ABSTRACT
Background: Clonidine is a centrally acting anti-adrenergic agent that may have applications in post-traumatic stress disorder (PTSD), particularly for sleep.
Objective: In this systematic review, we aimed to summarize the effect of clonidine on sleep quality and duration, nightmares, and PTSD symptom severity in adults with PTSD.
Method: PubMed (Medline), Embase, PsycINFO, CINAHL, and clinicaltrials.gov were searched up to April 2023. Studies on clonidine use in adult PTSD patients reporting data on the effect on sleep, nightmares, and PTSD symptoms were included. A narrative summary and a meta-analysis of the study findings are presented.
Results: Ten reports, accounting for N = 569 patients with PTSD (145 on clonidine and 436 controls), were included in the final selection. There were four case reports, four observational studies, one non-blind clinical trial, and one crossover randomized controlled trial (RCT). Median clonidine dose was 0.15 mg/day (range: 0.1–0.5 mg/day). Median follow-up time was 31 days (range: 3 days to 19 months). The quality of the evidence was rated from very low to low. There was marked between-study heterogeneity and low power in the individual studies, but many reported improved sleep quality, nightmare reduction, and improvement of PTSD symptoms for patients treated with clonidine. Meta-analysis was only possible for two studies reporting the effect of clonidine on nightmares, and showed no difference from the comparator (i.e. prazosin or terazosin) (odds ratio: 1.16; 95% confidence interval: 0.66 to 2.05), potentially pointing towards non-inferiority between these medications.
Conclusions: Future research, such as well-powered RCTs, is needed to identify the efficacy in the lower dose range and the most suitable treatment group, and to obtain good evidence on the effects of clonidine in the treatment of sleep disorders related to PTSD.
HIGHLIGHTS
Post-traumatic stress disorder (PTSD) is associated with hyperarousal and sleep disorders, reflecting adrenergic nervous system involvement.
The use of anti-adrenergic drugs to target the sympathetic activation in PTSD is rational. However, previous reports on prazosin, a peripherally acting agent, yielded weak evidence.
Clonidine, a central adrenergic antagonist, shows promise in improving sleep, nightmares, and PTSD symptoms, but further research is needed because the quality of the current evidence is low.
Antecedentes: La clonidina es un agente antiadrenérgico de acción central que podría tener aplicaciones en el trastorno de estrés postraumático (TEPT), particularmente para el sueño.
Objetivo: En esta revisión sistemática el objetivo fue resumir el efecto de la clonidina sobre la calidad y duración del sueño, las pesadillas y la gravedad de los síntomas de TEPT en adultos con TEPT.Método: Se realizaron búsquedas en PubMed (Medline), Embase, PsycINFO, CINAHL y Clinicaltrials.gov hasta abril de 2023. Se incluyeron estudios sobre el uso de clonidina en pacientes adultos con TEPT informando datos sobre el efecto en el sueño, pesadillas y síntomas de TEPT. Se presenta un resumen narrativo y un metanálisis de los hallazgos del estudio.
Resultados: En la selección final se incluyeron diez comunicaciones, que representaban N = 569 pacientes con TEPT (145 con clonidina y 436 controles). Hubo 4 informes de casos, 4 estudios observacionales, 1 ensayo clínico no ciego y 1 ensayo clínico aleatorizado (ECA) cruzado. La dosis mediana de clonidina fue de 0,15 mg/día (rango: 0,1-0,5 mg/día). La mediana del tiempo de seguimiento fue de 31 días (entre 3 días y 19 meses). La calidad de la evidencia se calificó de muy baja a baja. Hubo una marcada heterogeneidad entre los estudios y un poder estadístico bajo en los estudios individuales, pero muchos informaron una mejor calidad del sueño, una reducción de las pesadillas y una mejoría de los síntomas de TEPT en los pacientes tratados con clonidina. El metanálisis solo fue posible para dos estudios que informaron el efecto de la clonidina sobre las pesadillas y no mostró diferencias con el comparador (es decir, prazosina o terazosina) (OR: 1,16; IC del 95 %: 0,66; 2,05), potencialmente apuntando hacia una no inferioridad entre estos medicamentos.
Conclusiones: Se necesitan investigaciones futuras, como ECA de suficiente poder, para identificar la eficacia en el rango de dosis más bajo, el grupo de tratamiento más adecuado y obtener buena evidencia de los efectos de la clonidina para el tratamiento de los trastornos del sueño relacionados con el TEPT.
1. Introduction
Post-traumatic stress disorder (PTSD) is a debilitating condition with an estimated lifetime prevalence of 6.8%, and has a significant impact on individuals’ quality of life (NIMH, Citation2022). Treatment guidelines generally prioritize psychotherapy, with a secondary role for medication. The efficacy of treatment is often limited and less than half of patients achieve full recovery after 2 years (Rosellini et al., Citation2018). Despite the psychological nature of trauma, patients with PTSD frequently experience physical symptoms such as stress-related pains, hypervigilance, and, frequently, sleep disorders. The hippocampus, amygdala, and locus coeruleus in the brain show neurochemical changes in noradrenergic and serotonin pathways that contribute to these physical symptoms. In particular, the sympathetic nervous system neurotransmitter noradrenaline (norepinephrine) is involved in the sleep disorders often seen in PTSD patients (Wingenfeld et al., Citation2015).
