ABSTRACT
Background: Oculomotor movements have been shown to aid in the retrieval of episodic memories, serving as sensory cues that engage frontoparietal brain regions to reconstruct visuospatial details of a memory. Frontoparietal brain regions not only are involved in oculomotion, but also mediate, in part, the retrieval of autobiographical episodic memories and assist in emotion regulation.
Objective: We sought to investigate how oculomotion influences retrieval of traumatic memories by examining patterns of frontoparietal brain activation during autobiographical memory retrieval in post-traumatic stress disorder (PTSD) and in healthy controls.
Method: Thirty-nine participants (controls, n = 19; PTSD, n = 20) recollected both neutral and traumatic/stressful autobiographical memories while cued simultaneously by horizontal and vertical oculomotor stimuli. The frontal (FEF) and supplementary (SEF) eye fields were used as seed regions for psychophysiological interaction analyses in SPM12.
Results: As compared to controls, upon retrieval of a traumatic/stressful memory while also performing simultaneous horizontal eye movements, PTSD showed: i) increased SEF and FEF connectivity with the right dorsolateral prefrontal cortex, ii) increased SEF connectivity with the right dorsomedial prefrontal cortex, and iii) increased SEF connectivity with the right anterior insula. By contrast, as compared to PTSD, upon retrieval of a traumatic/stressful memory while also performing simultaneous horizontal eye movements, controls showed: i) increased FEF connectivity with the right posterior insula and ii) increased SEF connectivity with the precuneus.
Conclusions: These findings provide a neurobiological account for how oculomotion may influence the frontoparietal cortical representation of traumatic memories. Implications for eye movement desensitization and reprocessing are discussed.
HIGHLIGHTS
• Traumatic/stressful memory retrieval while performing horizontal eye movements engages frontoparietal regions involved in autobiographical memory retrieval and emotion regulation that show further connectivity with the right frontal and supplementary eye fields.• Dissociation may compromise the oculomotor frontoparietal network’s ability to recruit the right dorsolateral prefrontal cortex for use in top–down emotion regulation.• Oculomotion may influence the frontoparietal cortical representation of traumatic memories.• These findings may have implications for eye movement desensitization and reprocessing therapies.
Antecedentes: Se ha visto que los movimientos óculomotores ayudan a la recuperación de memorias episódicas, sirviendo como señales sensoriales que envuelven las regiones cerebrales frontoparietales para reconstruir detalles visuoespaciales. Las regiones cerebrales frontoparietales no solo están involucradas críticamente en el movimiento ocular, pero ellos también median, en parte, la recuperación de la memoria episódica autobiográfica y ayudan en la regulación emocional.
Objetivo: Buscamos investigar cómo el movimiento ocular influye en la recuperación de la memoria traumática al examinar patrones de activación cerebral frontoparietales durante la recuperación de la memoria autobiográfica en trastorno de estrés postraumático (TEPT) y controles sanos.
Método: Se recolectaron en treinta y nueve participantes (controles, n= 19; TEPT, n=20): (i) neutral; y (ii) memorias autobiográficas traumáticas/estresantes mientras se señalaba simultáneamente por estímulos oculomotores horizontales y verticales. Se usaron los campos oculares frontal (FEF por sus siglas en inglés) y suplementario (SEF por sus siglas en inglés) como regiones bases para el análisis de interacción psico fisiológica en SPM12.
Resultados: En comparación con los controles, al recuperar una memoria traumática/estresante mientras se realizan simultáneamente movimientos oculares horizontales, el TEPT mostró: (i) SEF aumentado y conectividad FEF con la corteza prefrontal dorsolateral derecha, (ii) conectividad SEF aumentada con la corteza prefrontal dorsomedial derecha y (iii) conectividad SEF aumentada con la ínsula anterior derecha. En contraste, al compararlo con TEPT, al recuperar una memoria traumática/estresante mientras se realizan simultáneamente movimientos oculares horizontales, los controles mostraron: (i) conectividad FEF aumentada con la región posterior derecha de la ínsula y (ii) conectividad SEF aumentada con el precuneo
Conclusiones: Estos hallazgos proveen un base neurobiológica de cómo los movimientos oculares pueden influir en la representación cortical frontoparietal de las memorias traumáticas. Se discuten las implicaciones del reprocesamiento y desensibilización por movimientos oculares.
