which this is insufficient, long, slow outbreaths to elicit a parasympathetic down-regulation of arousal (Komori, Citation2018) termed ‘release breaths’ are encouraged. Sessions are structured using the Orienting-Tension-(Shock)-Affect sequence in the below manner.
Identifying the target: After initial greetings with the therapist, participants are first asked to identify a recent trigger or a past traumatic experience. Once the activating event is identified, the therapist assists the participant in identifying a moment of maximal attentional capture or ‘core’ of the experience; for example, a look from or posture of the perpetrator during a physical or sexual assault. This is referred to as the ‘target’ or ‘activating stimulus’.
Orienting to the present: Once a distressing recent or past memory is identified, the participant is asked to let go of that memory while redirecting their attention to their body in gravitational space. The therapist verbally guides the participant in noticing their bodily position between the ground and sky, their posture, the sensation of gravity holding them in their chair, any sounds near or far, or visual stimuli. Participants are encouraged to close their eyes; if that feels uncomfortable, they are instead encouraged to lower their gaze to their lap to avoid activating neural circuits related to external visual or social stimulation. Next, participants are guided in releasing as much tension as possible from the head and neck area (‘try to release any tension that you can from the muscles in your forehead, scalp, cheeks and mouth, jaws and neck … ’).
‘O’: Orienting to the traumatic trigger/event: The therapist then asks the participant to bring the target to mind in an effort to direct attention towards the activating stimulus.
‘T’: Tension: The therapist then asks the participant to notice any tension that immediately emerges into the head, face, or neck. The therapist allows time for the participant to respond, proceeding slowly. If the participant immediately demonstrates or experiences strong affect without first noticing a change in tension, they are guided back to identifying any tension in the head, face, and neck. Once the tension is identified, almost always in the muscles around the eyes, the forehead or the back of the neck, participants are guided in simply noticing it, taking their time, and allowing the next stage to emerge naturally.
Shock: Those participants who experience a subsequent pre-affective shock response (e.g.., shoulder or upper torso bracing tension, a pulling sensation behind the eyes, a deeply disorienting surge of energy through the body, or a sudden rigidity of parts of the body) are guided in allowing this response to occur. The therapist ensures the participant finds these experiences to be manageable, and if not, guides them back to noticing the orienting tension. The therapist provides minimal, but attuned, commentary on the individual’s physical experience and encourages them to allow the response to occur instead of attempting to block or ease it.
Affect: Affect is allowed to flow naturally once the O-T-(S) components of the sequence have been identified and deepened. Here, initial affect experienced by the participant such as rage, grief, fear, or shame is identified with support from the therapist. Participants are encouraged to remain with their emotion and allow processing to flow. If memories linked with the sequence arise, the therapist encourages the participant to let these memories ‘play in their minds’. If there is any tendency towards emotional overwhelm at this point, direction can be provided to reorient back to the awareness of the initial anchor identified in the orienting tension, such that the intensity of vehement emotions does not overwhelm the capacity to process through to completion.
Closure of the session: Typically, tensions and affect decrease in intensity by the end of the session. With any residual tension, the participant is encouraged to use release breathing, neck stretches/movement, and self-massage of any tense areas. Participants are also asked whether a new perspective of themselves had emerged as a result of the session; if so, they are encouraged to remain aware of this new perspective after the session.
2.3. Statistical analyses
All statistical analyses were conducted in SPSS (v. 28.0.0.0). Fisher’s exact test and independent samples t-tests were utilized to determine any between-group differences in categorical and continuous variables, respectively (two-tailed, p < .05). Longitudinal changes in CAPS-5 total and subscale scores (re-experiencing, avoidance, negative alterations in cognition and mood, alterations in arousal/reactivity) were analyzed using linear mixed models with restricted maximum likelihood (REML) estimation including subjects lost to 3-month follow-up to maximize power and model efficiency. A diagonal covariance structure was used as the repeated covariance type. We analyzed a main effect of time (pre-DBR/WL, post-DBR/WL, 3-month follow-up) as well as group (DBR, WL) x time interactions, where significant interactions could be interpreted as a differential treatment effect. Split file was employed to examine repeated measures within DBR and WL groups separately. Within each CAPS-5 score/subscore analysis, Bonferroni corrections were applied to adjust significance levels in consideration of the six pairwise comparisons per group (p < .05/6, or p < .008). Percent change/reduction was calculated as [(original score-observed score)/original score x 100] and effect sizes were derived as Cohen’s d.
3. Results
1. Clinical and demographic information.
Demographic information for each group is presented in . Overall, the sample was predominantly female (91%) and White (84%) with minimum college or some university education (94%). At baseline, no significant differences were found between groups for CAPS-5 severity scores, nor were there significant differences in the proportion of individuals classified as dissociative subtype (28% WL, 35% DBR). 89% of participants (86% DBR, 92% WL) were exposed to childhood adversity as determined by total CTQ scores above 35, a cut-off utilized in previous studies (Rukiye & Gonenir Erbay, Citation2018; Yaşar et al., Citation2020); this proportion is consistent with the 87% of participants who scored above cut-off on one or more CTQ subscales (67% scored above cut-off on more than one subscale). In addition to childhood trauma, some individuals experienced direct exposure to traumatic events in adulthood including work-related trauma (31% DBR, 16% WL), physical assault (3% DBR, 4% WL), sexual assault (3% DBR, 4% WL), medical trauma (0% DBR, 4% WL), and interpersonal trauma (62% DBR, 64% WL); most individuals (83% DBR, 84% WL) experienced multiple trauma types (e.g. childhood trauma and work-related trauma). There were no significant between-group differences in trauma type or in early adverse childhood experiences as reflected by CTQ total and subscale scores. Further, no significant differences emerged in proportions of individuals a) currently on a psychotropic medication regime across drug classes (antidepressants, sedatives, atypical antipsychotics, mood stabilizers, anticonvulsants, other) nor b) with co-existing psychiatric conditions, apart from higher panic disorder prevalence in the DBR group (p = .03).