Previous studies have examined the effect of peripheral anti-adrenergic agents for PTSD, such as propranolol and prazosin. However, the quality of the evidence for their efficacy was insufficient to support their use in routine clinical practice (Steenen et al., Citation2016; Zhang et al., Citation2020). In contrast, central adrenergic inhibition by anti-adrenergic drugs such as clonidine and guanfacine, which stimulate presynaptic alpha-2 adrenoceptors and thus inhibit adrenergic tone, may be beneficial for treating PTSD-related sleep disorders (Davis et al., Citation2008; Jagtiani et al., Citation2024; Khan et al., Citation1999; Raskind et al., Citation2018; Zhang et al., Citation2020). Clonidine inhibits the release of noradrenaline in the locus coeruleus and has shown promising results in case reports, retrospective chart reviews, and pilot studies for treating sleep disorders and overall symptoms in PTSD patients (Alao et al., Citation2012; Bange & Melvin, Citation2022; Burek et al., Citation2021; Kinzie et al., Citation1994). Recent studies suggest that clonidine may also restore rapid eye movement (REM) sleep and have an impact on emotional memory consolidation (Jang et al., Citation2022; Miyazaki et al., Citation2004; Saggu et al., Citation2023), but good-quality research and solid evidence are lacking with respect to the effectiveness and tolerability of clonidine in PTSD. Although clonidine has been registered for medical use for several decades, it is not yet approved for treating PTSD-related symptoms.
We aimed to systematically review the literature on the use of clonidine in PTSD, including both qualitative and quantitative sleep evaluation, nightmares, and overall PTSD symptom level.
2. Method
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., Citation2021). The protocol of this study was registered with PROSPERO (registered under number CRD42023418049).
2.1. Data sources and search strategy
We searched the PubMed (Medline), Embase, PsycINFO, CINAHL, and clinicaltrials.gov databases until 10 April 2023, using a combination of terms related to ‘PTSD’ OR ‘post-traumatic stress disorder*’ AND ‘clonidine’. In addition, cross-references were checked. No restrictions regarding the language of publication or publication date were set.
2.2. Eligibility criteria
We included studies reporting data on clonidine use for adults (aged ≥ 18 years) suffering from PTSD, without restrictions on the language of the publication or setting of the enrolment. Studies that considered a sample of healthy volunteers or adults with psychiatric diagnosis other than PTSD were excluded. The primary focus was randomized controlled trials (RCTs), and in the absence of such evidence, we also included data from observational studies and case reports, although these studies usually provide data of lower quality. We excluded qualitative studies and reviews, although the reference lists of the identified reviews were screened for potentially relevant studies missed in the electronic database search. We only included studies published in peer-reviewed journals, excluding conference abstracts and dissertations. If data from the same sample were published in multiple works, we considered only the study reporting more exhaustive information. Sample overlap was ruled out through a careful check of the registration codes as well as the place and year(s) of sampling.
2.3. Condition and outcomes of interest
PTSD diagnosis had to be defined according to standard operational diagnostic criteria, i.e. according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) (American Psychiatric Association, Citation2013) or the International Classification of Diseases (ICD) (WHO, Citation2004). The primary outcome was assessment of sleep, considering both sleep quality and duration; the secondary outcome was PTSD-related nightmares. These were considered when measured using validated tools or self-reports. Additional outcomes were PTSD symptom severity, measured with specific psychometric tools, and analysis of the safety and tolerability of the treatment with clonidine, assessed through evaluation of the rate of overall adverse events, dropouts due to any cause, dropouts due to severe adverse events, and death.
2.4. Data collection and extraction
Two authors (MM and PG) working independently reviewed the titles and abstracts of the retrieved articles. The initial screening was followed by the analysis of full texts to check compliance with the inclusion and exclusion criteria. All disagreements were explored until consensus was reached, and if consensus was not possible, another member of the team (MPB) was consulted. A standardized form was used for data extraction. For each eligible trial, two review authors (MM and PG) independently extracted the following information: (1) study characteristics (first author’s last name, year of publication, country, study setting and design, eligibility criteria, number of participants); (2) participants’ characteristics (age, sex, PTSD diagnosis and stage of illness, symptom severity at baseline, ongoing psychiatric treatment); (3) intervention details (clonidine treatment and, where applicable, the comparator used, prescribed dosage and range, frequency of administration, route of administration, cointerventions); and (4) outcome measures of interest, duration of the follow-up, and time of data collection. Extraction sheets for each study were cross-checked for consistency and any disagreement was resolved by discussion within the research group.
2.5. Statistical analyses
Where possible, we summarized quantitative data among studies using meta-analyses. We used inverse-variance models with random effects to summarize both continuous and dichotomous outcome data (DerSimonian & Laird, Citation1986). For continuous outcome data, we calculated the Hedges’ g standardized mean differences (SMDs) and the corresponding 95% confidence intervals (CIs); for dichotomous outcome data, we calculated the pooled odds ratios (ORs) and the corresponding 95% CIs (Higgins et al., Citation2021). We used data from the intention-to-treat analyses for both continuous and dichotomous outcomes. The results were summarized using forest plots. Standard Q tests and the I2 statistic (i.e. the percentage of variability in prevalence estimates attributable to heterogeneity rather than sampling error or chance, with values of I2 75% indicating high heterogeneity) were used to assess between-study heterogeneity (Higgins & Thompson, Citation2002). When the meta-analysis included at least 10 studies (Sterne et al., Citation2011), we performed funnel plot analysis and the Egger test to test for publication bias. If analyses showed a significant risk of publication bias, we used the trim-and-fill method to estimate the number of missing studies and the adjusted effect size (Duval & Tweedie, Citation2000). Meta-regression analysis was performed to examine sources of between-study heterogeneity on a range of prespecified study characteristics (i.e. clonidine dose, length of follow-up, use as add-on or monotherapy, sex, age, and treatment resistance). The analyses were performed using the meta and metafor packages in R (Balduzzi et al., Citation2019; RStudio Team, Citation2021; Schwarzer, Citation2021). Statistical tests were two sided and used a significance threshold of p < .05.
2.6. Risk of bias assessment and GRADE
The risk of bias in the included studies was independently assessed by two reviewers (MM and PG), using the Cochrane risk of bias tool (Higgins et al., Citation2011). All disagreements were discussed until consensus was reached, and if necessary, another member of the team (MPB) was consulted. Each item on the risk of bias assessment was scored as high, low, or unclear, and the GRADE tool was used to assess the overall certainty of evidence (Schünemann et al., Citation2013).
3. Results
3.1. Study characteristics
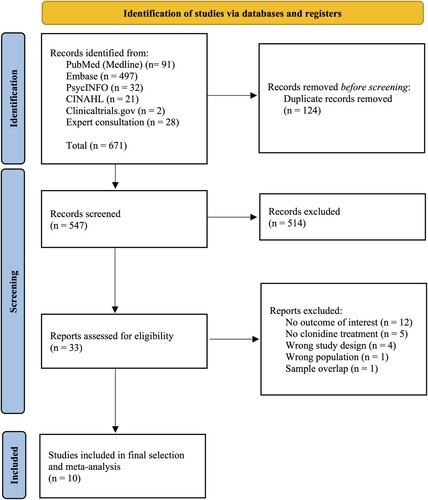
summarizes the paper selection process. From 671 records screened on title and abstract, 33 full texts were analysed. The review process led to the selection of 10 studies (Alao et al., Citation2012; Alexander & Kuntz, Citation2012; Bange & Melvin, Citation2022; Burek et al., Citation2021; Detweiler et al., Citation2016; Hansenne et al., Citation1991; Kinzie et al., Citation1994; Kinzie & Leung, Citation1989; Ouyang et al., Citation2015; Wendell & Maxwell, Citation2015; Ziegenhorn et al., Citation2009), referring to 10 different samples, leading to a total of 569 participants (i.e. 145 clonidine and 436 controls), which were included in the quantitative synthesis.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. The figure shows the flowchart of the review phases, reporting the number of records identified, screened, and included. The main reason for exclusion among the full texts assessed was the absence of outcome data of interest for this review.
On average across the studies, the percentage of female participants was 35.9% (range: 0–100%). The mean age of participants across the studies was 44.5 years (SD = 13.6), ranging from 20 to 82 years old, and the median age across the studies was 43 years. Four out of 10 studies (40%) considered a population of veterans. The selected studies were conducted in the USA (n = 9; 90%) and Germany (n = 1 each; 10%). The year of publication ranged from 1989 to 2022. Concerning the study design, there were four case reports (40%), corresponding to five patients, three retrospective studies on data from clinical charts (30%), one prospective observational study (10%), one non-blind clinical trial (10%), and one crossover RCT (10%). A comparator was used in four studies (40%); of these, two studies used another alpha-lytic agent (e.g. prazosin or terazosin) or second generation antipsychotics or antidepressants, one study used a placebo, and one considered a subtherapeutic clonidine dose (i.e. < 0.1 mg/day). The mean clonidine dose implemented across the studies was 0.19 mg/day (SD = 0.12) and the median was 0.15 mg/day, ranging from 0.1 to 0.5 mg/day. The route of administration was oral in nine studies (90%), generally at bedtime; if the dose was higher than 0.15 mg/day, it was administered two or three times per day. Only one study (10%) used intravenous administration of 0.15 mg/day of clonidine. Four studies (40%) reported outcome data on sleep quality, assessed through patients self-reporting, two studies (20%) provided data on sleep amount, assessed though sleep latency reduction, seven studies (70%) assessed self-reported change in nightmares, and one study also provided polysomnography measurement, and five studies (50%) evaluated PTSD symptom severity after clonidine treatment.
All study characteristics are summarized in .
Table 1. Characteristics of the included studies.
3.2. Analysis of the effect of clonidine on sleep quality
Four studies reported outcome data about the effect of clonidine on self-reported sleep quality among adult PTSD patients. Only one of these implemented a placebo control group in a 6 week crossover RCT, on a population of adults with borderline personality disorder and PTSD, reporting an improvement in restorative sleep quality following clonidine treatment at the dosage of 0.45 mg/day in two administrations (0.15 mg in the morning and 0.3 mg at bedtime), although this was not statistically significant (Z-score = −1.83; p = .068). The other three studies did not implement a control group. One prospective observational study on nine PTSD participants reported improved sleep quality in six (66.7%) after 12 months of treatment with clonidine 0.1 mg twice a day plus imipramine. A second study, a non-blinded clinical trial on four PTSD patients, reported an improvement in self-reported sleep quality after 2 weeks of clonidine treatment at 0.2–0.3 mg/day. A third study was a case report of two veteran patients whose sleep quality improved after 2 weeks of treatment with clonidine 0.3 mg/day.
3.3. Analysis of the effect of clonidine on sleep duration
Two studies reported outcome data on sleep duration. One of these was a 6 week crossover RCT on borderline patients with PTSD. The authors measured the latency in sleep, finding a statistically significantly greater subjective improvement following clonidine treatment than on placebo (Z-score = −2.19; p = .028). The other was a non-blind clinical trial on four PTSD patients, finding no change in sleep latency measured with polysomnography over 2 weeks of clonidine treatment.