Table 1. Clinical and demographic information for individuals in the Waitlist and Active Treatment (DBR) conditions.
2. Between-group analysis.
No significant difference in CAPS total scores (p = 0.508; Cohen’s d = 0.18) was found between the DBR and WL groups at the pre-treatment time point. No significant differences were found for any CAPS subscale score at baseline apart from higher alterations in arousal/reactivity in the WL group (p = .036; Cohen’s d = 0.59). At post-DBR/WL, significantly lower scores in the DBR group as compared to WL were found for CAPS total severity (p < .001, Cohen’s d = 1.17) (, ), the re-experiencing subscale (p = .005, Cohen’s d = 0.80), the avoidance subscale (p < .001, Cohen’s d = 1.39), the negative alterations in cognition/mood (NACM) subscale (p = .004, Cohen’s d = 0.81), and the alterations in arousal/reactivity subscale (p < .001, Cohen’s d = 1.09) (). Significantly lower scores in the DBR group were maintained at 3MFU for CAPS total severity (p < .001, Cohen’s d = 1.18), the re-experiencing subscale (p < .001, Cohen’s d = 1.10), the avoidance subscale (p < .001, Cohen’s d = 1.35), the NACM subscale (p = .005, Cohen’s d = 0.88), and the alterations in arousal/reactivity subscale (p < .001, Cohen’s d = 1.14) ().
Figure 3. Between-group differences in PTSD symptom severity (CAPS-5) scores within assessment timepoints. Error bars indicate standard deviation around the estimated marginal means; *Indicates significance at p < .05; **Indicates significance at p < .001; CAPS = Clinician Administered PTSD Scale (5th version); DBR = Deep Brain Reorienting; PTSD = Post-traumatic stress disorder; WL = Waitlist
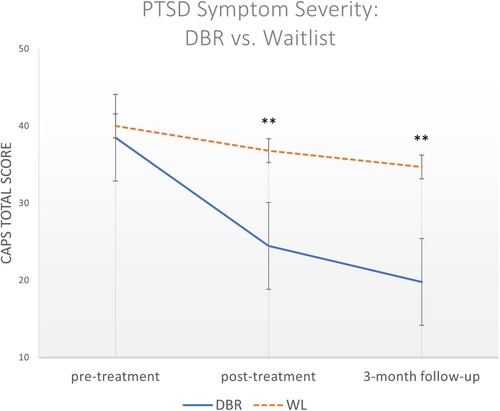
Table 2. Descriptive statistics for (A) between-group differences and (B) within-group differences across timepoints on CAPS total severity scores. (A) Independent sample two-sided t-tests were performed for estimated marginal mean CAPS total scores and Fisher’s exact tests were performed for presence/absence of PTSD diagnosis with a statistical significance threshold of p < 0.05. (B) Linear mixed model results for pairwise comparisons of timepoints conducted with a Bonferroni-corrected statistical significance threshold of p < .008.
Table 3. Descriptive statistics for (A) between-group differences and (B) within-group differences across timepoints on CAPS-5 subscale scores. (A) Independent sample two-sided t-tests were performed for estimated marginal mean CAPS subscale scores (B) Linear mixed model results for pairwise comparisons of timepoints conducted with a Bonferroni-corrected statistical significance threshold of p < .008.
3. Within-group analyses.
3.1. CAPS total.
Linear mixed models revealed a significant main effect of time (F(2, 84.93) = 21.056, p < .001) and a significant interaction effect of group × time (F(3, 49.015) = 12.511, p < .001). presents CAPS total score differences within the DBR and WL groups. While small to medium reductions were seen over time in the WL group, more significant reductions were seen in the DBR group. For instance, the WL group showed a 8.0% reduction from pre-WL to post-WL (p = .14) and a 15.1% reduction from pre-WL to 3-month follow-up (p = .06), while the DBR group showed Bonferroni-corrected differences between pre-DBR and post-DBR (p < .001; 36.6% reduction) and pre-DBR and 3-month follow-up (p < .001; 48.6% reduction). Reductions were not significant between post-treatment and 3MFU for either group.
3.2. CAPS re-experiencing.
A significant main effect of time (F(2, 87.48) = 18.38, p < .001) and a significant interaction for group × time (F(3, 49.02) = 7.601, p < .001) emerged. No decreases of corrected significance were found for re-experiencing symptoms in the WL group. In the DBR group, significant decreases were found between pre-DBR and post-DBR (p < .001), and pre-DBR to 3MFU (p < .001) ().
3.3. CAPS avoidance.
A significant main effect of time (F(2, 84.40) = 17.26, p < .001) and a significant interaction for group × time (F(3, 49.02) = 14.738, p < .001) emerged. No decreases of corrected significance were found for avoidance in the WL group. In the DBR group, significant decreases were found between pre-DBR and post-DBR (p < .001), and pre-DBR to 3MFU (p < .001) ().
3.4. CAPS negative alterations in cognition and mood.
A significant main effect of time (F(2, 89.95)) = 13.84, p < .001 and a significant interaction for group x time (F(3, 49.02) = 5.89, p = .002) emerged. No decreases of corrected significance were found for negative alterations in cognition/mood in the WL group. In the DBR group, significant decreases were found between pre-DBR and post-DBR (p < .001), and pre-DBR to 3MFU (p < .001) ().
3.5. CAPS alterations in arousal/reactivity.
A significant main effect of time (F(2, 92.41)) = 8.65, p < .001 and a significant interaction for group x time (F(3, 49.02) = 11.83, p < .001) emerged. No decreases of corrected significance were found for alterations in arousal/reactivity in the WL group. In the DBR group, significant decreases were found between pre-DBR and post-DBR (p < .001), and pre-DBR to 3MFU (p < .001) ().
4. Discussion
We conducted an interim analysis of PTSD symptom severity following eight weekly sessions of a novel therapeutic intervention, Deep Brain Reorienting (DBR). Greater attenuation of symptoms in the active treatment DBR group as compared to the matched waitlist group points strongly towards the potential effectiveness of DBR in the treatment of PTSD. Here, clinical assessment of PTSD symptom severity, as measured by the CAPS-5, revealed large between-group effect sizes at post-treatment (Cohen’s d = 1.17) and at 3-month follow-up (3MFU) (Cohen’s d = 1.18). Notably, the DBR group showed an overall 36.6% reduction in PTSD symptoms from pre-treatment to post-treatment and a 48.6% reduction from pre-treatment to 3MFU, which contrasted with WL reductions of 8.0% and 15.1%, respectively. These reductions in the DBR group corresponded with 14 out of 29 individuals no longer meeting criteria for PTSD post-treatment (48.3%) and 12 out of the 23 individuals who completed the 3MFU (52.2%); only one participant in the WL group no longer met criteria at both post-WL (4.0%) and 3MFU (4.3%). Overall attrition was minimal with a single participant dropping out of the DBR condition; this corresponds with a 4.3% dropout rate that compares very favourably with an average dropout rate of up to 18% or more in other trauma-focused treatments (Imel et al., Citation2013; Lewis et al., Citation2020; Roberts et al., Citation2022). Although collection of behavioural data from a larger sample size is warranted, these preliminary results suggest strongly that the effectiveness of DBR may be comparable to current gold-standard treatments for individuals with PTSD (Bisson et al., Citation2007; Bradley et al., Citation2005; McLean et al., Citation2022; Watts et al., Citation2013) with the additional benefit of lower attrition despite its trauma-centric nature.
The CAPS-5 measures the core symptomatology of PTSD in the domains of re-experiencing, avoidance, negative alterations in cognitions and mood, and alterations in arousal and reactivity (American Psychiatric Association, Citation2013), all of which showed significant attenuation after DBR treatment as compared to waitlist control. These symptoms are oftentimes refractory despite attempts to intellectually rationalize or contextualize one’s thoughts and feelings (Corrigan & Hull, Citation2015). In juxtaposition, subcortical and pre-verbal regions such as the superior colliculi (SC), locus coeruleus (LC), and periaqueductal grey (PAG) play critical roles in subconscious orienting toward or away from salient stimuli, modulating arousal, and directing active or passive defense responses. Though currently speculative, PTSD-related symptoms may be rooted in functional alterations to these phylogenetically and ontogenetically foundational structures, creating an explanatory account for how DBR reduces psychiatric and somatic symptoms related to an event or events that elicited primal threat and/or early attachment disruption. Accordingly, the therapeutic focus of DBR is on targeted, tracked responses theoretically emanating from the SC, LC, and PAG in temporal succession, and on nonverbal somatic responses as a means of undoing a mechanistic involvement and adaptation of key brainstem and midbrain structures in mediating PTSD symptoms (Bandler & Keay, Citation1996; Breton-Provencher et al., Citation2021; Jansen et al., Citation1998; Li et al., Citation2018; van der Kolk, Citation2002).
In line with common factors theory (Rosenzweig, Citation1936; Wampold, Citation2015), there are also key elements involved in the administration of DBR that are common to other forms of psychotherapy that likely contribute toward its effectiveness. Importantly, the establishment of a trusted alliance with the therapist, the adherence to a treatment protocol tailored toward alleviating specific symptomatology, and the resulting expectations of betterment are ingredients shared with other widely utilized treatment approaches (Wampold, Citation2015). This contrasts with a view that the effectiveness of DBR, or any other form of psychotherapy, is best explained only by a specified procedure (Benish et al., Citation2008). When compared with other treatments, however, the stipulated tracking of midbrain and brainstem responses as is exclusive to DBR may account, in part, for its unique contributions in attenuating PTSD-related symptoms. Further studies comparing the effectiveness of DBR with other treatment approaches will serve to clarify to what extent the hypothesized mechanistic underpinnings of DBR, namely subcortical circuits involving the LC, SC, and PAG, contribute to treatment efficacy.
5. Limitations and future directions
Firstly, this analysis was limited to behavioural data and thus we cannot confirm involvement of subcortical neural mechanisms responsible for DBR treatment efficacy. As a result of unforeseen roadblocks to imaging data collection, including COVID-19 restrictions and a temporarily inoperative 7 T scanner due to magnet quench, fMRI analyses remain underpowered preventing imaging analyses at present. Planned future analyses of neuroimaging data pre- and post-DBR treatment will elucidate whether these behavioural changes map onto neurobiological differences instantiated through DBR. Second, our sample size is relatively small and predominantly White and female, necessitating efforts toward sex/gender and ethnic inclusivity in future studies with larger samples. We also excluded participants with pronounced identity instability, suicidality with active intent/plan, and co-existing bipolar, psychotic, or active substance use disorders. Thus, future studies are needed to generalize our findings to individuals with PTSD who experience additionally any of these exclusion criteria. Although our DBR and WL groups were equivalent for CAPS-total scores at baseline, groupwise differences in CAPS-alterations in arousal/reactivity subscale scores reached statistical significance; thus, our ability to interpret post-DBR groupwise differences in arousal/reactivity-related symptomatology specifically is limited. Finally, we compared the efficacy of DBR only to a waitlist condition, and future studies are required to evaluate the relative efficacy of DBR in comparison to, or in adjacency with, current gold-standard treatments. Research into DBR as an adjunctive treatment to optimize other forms of psychotherapeutic intervention, including verbally dominant or ‘top-down’ approaches, will be essential in continued efforts toward helping individuals reach a stage of transformational healing from trauma.