3.4. Analysis of the effect of clonidine on nightmares
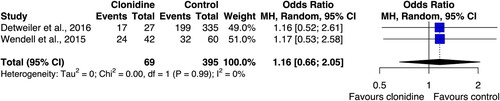
Seven studies investigated the effect of clonidine on PTSD-related nightmares. The outcome was measured through self-reporting in all of the studies, and one study also provided measurement through sleep recordings. Three studies were observational, three were case reports, and one was a non-blinded clinical trial. Two of the observational studies implemented a control group using active comparators (Detweiler et al., Citation2016; Wendell & Maxwell, Citation2015): one used other alpha-adrenergic agents (i.e. prazosin or terazosin) or second generation antipsychotics (i.e. risperidone or quetiapine) or antidepressants (i.e. trazodone or mirtazapine); the other used prazosin only. Both studies were retrospective clinical chart reviews from two veteran clinics in different states in the USA. The dose of clonidine ranged from 0.1 to 0.4 mg/day. The meta-analysis of these studies is displayed in . There was no significant difference between clonidine and the comparators in reducing the PTSD-related nightmares (OR: 1.16, 95% CI: 0.66 to 2.05), while the level of between-study heterogeneity was low (I2 = 0%).
Figure 2. Forest plot of the effect of clonidine on post-traumatic stress disorder (PTSD)-related nightmares. The figure shows the effect size of the two studies included in the meta-analysis and their pooled estimate, with relative 95% confidence intervals (95% CI) (Detweiler et al., Citation2016; Wendell & Maxwell, Citation2015). The confidence intervals for all estimates cross the line of no effect, indicating no statistical significance. The two studies have similar weights in the analysis. The meta-analysis includes 69 participants treated with clonidine and 395 controls; MH = Mantel Haenszel.
Among the studies that did not implement a control group, one was a prospective study reporting an improvement in nightmares in seven out of nine PTSD patients (77.8%), and the other was a non-blind clinical trial with four PTSD patients reporting reductions in PTSD-related nightmares, both self-reported and measured with sleep recordings. The remaining three studies were case reports, corresponding to four patients, all of whom improved after clonidine treatment at a dose ranging from 0.1 to 0.3 mg/day. Notably, one of these studies reported a sustained beneficial effect after 4 months of treatment.
3.5. Analysis of the effect of clonidine on PTSD symptom severity
Five studies evaluated the effect of clonidine on PTSD symptom severity. Each of the studies had a different study design. One was a crossover RCT of clonidine and placebo for borderline patients, of whom a subpopulation had comorbid PTSD. This study found a significantly greater improvement in PTSD symptoms following clonidine treatment than while on placebo in the PTSD-affected subsample (Z-score = −2.67; p = .008). The second study was a retrospective analysis of the electronic medical charts from a veteran clinic in the USA, comparing changes in PTSD symptoms after clonidine treatment at different dosages, ranging from 0.05 to 0.5 mg/day. This study yielded evidence supporting the effectiveness of clonidine on PTSD symptoms measured on the Clinical Global Impression scale for doses greater than 0.05 mg/day. Notably, by comparing the rate of improvement between those treated with clonidine < 0.1 mg/day and those with higher dosages, the odds of improvement was significantly increased for the higher dosage group (OR: 4.90; 95% CI 1.72 to 14.0). However, no significant gain in the treatment effect was obtained for clonidine dosages ≥ 0.25 mg/day over the dose between 0.15 and 0.20 mg/day, as demonstrated by similar rates of patients showing improvements in the two groups (85% and 83%, respectively). A third prospective observational study, without a control group, reported improvement in PTSD symptoms, measured with the Diagnostic and Statistical Manual of Mental Disorders, third edition (DSM-III) PTSD checklist, following more than 12 months of clonidine treatment at 0.1 mg/day, in six out of nine participants (66.7%). The fourth study, a non-blind clinical trial with four PTSD patients, found self-reported improvement in irritability and arousal after 2 weeks of treatment with clonidine, but no effect on mood. Finally, the fifth study was a case report of one patient, showing a marked decrease in PTSD symptoms on the Impact of Event Scale – Revised (IES-R) following clonidine 0.15 mg intravenous infusion (the baseline IES-R score was 45; after 3 weeks it was 4).
The narrative summary of the findings of these studies is reported in .
Table 2. Narrative summary of the studies’ findings.
3.6. Analysis of safety and tolerability of clonidine
Two studies (20%) provided detailed data on the safety and tolerability of clonidine treatment. Both studies were retrospective reviews of medical electronic charts from two different veteran clinics. One study did not report safety and tolerability data in a case–control fashion; however, clonidine showed a 22.8% rate of side effects and half of the participants experiencing side effects discontinued the treatment. The most reported side effects were sleepiness/grogginess (8.9% of participants receiving clonidine), lightheadedness/dizziness (6.3% of participants receiving clonidine), and gastrointestinal disturbances (3.8% of participants receiving clonidine). In the other study, safety data were available for both clonidine and the comparator, which was prazosin. No significant difference in the risk of dropouts was found between clonidine and prazosin (dropout due to any cause OR: 0.88; 95% CI: 0.40 to 1.93; dropout due to adverse events OR: 1.62; 95% CI: 0.66 to 3.98). However, the risk of any adverse effect was higher during clonidine treatment (OR: 2.30; 95% CI: 1.01 to 5.25), although the lower bound of the OR confidence interval was very close to 1. Notably, no deaths or other serious adverse events were reported in either study.