6. Conclusions
This interim analysis from the first randomized clinical trial of DBR strongly supports its efficacy in reducing PTSD-related symptoms. DBR, a psychotherapeutic intervention that harnesses emerging understandings of the centrality of brainstem and midbrain neural systems in the development of and natural healing from trauma-related conditions (Corrigan & Christie-Sands, Citation2020), is a novel and unique approach that considers and integrates animal research, human neuroimaging research, and clinical practice. DBR has taken shape as a nonverbal, somatic methodology targeting a predictable temporal sequence of subcortical events that is traceable by a trained therapist and slowed down to allow for the clearance of unresolved defensive and/or affective responses cemented during a past traumatic event. Although preliminary, we demonstrate here that PTSD severity was significantly reduced in individuals who received internet-based DBR therapy as compared to a waitlist condition not only immediately post-treatment but also at a 3-month follow-up (3MFU) time point with large effect sizes that are comparable to those typically found with gold-standard psychological treatments for PTSD. Additionally, 48.3% of individuals no longer met PTSD criteria post-DBR, which was maintained at 3MFU. A low attrition rate in the active treatment group also suggests that DBR is generally well-tolerated. These findings show promise in the potential for DBR to have robust, lasting effects of similar magnitude to contemporary evidence-based treatments for PTSD. Future analyses, including the use of fMRI analyses pre – and post-DBR treatment, are warranted to ground these theoretical concepts in demonstrable neurobiological changes.
Acknowledgements
We would like to thank all the individuals who willingly participated in the study. We are also grateful to our dedicated research team, including Suzy Southwell and Nancy Mazza.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data analysed in the current study are available from the corresponding author (RAL) on reasonable request.
Additional information
Funding
References
- Akiki, T. J., Averill, C. L., & Abdallah, C. G. (2017). A network-based neurobiological model of PTSD: Evidence from structural and functional neuroimaging studies. Current Psychiatry Reports, 19(11), 1–10. https://doi.org/10.1007/s11920-017-0840-4
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th Edn). American Psychiatric Publishing.
- Bandler, R., & Keay, K. A. (1996). Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Progress in Brain Research, 107, 285–300. https://doi.org/10.1016/S0079-6123(08)61871-3
- Bao, W., Gao, Y., Cao, L., Li, H., Liu, J., Liang, K., Hu, X., Zhang, L., Hu, X., Gong, Q., & Huang, X. (2021). Alterations in large-scale functional networks in adult posttraumatic stress disorder: A systematic review and meta-analysis of resting-state functional connectivity studies. Neuroscience & Biobehavioral Reviews, 131, 1027–1036. https://doi.org/10.1016/j.neubiorev.2021.10.017
- Benish, S. G., Imel, Z. E., & Wampold, B. E. (2008). The relative efficacy of bona fide psychotherapies for treating post-traumatic stress disorder: A meta-analysis of direct comparisons. Clinical Psychology Review, 28(5), 746–758. https://doi.org/10.1016/j.cpr.2007.10.005
- Bernstein, D., & Fink, L. (1998). Childhood Trauma Questionnaire: A Retrospective Self- Report: Manual. Psychological Corporation.
- Besser, A., & Neria, Y. (2012). When home isn’t a safe haven: Insecure attachment orientations, perceived social support, and PTSD symptoms among Israeli evacuees under missile threat. Psychological Trauma: Theory, Research, Practice, and Policy, 4(1), 34–46. https://doi.org/10.1037/a0017835
- Bisson, J. I., Ehlers, A., Matthews, R., Pilling, S., Richards, D., & Turner, S. (2007). Psychological treatments for chronic post-traumatic stress disorder. British Journal of Psychiatry, 190(2), 97–104. https://doi.org/10.1192/bjp.bp.106.021402
- Blake, D. D., Weathers, F. W., Nagy, L. M., Kaloupek, D. G., Gusman, F. D., Charney, D. S., & Keane, T. M. (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90. https://doi.org/10.1002/jts.2490080106
- Bongaerts, H., Voorendonk, E. M., van Minnen, A., Rozendaal, L., Telkamp, B. S. D., & de Jongh, A. (2022). Fully remote intensive trauma-focused treatment for PTSD and complex PTSD. European Journal of Psychotraumatology, 13(2), 2103287. https://doi.org/10.1080/20008066.2022.2103287
- Bradley, R., Greene, J., Russ, E., Dutra, L., & Westen, D. (2005). A multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry, 162(2), 214–227. https://doi.org/10.1176/appi.ajp.162.2.214
- Breton-Provencher, V., Drummond, G. T., & Sur, M. (2021). Locus coeruleus norepinephrine in learned behavior: Anatomical modularity and spatiotemporal integration in targets. Frontiers in Neural Circuits, 15, 1–11. https://doi.org/10.3389/fncir.2021.638007
- Cauzzo, S., Singh, K., Stauder, M., Garcia-Gomar, M. G., Vanello, N., Passino, C., Staab, J., Indovina, I., & Bianciardi, M. (2022). Functional connectome of brainstem nuclei involved in autonomic, limbic, pain and sensory processing in living humans from 7 Tesla resting state fMRI. NeuroImage, 250, 118925. https://doi.org/10.1016/j.neuroimage.2022.118925
- Comoli, E., Das Neves Favaro, P., Vautrelle, N., Leriche, M., Overton, P. G., & Redgrave, P. (2012). Segregated anatomical input to sub-regions of the rodent superior colliculus associated with approach and defense. Frontiers in Neuroanatomy, 6(9), 1–19. https://doi.org/10.3389/fnana.2012.00009
- Corneil, B. D., Munoz, D. P., Chapman, B. B., Admans, T., & Cushing, S. L. (2008). Neuromuscular consequences of reflexive covert orienting. Nature Neuroscience, 11(1), 13–15. https://doi.org/10.1038/nn2023
- Corneil, B. D., Olivier, E., & Munoz, D. P. (2002). Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulation parameters. Journal of Neurophysiology, 88(4), 1980–1999. https://doi.org/10.1152/jn.2002.88.4.1980
- Corrigan, F. M., & Christie-Sands, J. (2020). An innate brainstem self-other system involving orienting, affective responding, and polyvalent relational seeking: Some clinical implications for a “Deep Brain Reorienting” trauma psychotherapy approach. Medical Hypotheses, 136(109502), 1–10. https://doi.org/10.1016/j.mehy.2019.109502
- Corrigan, F. M., & Hull, A. M. (2015). Recognition of the neurobiological insults imposed by complex trauma and the implications for psychotherapeutic interventions. BJPsych Bulletin, 39(2), 79–86. https://doi.org/10.1192/pb.bp.114.047134
- Cusack, K., Jonas, D. E., Forneris, C. A., Wines, C., Sonis, J., Middleton, J. C., Feltner, C., Brownley, K. A., Olmsted, K. R., Greenblatt, A., Weil, A., & Gaynes, B. N. (2016). Psychological treatments for adults with posttraumatic stress disorder: A systematic review and meta-analysis. Clinical Psychology Review, 43, 128–141. https://doi.org/10.1016/j.cpr.2015.10.003
- Dean, P., Redgrave, P., & Westby, G. M. (1989). Event or emergency? Two response systems in the mammalian superior colliculus. Trends in Neurosciences, 12(4), 137–147. https://doi.org/10.1016/0166-2236(89)90052-0
- DesJardin, J. T., Holmes, A. L., Forcelli, P. A., Cole, C. E., Gale, J. T., Wellman, L. L., Gale, K., & Malkova, L. (2013). Defense-like behaviors evoked by pharmacological disinhibition of the superior colliculus in the primate. The Journal of Neuroscience, 33(1), 150–155. https://doi.org/10.1523/JNEUROSCI.2924-12.2013
- Fung, S. J., Manzoni, D., Chan, J. Y. H., Pompeiano, O., & Barnes, C. D. (1991). Locus coeruleus control of spinal motor output. In C. D. Barnes, & O. Pompeiano (Eds.), Progress in Brain Research (pp. 395–409). https://doi.org/10.1016/S0079-6123(08)63825-X.
- Gandhi, N. J., & Katnani, H. A. (2011). Motor functions of the superior colliculus. Annual Review of Neuroscience, 34(1), 205–231. https://doi.org/10.1146/annurev-neuro-061010-113728
- Gantt, W. H. (1944). Production of disturbances in behavior by natural emotional shocks; Traumatic and experimental war neuroses. In W. H. Gantt (Ed.), Experimental Basis for Neurotic Behavior: Origin and Development of Artificially Produced Disturbances of Behavior in Dogs (pp. 19–31). Paul B Hoeber/Harper & Brothers. https://doi.org/10.1037/11517-003.
- Graham, J. (1977). An autoradiographic study of the efferent connections of the superior colliculus in the cat. The Journal of Comparative Neurology, 173(4), 629–654. https://doi.org/10.1002/cne.901730403
- Greenwood, H., Krzyzaniak, N., Peiris, R., Clark, J., Scott, A. M., Cardona, M., Griffith, R., & Glasziou, P. (2022). Telehealth versus face-to-face psychotherapy for less common mental health conditions: Systematic review and meta-analysis of randomized controlled trials. JMIR Mental Health, 9(3), e31780. https://doi.org/10.2196/31780
- Halvorsen, JØ, Stenmark, H., Neuner, F., & Nordahl, H. M. (2014). Does dissociation moderate treatment outcomes of narrative exposure therapy for PTSD? A secondary analysis from a randomized controlled clinical trial. Behaviour Research and Therapy, 57, 21–28. https://doi.org/10.1016/j.brat.2014.03.010
- Harricharan, S., Nicholson, A. A., Densmore, M., Théberge, J., McKinnon, M. C., Neufeld, R. W. J., & Lanius, R. A. (2017). Sensory overload and imbalance: Resting-state vestibular connectivity in PTSD and its dissociative subtype. Neuropsychologia, 106, 169–178. https://doi.org/10.1016/j.neuropsychologia.2017.09.010
- Hoch, P. H. (1943). Psychopathology of the traumatic war neuroses. American Journal of Psychiatry, 100(1), 124–126. https://doi.org/10.1176/ajp.100.1.124
- Imel, Z. E., Laska, K., Jakupcak, M., & Simpson, T. L. (2013). Meta-analysis of dropout in treatments for posttraumatic stress disorder. Journal of Consulting and Clinical Psychology, 81(3), 394–404. https://doi.org/10.1037/a0031474
- Jansen, A. S. P., Farkas, E., Sams, J. M., & Loewy, A. D. (1998). Local connections between the columns of the periaqueductal gray matter: A case for intrinsic neuromodulation. Brain Research, 784(1-2), 329–336. https://doi.org/10.1016/S0006-8993(97)01293-6
- Kar, N. (2011). Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: A review. Neuropsychiatric Disease and Treatment, 7, 167–181. https://doi.org/10.2147/NDT.S10389
- Kearney, B. E., & Lanius, R. A. (2022). The brain-body disconnect: A somatic sensory basis for trauma-related disorders. Frontiers in Neuroscience, 16, 1–34. https://doi.org/10.3389/fnins.2022.1015749
- Keay, K., & Bandler, R. (2002). Distinct central representations of inescapable and escapable pain: Observations and speculation. Experimental Physiology, 87(2), 275–279. https://doi.org/10.1113/eph8702355
- Kleindienst, N., Limberger, M. F., Ebner-Priemer, U. W., Keibel-Mauchnik, J., Dyer, A., Berger, M., Schmahl, C., & Bohus, M. (2011). Dissociation predicts poor response to dialectical behavioral therapy in female patients with borderline personality disorder. Journal of Personality Disorders, 25(4), 432–447. https://doi.org/10.1521/pedi.2011.25.4.432
- Komori, T. (2018). The relaxation effect of prolonged expiratory breathing. Mental Illness, 10(1), 6–7. https://doi.org/10.1108/mi.2018.7669
- Kozlowska, K., Walker, P., McLean, L., & Carrive, P. (2015). Fear and the defense cascade: Clinical implications and management. Harvard Review of Psychiatry, 31(2), 60–82. https://doi.org/10.1097/HRP.0000000000000358
- Kurtz, R. (1990). Body-centered Psychotherapy: The Hakomi Method: The Integrated use of Mindfulness, Nonviolence, and the Body. LifeRhythm.