3.7. GRADE of the evidence
A summary of the risk of bias in all 10 trials is reported in , along with an assessment of the quality of the evidence. In the GRADE system, the evidence from RCTs is initially set to high, for observational studies to low, and for case reports to very low, and then several criteria can be used either to downgrade or to upgrade. We downgraded by one level when any of the sources of risk of bias were rated as ‘high’ or two were rated as ‘unclear’. Where a pooled estimate was calculated, we also considered imprecision and downgraded by one level where the 95% CI included the null value. The quality of the evidence was rated low for the crossover RCT, with concerns related to the blinding procedure, and very low for all of the other studies, which failed to provide a control group and to report complete information on the treatment effect and follow-up duration.
Table 3. Risk of bias of the included studies and grading of the evidence.
4. Discussion
This systematic review of the effectiveness of clonidine in the treatment of PTSD-related sleep disorders and other PTSD symptoms indicates that while clonidine may have a beneficial effect on sleep quality and duration, nightmare reduction, and overall PTSD symptom severity in adults with PTSD, the evidence is currently poor. Owing to marked heterogeneity in study design and study quality, and a lack of comparators, no firm conclusions can be drawn and further studies are needed. Moreover, there are not enough data to provide guidance on the optimal clonidine dose for treating PTSD-related sleep disorders. One study focusing on overall PTSD symptom change after clonidine treatment found that doses < 0.05 mg per day were not effective, and doses of 0.25 mg per day did not bring any additional benefits (Burek et al., Citation2021). Furthermore, there is still uncertainty regarding the superiority of clonidine over other PTSD treatments, particularly other anti-adrenergic drugs such as prazosin or terazosin. Our meta-analysis of two observational studies did not identify any significant differences between clonidine and other anti-adrenergic drugs in terms of treating nightmares. However, in their papers, the authors of these studies concluded that clonidine was indeed effective in improving nightmares (Detweiler et al., Citation2016; Wendell & Maxwell, Citation2015). One of the two studies reported a higher frequency of treatment success on clonidine than on prazosin, although the difference was not statistically significant (Detweiler et al., Citation2016). It is worth noting that the results of larger trials of prazosin for PTSD have been negative (Raskind et al., Citation2018; Zhang et al., Citation2020), while pooled estimates from some meta-analyses remained statistically significant with respect to the benefit of prazosin on PTSD symptoms and sleep disturbances (Reist et al., Citation2021; Singh et al., Citation2016).
In contrast to prazosin, clonidine acts mainly centrally, inhibiting noradrenaline release (Katic et al., Citation1972). Evidence suggests that this effect in the locus coeruleus may be related to the hypnotic effects of clonidine (Khan et al., Citation1999), as well as to decreased transmission of pain signals to the spine (Deyama et al., Citation2011) and relieving symptoms of anxiety and low mood (Lebow & Chen, Citation2016). Previous research suggested that people with PTSD display increased responsiveness of the noradrenergic system and altered sensitivity of pre-synaptic alpha-2 receptors, contributing to arousal symptoms such as hypervigilance and insomnia (Southwick et al., Citation1993). Moreover, the increased release of noradrenaline at the prefrontal cortex level has been related to the impairment of executive functions such as reality testing, whereas in the amygdala and hippocampus noradrenaline facilitated the retrieval of past traumatic memories (Southwick et al., Citation1999). These mechanisms may underlie nightmares and flashbacks, where the past memory is experienced as if it were occurring in the present. In that context, clonidine's ability to reduce nightmares and improve sleep may be related to a desensitization of the noradrenergic system via alpha-2 receptor agonism (Southwick et al., Citation1999). In addition, clonidine showed a dose-dependent effect on REM and non-REM sleep (Miyazaki et al., Citation2004), which has been related to improved memory consolidation (Lebow & Chen, Citation2016; Marin et al., Citation2020; Saggu et al., Citation2023; Schulz et al., Citation2002). However, further studies on the specific pathways through which clonidine acts are warranted (Marin et al., Citation2020; Schulz et al., Citation2002).
4.1. Limitations
It is important to acknowledge the limitations of the current review. First, there was a high level of heterogeneity among the studies included in this review, limiting the possibility to perform a meta-analysis of the individual study estimates, and reducing the strength of the conclusions. Secondly, the quality of the evidence was low, with many studies being observational studies or case reports, or having small sample sizes. Therefore, the role of clonidine in treating PTSD-related sleep disorders remains uncertain, underscoring the need for future research and, importantly, rigorous RCTs. Thirdly, there was a lack of reporting on the safety and tolerability of clonidine treatment for PTSD. Potential side effects of anti-adrenergic agents, including the risk of orthostatic hypotension, may limit their use, especially in patients with comorbid medical conditions. Thus, future research should aim to clarify the safety and tolerability of clonidine, with a focus on those at risk of adverse effects. The findings of the review suggest that the higher dosages of clonidine may be associated with a higher incidence of any adverse reaction compared with prazosin. However, the two drugs did not differ significantly in the rate of severe adverse effects and dropout. Finally, to ensure better quality evidence to guide clinical decision making, improved study designs and standardized reporting of outcomes are crucial. Strict adherence to the standards of reporting, such as the Consolidated Standards of Reporting Trials (CONSORT) (Cuschieri, Citation2019), will also enable meta-analyses to be performed, increasing the robustness of the conclusions drawn from these studies.
4.2. Implications for research and practice
To the best of our knowledge, this is the first systematic review of clonidine in PTSD. The findings highlight the need for further research in this area. Large, well-powered high-quality studies are needed to confirm the promising effects of clonidine on sleep quality, nightmares, and PTSD symptom reduction in adults with PTSD. Further investigation is also needed on the safety of clonidine for PTSD.
Given the persistent nature of sleep disorders and nightmares in PTSD, there is a significant medical need for new treatments. Previous trials of prazosin have demonstrated higher effectiveness when used in conjunction with psychotherapy (Raskind et al., Citation2013), and a similar debate pertains to the use of psychedelics [specifically concerning the use of 3,4-methylenedioxymethamphetamine (MDMA)-assisted therapy] in PTSD (Feduccia & Mithoefer, Citation2018; Smith et al., Citation2022). This suggests that future research could investigate the potential benefits of combining clonidine with PTSD-specific psychotherapies such as eye movement desensitization and reprocessing (EMDR) (Cuijpers et al., Citation2020), also bearing in mind preliminary evidence of the impact of clonidine in memory consolidation (Groch et al., Citation2011; Saggu et al., Citation2023).
5. Conclusions
There is a suggestion in the literature that clonidine is beneficial for treating PTSD-related sleep disorders and other PTSD symptoms, but good evidence is lacking. Further research is needed to establish the effectiveness, optimal dosing, and potential side effects of clonidine, and well as the most suitable population for this treatment.
Authors’ contributions
The authors had full access to all the data in this study, and all authors had the final responsibility for making the decision to submit this review for publication.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data included in this systematic review were obtained from publicly available sources, such as published articles and other relevant repositories. Detailed information on the search strategies and inclusion/exclusion criteria is provided in the Method section to facilitate replication and verification of the study findings. The data set generated and the codes for reproducing the analyses can be accessed here: https://github.com/MattiaMarchi/Clondine-for-PTSD/tree/main.
Additional information
Funding
References
- Alao, A., Selvarajah, J., & Razi, S. (2012). The use of clonidine in the treatment of nightmares among patients with co-morbid PTSD and traumatic brain injury. The International Journal of Psychiatry in Medicine, 44(2), 165–169. https://doi.org/10.2190/PM.44.2.g
- Alexander, S., & Kuntz, S. (2012). PTSD-related sleep disturbances: Is there evidence-based treatment? JAAPA: Journal of the American Academy of Physician Assistants, 25, 44–51. https://doi.org/10.1097/01720610-201209000-00008
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. (5th ed.).
- Balduzzi, S., Rücker, G., & Schwarzer, G. (2019). How to perform a meta-analysis with R: A practical tutorial. Evidence Based Mental Health, 22(4), 153–160. https://doi.org/10.1136/ebmental-2019-300117
- Bange, J. S., & Melvin, K. E. (2022). Clonidine use for the treatment of nightmares in posttraumatic stress disorder. Case Reports in Psychiatry, 2022, 5251406. https://doi.org/10.1155/2022/5251406
- Burek, G. A., Waite, M. R., Heslin, K., Liewen, A. K., Yaqub, T. M., & Larsen, S. E. (2021). Low-dose clonidine in veterans with posttraumatic stress disorder. Journal of Psychiatric Research, 137, 480–485. https://doi.org/10.1016/j.jpsychires.2021.03.008
- Cuijpers, P., Veen, S. C. v., Sijbrandij, M., Yoder, W., & Cristea, I. A. (2020). Eye movement desensitization and reprocessing for mental health problems: A systematic review and meta-analysis. Cognitive Behaviour Therapy, 49(3), 165–180. https://doi.org/10.1080/16506073.2019.1703801
- Cuschieri, S. (2019). The CONSORT statement. Saudi Journal of Anaesthesia, 13(5), 27–S30. https://doi.org/10.4103/sja.SJA_559_18
- Davis, L. L., Ward, C., Rasmusson, A., Newell, J. M., Frazier, E., & Southwick, S. M. (2008). A placebo-controlled trial of guanfacine for the treatment of posttraumatic stress disorder in veterans. Psychopharmacology Bulletin, 41, 8–18.
- DerSimonian, R., & Laird, N. (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188. https://doi.org/10.1016/0197-2456(86)90046-2
- Detweiler, M. B., Pagadala, B., Candelario, J., Boyle, J. S., Detweiler, J. G., & Lutgens, B. W. (2016). Treatment of post-traumatic stress disorder nightmares at a Veterans Affairs Medical Center. Journal of Clinical Medicine, 5(12), 117. https://doi.org/10.3390/jcm5120117
- Deyama, S., Ide, S., Kondoh, N., Yamaguchi, T., Yoshioka, M., & Minami, M. (2011). Inhibition of noradrenaline release by clonidine in the ventral bed nucleus of the stria terminalis attenuates pain-induced aversion in rats. Neuropharmacology, 61(1-2), 156–160. https://doi.org/10.1016/j.neuropharm.2011.03.023
- Duval, S., & Tweedie, R. (2000). Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463. https://doi.org/10.1111/j.0006-341X.2000.00455.x
- Feduccia, A. A., & Mithoefer, M. C. (2018). MDMA-assisted psychotherapy for PTSD: Are memory reconsolidation and fear extinction underlying mechanisms? Progress in Neuro-Psychopharmacology and Biological Psychiatry, 84, 221–228. https://doi.org/10.1016/j.pnpbp.2018.03.003
- Groch, S., Wilhelm, I., Diekelmann, S., Sayk, F., Gais, S., & Born, J. (2011). Contribution of norepinephrine to emotional memory consolidation during sleep. Psychoneuroendocrinology, 36(9), 1342–1350. https://doi.org/10.1016/j.psyneuen.2011.03.006
- Hansenne, M., Pitchot, W., & Ansseau, M. (1991). The clonidine test in posttraumatic stress disorder. American Journal of Psychiatry, 148, 810–811. https://doi.org/10.1176/ajp.148.6.810b
- Higgins, J. P. T., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., Savović, J., Schulz, K. F., Weeks, L., & Sterne, J. A. C. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343, d5928. https://doi.org/10.1136/bmj.d5928
- Higgins, J. P. T., & Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine, 21(11), 1539–1558. https://doi.org/10.1002/sim.1186
- Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., & Welch, V. (2021). Cochrane handbook for systematic reviews of interventions, version 6.2. ed. (updated February 2021). Cochrane.