- Lanius, R. A., Bluhm, R. L., Coupland, N. J., Hegadoren, K. M., Rowe, B., Théberge, J., Neufeld, P. C., Williamson, P. C., & Brimson, M. (2010). Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatrica Scandinavica, 121(1), 33–40. https://doi.org/10.1111/j.1600-0447.2009.01391.x
- Lanius, R. A., Boyd, J. E., McKinnon, M. C., Nicholson, A. A., Frewen, P., Vermetten, E., Jetly, R., & Spiegel, D. (2018). A review of the neurobiological basis of trauma-related dissociation and its relation to cannabinoid- and opioid-mediated stress response: A transdiagnostic, translational approach. Current Psychiatry Reports, 20(12), 118. https://doi.org/10.1007/s11920-018-0983-y
- Lanius, R. A., Rabellino, D., Boyd, J. E., Harricharan, S., Frewen, P. A., & McKinnon, M. C. (2017). The innate alarm system in PTSD: Conscious and subconscious processing of threat. Current Opinion in Psychology, 14, 109–115. https://doi.org/10.1016/j.copsyc.2016.11.006
- Levine, P. A. (2010). In an Unspoken Voice: How the Body Releases Trauma and Restores Goodness. North Atlantic Books.
- Lewis, C., Roberts, N. P., Gibson, S., & Bisson, J. I. (2020). Dropout from psychological therapies for post-traumatic stress disorder (PTSD) in adults: Systematic review and meta-analysis. European Journal of Psychotraumatology, 11(1), 1709709. https://doi.org/10.1080/20008198.2019.1709709
- Li, L., Feng, X., Zhou, Z., Zhang, H., Shi, Q., Lei, Z., Shen, P., Yang, Q., Zhao, B., Chen, S., Li, L., Zhang, Y., Wen, P., Lu, Z., Li, X., Xu, F., & Wang, L. (2018). Stress accelerates defensive responses to looming in mice and involves a locus coeruleus superior colliculus projection. Current Biology, 28(6), 859–871. https://doi.org/10.1016/j.cub.2018.02.005
- Liddell, B. J., Brown, K. J., & Kemp, A. H. (2005). A direct brainstem-amygdala-cortical “alarm” system for subliminal signals of fear. NeuroImage, 24(1), 235–243. https://doi.org/10.1016/j.neuroimage.2004.08.016
- May, P. J. (2006). The mammalian superior colliculus: Laminar structure and connections. Progress in Brain Research, 151, 321–378. https://doi.org/10.1016/S0079-6123(05)51011-2
- McLean, C. P., Levy, H. C., Miller, M. L., & Tolin, D. F. (2022). Exposure therapy for PTSD: A meta-analysis. Clinical Psychology Review, 91, 102115. https://doi.org/10.1016/j.cpr.2021.102115
- Mendes, D. D., Mello, M. F., Ventura, P., Passarela, C. D. M., & Mari, J. D. J. (2008). A systematic review on the effectiveness of cognitive behavioral therapy for posttraumatic stress disorder. The International Journal of Psychiatry in Medicine, 38(3), 241–259. https://doi.org/10.2190/PM.38.3.b
- Meredith, M. A., Wallace, M. T., & Stein, B. E. (1992). Visual, auditory, and somatosensory convergence in output neurons of the cat superior colliculus: Multisensory properties of the tecto-reticulo-spinal projection. Experimental Brain Research, 88(1), 181–186. https://doi.org/10.1007/BF02259139
- Merker, B. (2013). The efference cascade, consciousness, and its self: Naturalizing the first person pivot of action control. Frontiers in Psychology, 4, 501. https://doi.org/10.3389/fpsyg.2013.00501
- Michelson, L., June, K., Vives, A., Testa, S., & Marchione, N. (1998). The role of trauma and dissociation in cognitive-behavioral psychotherapy outcome and maintenance for panic disorder with agoraphobia. Behaviour Research and Therapy, 36(11), 1011–1050. https://doi.org/10.1016/S0005-7967(98)00073-4
- Mikulincer, M., Shaver, P. R., & Solomon, Z. (2015). An attachment perspective on traumatic and posttraumatic reactions. In M. Safir, H. Wallach, & A. Rizzo (Eds.), Future Directions in Post-Traumatic Stress Disorder: Prevention, Diagnosis, and Treatment (pp. 79–96). Springer). https://doi.org/10.1007/978-1-4899-7522-5_4.