- Jagtiani, A., Gandhi, R., Banga, A., Blacker, J., Joshi, R., Bollu, B., & Kashyap, R. (2024). Alpha-2 agonists in children and adolescents with post-traumatic stress disorder: A systematic review. Cureus, 16, e53009. https://doi.org/10.7759/cureus.53009
- Jang, Y.-J., Choi, H., Han, T. S., Sung, D., Woo, J. Y., Kim, T.-H., & Park, M.-H. (2022). Effectiveness of clonidine in child and adolescent sleep disorders. Psychiatry Investigation, 19(9), 738–747. https://doi.org/10.30773/pi.2022.0117
- Katic, F., Lavery, H., & Lowe, R. D. (1972). The central action of clonidine and its antagonism. British Journal of Pharmacology, 44(4), 779–787. https://doi.org/10.1111/j.1476-5381.1972.tb07315.x
- Khan, Z. P., Ferguson, C. N., & Jones, R. M. (1999). Alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia, 54(2), 146–165. https://doi.org/10.1046/j.1365-2044.1999.00659.x
- Kinzie, J. D., & Leung, P. (1989). Clonidine in Cambodian patients with posttraumatic stress disorder. The Journal of Nervous and Mental Disease, 177(9), 546–550. https://doi.org/10.1097/00005053-198909000-00005
- Kinzie, J. D., Sack, R. L., & Riley, C. M. (1994). The polysomnographic effects of clonidine on sleep disorders in posttraumatic stress disorder: A pilot study with Cambodian patients. The Journal of Nervous and Mental Disease, 182(10), 585–587. https://doi.org/10.1097/00005053-199410000-00010
- Lebow, M. A., & Chen, A. (2016). Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21(4), 450–463. https://doi.org/10.1038/mp.2016.1
- Marin, F. N., Franzen, J. M., Troyner, F., Molina, V. A., Giachero, M., & Bertoglio, L. J. (2020). Taking advantage of fear generalization-associated destabilization to attenuate the underlying memory via reconsolidation intervention. Neuropharmacology, 181, 108338. https://doi.org/10.1016/j.neuropharm.2020.108338
- Miyazaki, S., Uchida, S., Mukai, J., & Nishihara, K. (2004). Clonidine effects on all-night human sleep: Opposite action of low- and medium-dose clonidine on human NREM-REM sleep proportion. Psychiatry and Clinical Neurosciences, 58(2), 138–144. https://doi.org/10.1111/j.1440-1819.2003.01207.x
- NIMH, N.I. of M.H. (2022). Post-Traumatic Stress Disorder (PTSD) [www document]. National Institute of Mental Health (NIMH). Retrieved December 22, 2022, from https://www.nimh.nih.gov/health/statistics/post-traumatic-stress-disorder-ptsd.
- Ouyang, S., Hyatt, J., & Sordo, E. (2015). Use of clonidine in a patient intolerant to prazosin for post-traumatic stress disorder nightmares: A case report. Journal of Pharmacy Practice, 28, 374–375. https://doi.org/10.1177/0897190015582204
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71. https://doi.org/10.1136/bmj.n71
- Raskind, M. A., Peskind, E. R., Chow, B., Harris, C., Davis-Karim, A., Holmes, H. A., Hart, K. L., McFall, M., Mellman, T. A., Reist, C., Romesser, J., Rosenheck, R., Shih, M.-C., Stein, M. B., Swift, R., Gleason, T., Lu, Y., & Huang, G. D. (2018). Trial of prazosin for post-traumatic stress disorder in military veterans. New England Journal of Medicine, 378(6), 507–517. https://doi.org/10.1056/NEJMoa1507598
- Raskind, M. A., Peterson, K., Williams, T., Hoff, D. J., Hart, K., Holmes, H., Homas, D., Hill, J., Daniels, C., Calohan, J., Millard, S. P., Rohde, K., O’Connell, J., Pritzl, D., Feiszli, K., Petrie, E. C., Gross, C., Mayer, C. L., Freed, M. C., … Peskind, E. R. (2013). A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. American Journal of Psychiatry, 170(9), 1003–1010. https://doi.org/10.1176/appi.ajp.2013.12081133
- Reist, C., Streja, E., Tang, C. C., Shapiro, B., Mintz, J., & Hollifield, M. (2021). Prazosin for treatment of post-traumatic stress disorder: A systematic review and meta-analysis. CNS Spectrums, 26(4), 338–344. https://doi.org/10.1017/S1092852920001121
- Rosellini, A. J., Liu, H., Petukhova, M. V., Sampson, N. A., Aguilar-Gaxiola, S., Alonso, J., Borges, G., Bruffaerts, R., Bromet, E. J., de Girolamo, G., de Jonge, P., Fayyad, J., Florescu, S., Gureje, O., Haro, J. M., Hinkov, H., Karam, E. G., Kawakami, N., Koenen, K. C., … Kessler, R. C. (2018). Recovery from DSM-IV post-traumatic stress disorder in the WHO World Mental Health surveys. Psychological Medicine, 48(3), 437–450. https://doi.org/10.1017/S0033291717001817
- RStudio Team. (2021). RStudio: Integrated development environment for R.
- Saggu, S., Chen, Y., Cottingham, C., Rehman, H., Wang, H., Zhang, S., Augelli-Szafran, C., Lu, S., Lambert, N., Jiao, K., Lu, X.-Y., & Wang, Q. (2023). Activation of a novel α2AAR-spinophilin-cofilin axis determines the effect of α2 adrenergic drugs on fear memory reconsolidation. Molecular Psychiatry, 28(2), 588–600. https://doi.org/10.1038/s41380-022-01851-w
- Schulz, B., Fendt, M., & Schnitzler, H.-U. (2002). Clonidine injections into the lateral nucleus of the amygdala block acquisition and expression of fear-potentiated startle. European Journal of Neuroscience, 15(1), 151–157. https://doi.org/10.1046/j.0953-816x.2001.01831.x
- Schünemann, H., Brożek, J., Guyatt, G., & Oxman, A. (2013). GRADE handbook for grading quality of evidence and strength of recommendations. [www document]. Retrieved July 6, 2022, from https://gdt.gradepro.org/app/handbook/handbook.html
- Schwarzer, G. (2021). Meta: General package for meta-analysis.
- Singh, B., Hughes, A. J., Mehta, G., Erwin, P. J., & Parsaik, A. K. (2016). Efficacy of prazosin in posttraumatic stress disorder: A systematic review and meta-analysis. The Primary Care Companion for CNS Disorders, 18(6). https://doi.org/10.4088/PCC.16l01978
- Smith, K. W., Sicignano, D. J., Hernandez, A. V., & White, C. M. (2022). MDMA-Assisted Psychotherapy for treatment of posttraumatic stress disorder: A systematic review with meta-analysis. The Journal of Clinical Pharmacology, 62(4), 463–471. https://doi.org/10.1002/jcph.1995
- Southwick, S. M., Bremner, J. D., Rasmusson, A., Morgan, C. A., Arnsten, A., & Charney, D. S. (1999). Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biological Psychiatry, 46(9), 1192–1204. https://doi.org/10.1016/S0006-3223(99)00219-X
- Southwick, S. M., Krystal, J. H., Morgan, C. A., Johnson, D., Nagy, L. M., Nicolaou, A., Heninger, G. R., & Charney, D. S. (1993). Abnormal noradrenergic function in posttraumatic stress disorder. Archives of General Psychiatry, 50(4), 266–274. https://doi.org/10.1001/archpsyc.1993.01820160036003
- Steenen, S. A., van Wijk, A. J., van der Heijden, G. J., van Westrhenen, R., de Lange, J., & de Jongh, A. (2016). Propranolol for the treatment of anxiety disorders: Systematic review and meta-analysis. Journal of Psychopharmacology, 30(2), 128–139. https://doi.org/10.1177/0269881115612236
- Sterne, J. A. C., Sutton, A. J., Ioannidis, J. P. A., Terrin, N., Jones, D. R., Lau, J., Carpenter, J., Rücker, G., Harbord, R. M., Schmid, C. H., Tetzlaff, J., Deeks, J. J., Peters, J., Macaskill, P., Schwarzer, G., Duval, S., Altman, D. G., Moher, D., & Higgins, J. P. T. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ, 343, d4002. https://doi.org/10.1136/bmj.d4002
- Wendell, K. R., & Maxwell, M. L. (2015). Evaluation of clonidine and prazosin for the treatment of nighttime posttraumatic stress disorder symptoms. Federal Practitioner, 32, 8–14.
- WHO. (2004). ICD-10 : international statistical classification of diseases and related health problems: tenth revision.
- Wingenfeld, K., Whooley, M. A., Neylan, T. C., Otte, C., & Cohen, B. E. (2015). Effect of current and lifetime posttraumatic stress disorder on 24-hour urinary catecholamines and cortisol: Results from the Mind Your Heart Study. Psychoneuroendocrinology, 52, 83–91. https://doi.org/10.1016/j.psyneuen.2014.10.023
- Zhang, Y., Ren, R., Sanford, L. D., Yang, L., Ni, Y., Zhou, J., Zhang, J., Wing, Y.-K., Shi, J., Lu, L., & Tang, X. (2020). The effects of prazosin on sleep disturbances in post-traumatic stress disorder: A systematic review and meta-analysis. Sleep Medicine, 67, 225–231. https://doi.org/10.1016/j.sleep.2019.06.010
- Ziegenhorn, A. A., Roepke, S., Schommer, N. C., Merkl, A., Danker-Hopfe, H., Perschel, F. H., Heuser, I., Anghelescu, I. G., & Lammers, C. H. (2009). Clonidine improves hyperarousal in borderline personality disorder with or without comorbid posttraumatic stress disorder: A randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychopharmacology, 29(2), 170–173. https://doi.org/10.1097/JCP.0b013e31819a4bae