- Mobbs, D., Petrovic, P., Marchnat, J., Hassabis, D., Weiskopf, N., Seymour, B., Dolan, R. J., & Frith, C. (2007). When fear is near: Threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science, 317(5841), 1079–1083. https://doi.org/10.1126/science.1144298
- Moleen, G. A. (1930). Influence of emotional shock on the gastro-intestinal tract in the psychoneuroses. JAMA: The Journal of the American Medical Association, 95(13), 910–913. https://doi.org/10.1001/jama.1930.02720130006002
- Ogden, P., Pain, C., & Fisher, J. (2006). A sensorimotor approach to the treatment of trauma and dissociation. Psychiatric Clinics of North America, 29(1), 263–279. https://doi.org/10.1016/j.psc.2005.10.012
- Ogle, C. M., Rubin, D. C., & Siegler, I. C. (2015). The relation between insecure attachment and posttraumatic stress: Early life versus adulthood traumas. Psychological Trauma: Theory, Research, Practice, and Policy, 7(4), 324–332. https://doi.org/10.1037/tra0000015
- Panksepp, J. (1998). The periconscious substrates of consciousness: Affective states and the evolutionary origins of the self. Journal of Consciousness Studies, 5(5-6), 566–582.
- Pavlou, A., & Casey, M. (2010). Simulating the effects of cortical feedback in the superior colliculus with topographic maps. The 2010 International Joint Conference on Neural Networks (IJCNN), Barcelona, Spain: 1-8. https://doi.org/10.1109/IJCNN.2010.5596839.
- Pitman, R. K., Altman, B., Greenwald, E., Longpre, R. E., Macklin, M. L., Poiré, R. E., & Steketee, G. S. (1991). Psychiatric complications during flooding therapy for posttraumatic stress disorder. The Journal of Clinical Psychiatry, 52(1), 17–20.
- Poletti, B., Tagini, S., Brugnera, A., Parolin, L., Pievani, L., Ferrucci, R., Compare, A., & Silani, V. (2020). Telepsychotherapy: A leaflet for psychotherapists in the age of COVID-19. A review of the evidence. Counselling Psychology Quarterly, 34(3-4), 352–367. https://doi.org/10.1080/09515070.2020.1769557
- Price, M., Kearns, M., Debra, H., & Rothbaum, B. O. (2014). Emergency department predictors of posttraumatic stress reduction for trauma-exposed individuals with and without an early intervention. Journal of Consulting and Clinical Psychology, 82(2), 336–341. https://doi.org/10.1037/a0035537
- Proudfit, H. K., & Clark, F. M. (1991). The projections of locus coeruleus neurons to the spinal cord. In C. D. Barnes, & O. Pompeiano (Eds.), Progress in Brain Research (Vol. 88: pp. 123–141). https://doi.org/10.1016/S0079-6123(08)63803-0.
- Rabellino, D., Densmore, M., Frewen, P. A., Théberge, J., & Lanius, R. A. (2016). The innate alarm circuit in post-traumatic stress disorder: Conscious and subconscious processing of fear- and trauma-related cues. Psychiatry Research: Neuroimaging, 248, 142–150. https://doi.org/10.1016/j.pscychresns.2015.12.005
- Rabellino, D., Tursich, M., Frewen, P. A., Daniels, J. K., Densmore, M., Théberge, J., & Lanius, R. A. (2015). Intrinsic connectivity networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli. Acta Psychiatrica Scandinavica, 132(5), 365–378. https://doi.org/10.1111/acps.12418
- Roberts, N. P., Lotzin, A., & Schäfer, I. (2022). A systematic review and meta-analysis of psychological interventions for comorbid post-traumatic stress disorder and substance use disorder. European Journal of Psychotraumatology, 13(1), 2041831. https://doi.org/10.1080/20008198.2022.2041831
- Roche, D. N., Runtz, M. G., & Hunter, M. A. (1999). Adult attachment: A mediator between child sexual abuse and later psychological adjustment. Journal of Interpersonal Violence, 14(2), 187–207. https://doi.org/10.1177/088626099014002006
- Rosenzweig, S. (1936). Some implicit common factors in diverse methods of psychotherapy. American Journal of Orthopsychiatry, 6(3), 412–415. https://doi.org/10.1111/j.1939-0025.1936.tb05248.x
- Rukiye, A. Y., & Gonenir Erbay, L. (2018). Relationship between childhood trauma and suicide probability in obsessive-compulsive disorder. Psychiatry Research, 261, 132–136. https://doi.org/10.1016/j.psychres.2017.12.054
- Sahibzada, N., Dean, P., & Redgrave, P. (1986). Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. The Journal of Neuroscience, 6(3), 723–733. https://doi.org/10.1523/JNEUROSCI.06-03-00723.1986
- Samuels, E. R., & Szabadi, E. (2008). Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part I: Principles of functional organization. Current Neuropharmacology, 6(3), 235–253. https://doi.org/10.2174/157015908785777229
- Sara, S. J. (2009). The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews Neuroscience, 10, 211–223. https://doi.org/10.1038/nrn2573
- Sara, S. J., & Bouret, S. (2012). Orienting and reorienting: The locus coeruleus mediates cognition through arousal. Neuron, 76(1), 130–141. https://doi.org/10.1016/j.neuron.2012.09.011
- Schauer, M., & Elbert, T. (2015). Dissociation following traumatic stress. Journal of Psychology, 218(2), 109–127. https://doi.org/10.1027/0044-3409/a000018
- Schottenbauer, M. A., Glass, C. R., Arnkoff, D. B., Tendick, V., & Hafter Gray, S. (2008). Nonresponse and dropout rates in outcome studies in PTSD: Review and methodological considerations. Psychiatry: Interpersonal and Biological Processes, 71(2), 134–168. https://doi.org/10.1521/psyc.2008.71.2.134
- Schulz, K. F., Altman, D. G., & Moher, D., for the CONSORT Group. (2010). CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials.
- Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., Hergueta, T., Baker, R., & Dunbar, G. C. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(Suppl 20), 22–33.
- Sparks, D. L. (1988). Neural cartograph: Sensory and motor maps in the superior colliculus. Brain, Behavior and Evolution, 31(1), 49–56. https://doi.org/10.1159/000116575
- Spitzer, C., Barnow, S., Freyberger, H. J., & Grabe, H. J. (2007). Dissociation predicts symptom-related treatment outcome in short-term inpatient psychotherapy. Australian & New Zealand Journal of Psychiatry, 41(8), 682–687. https://doi.org/10.1080/00048670701449146
- Sripada, R. K., King, A. P., Welsh, R. C., Garfinkel, S. N., Wang, X., Sripada, C. S., & Liberzon, I. (2012). Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Medicine, 74(9), 904–911. https://doi.org/10.1097/PSY.0b013e318273bf33
- Steuwe, C., Daniels, J. K., Frewen, P. A., Densmore, M., pannasch, S., Beblo, T., Reiss, J., & Lanius, R. A. (2014). Effect of direct eye contact in PTSD related to interpersonal trauma: An fMRI study of activation of an innate alarm system. Social Cognitive and Affective Neuroscience, 9(1), 88–97. https://doi.org/10.1093/scan/nss105
- Tamietto, M., & de Gelder, B. (2010). Neural bases of the non-conscious perception of emotional signals. Nature Reviews Neuroscience, 11(10), 697–709. https://doi.org/10.1038/nrn2889
- Terpou, B. A., Densmore, M., Théberge, j., Frewen, P., McKinnon, M. C., Nicholson, A. A., & Lanius, R. A. (2020). The hijacked self: Disrupted functional connectivity between the periaqueductal gray and the default mode network in posttraumatic stress disorder using dynamic causal modeling. NeuroImage: Clinical, 27, 102345. https://doi.org/10.1016/j.nicl.2020.102345
- Terpou, B. A., Densmore, M., Thome, J., Frewen, P., McKinnon, M. C., & Lanius, R. A. (2019). The innate alarm system and subliminal threat presentation in posttraumatic stress disorder: Neuroimaging of the midbrain and cerebellum. Chronic Stress, 3, 1–13. https://doi.org/10.1177/2470547018821496
- van der Kolk, B. A. (2002). Beyond the talking cure: Somatic experience and subcortical imprints in the treatment of trauma. In F. Shapiro (Ed.), EMDR as an Integrative Psychotherapy Approach: Experts of Diverse Orientations Explore the Paradigm Prism (pp. 57–83). American Psychological Association. https://doi.org/10.1037/10512-003.
- van Minnen, A., Arntz, A., & Keijsers, G. P. J. (2002). Prolonged exposure in patients with chronic PTSD: Predictors of treatment outcome and dropout. Behaviour Research and Therapy, 40(4), 439–457. https://doi.org/10.1016/S0005-7967(01)00024-9
- Wallace, M. T., Wilkinson, L. K., & Stein, B. E. (1996). Representation and integration of multiple sensory inputs in primate superior colliculus. Journal of Neurophysiology, 76(2), 1246–1266. https://doi.org/10.1152/jn.1996.76.2.1246
- Wampold, B. E. (2015). How important are the common factors in psychotherapy? An update. World Psychiatry, 14(3), 270–277. https://doi.org/10.1002/wps.20238
- Warner, E., Spinazzola, J., Westcott, A., Gunn, C., & Hodgdon, H. (2014). The body can change the score: Empirical support for somatic regulation in the treatment of traumatized adolescents. Journal of Child & Adolescent Trauma, 7(4), 237–246. https://doi.org/10.1007/s40653-014-0030-z
- Watts, B. V., Schnurr, P. P., Mayo, L., Young-Xu, Y., Weeks, W. B., & Friedman, M. J. (2013). Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. The Journal of Clinical Psychiatry, 74(6), e541–e550. https://doi.org/10.4088/JCP.12r08225
- Weathers, F. W., Blake, D. D., Schnurr, P. P., Kaloupek, D. G., Marx, B. P., & Keane, T. M. (2013). The clinician-administered PTSD scale for DSM-5 (CAPS-5). Interview available from the National Center for PTSD at www.ptsd.va.gov.
- Woodhouse, S., Ayers, S., & Field, A. P. (2015). The relationship between adult attachment style and post-traumatic stress symptoms: A meta-analysis. Journal of Anxiety Disorders, 35, 103–117. https://doi.org/10.1016/j.janxdis.2015.07.002
- Yasui, Y., Tsumori, T., Ando, A., Domoto, T., Kayahara, T., & Nakano, K. (1994). Descending projections from the superior colliculus to the reticular formation around the motor trigeminal nucleus and the parvicellular reticular formation of the medulla oblongata in the rat. Brain Research, 656(2), 420–426. https://doi.org/10.1016/0006-8993(94)91489-3
- Yaşar, A. B., Sayman, C., Taycan, S. E., Çetinkaya, Y., Gündüz, A., & Tireli, H. (2020). The association between temperament features and childhood traumas in patients with juvenile myoclonic epilepsy. Turkish Journal of Medical Sciences, 50(5), https://doi.org/10.3906/sag-1912-18
- Zoet, H. A., Wagenmans, A., van Minnen, A., & de Jongh, A. (2018). Presence of the dissociative subtype of PTSD does not moderate the outcome of intensive trauma focused treatment for PTSD. European Journal of Psychotraumatology, 9(1), 1468707. https://doi.org/10.1080/20008198.2018.1468707