背景:动眼神经运动已被证明有助于恢复情景记忆,作为感觉线索使前额脑区能够重建记忆的视觉空间细节。前额脑区不仅涉及眼动,而且还部分中介了自传情景记忆的提取,并协助情绪调节。
目的:我们通过考察在患有创伤后应激障碍(PTSD)和健康对照被试中自传记忆提取过程中的额顶脑区激活模式,探讨眼动如何影响创伤记忆的恢复。
方法:39名被试(对照组,n = 19; PTSD,n = 20)对中性和创伤/应激的自传记忆进行回忆,同时有刺激提示执行水平和垂直的眼动。额叶眼区(FEF)和辅助眼区(SEF)用作SPM12中心理生理相互分析的种子(seed)区。
结果:与对照组相比,在提取创伤/应激记忆同时进行水平眼球运动时,PTSD患者显示:i)右背外侧前额叶皮质(right dorsolateral prefrontal cortex)与SEF和FEF连通性增加,ii)右背内侧前额叶皮质(right dorsomedial prefrontal cortex)与SEF连通性增加,和 iii)右前脑岛和SEF连通性增加。与PTSD相比,对照组在检索创伤/应激记忆同时也执行水平眼球运动时,则显示出:i)与右后脑岛和FEF连通性增加,和 ii)与楔前叶的SEF连通性增加。
结论:这些发现提供了一个神经生物学上关于眼动可能如何影响创伤记忆的额顶皮质表现的解释,并讨论了其对眼球运动脱敏和再加工的启示。
1. Introduction
In post-traumatic stress disorder (PTSD), traumatic memories tend to be re-experienced as flashbacks of sensory elements of the memory (images, sounds, smells, or physical sensations) that are accompanied by intense negative affect (Brewin, Huntley, & Whalley, Citation2012; Ehlers, Hackmann, & Michael, Citation2004; van der Kolk & Fisler, Citation1995). To reduce frequent re-experiencing of traumatic memories and their associated negative affect in PTSD, therapeutic strategies such as eye movement desensitization and reprocessing (EMDR) use eye movements in an attempt to facilitate the reprocessing of traumatic memories (Shapiro, Citation1989; van der Kolk et al., Citation2007). Eye movements, i.e. oculomotion, have been shown to reduce not only sympathetic activity upon retrieval of a traumatic memory (Barrowcliff, Gray, Freeman, & MacCulloch, Citation2004), but also to diminish intrusive memories and the vividness associated with them (Andrade, Kavanagh, & Baddeley, Citation1997; Barrowcliff et al., Citation2004; Cotter, Meysner, & Lee, Citation2017). To date, however, little is known about the possible neurobiological underpinnings of these effects. In this pilot study, we examine specifically the relation between oculomotion and episodic memory by investigating patterns of brain activation in healthy controls and individuals with PTSD during retrieval of traumatic/stressful and neutral memories while performing simultaneously contrasting patterns of oculomotor movements (i.e. saccadic, smooth pursuit, stationary dot fixation). Here, we propose several key neural networks and brain regions central not only to episodic memory retrieval, but also to oculomotion and to accompanying emotional regulation strategies, that may heighten reprocessing of traumatic memories during EMDR.
1.1. Dorsal attentional network
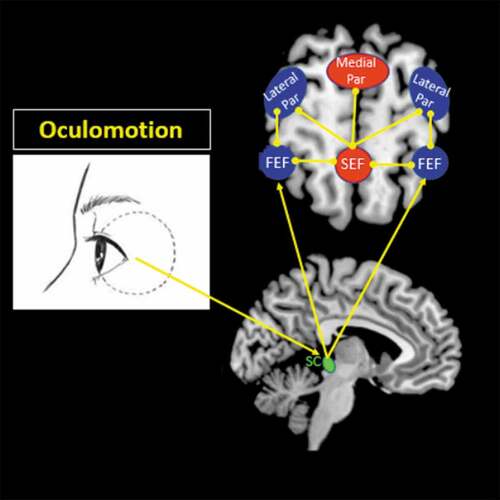
The dorsal attentional network consists of dorsal frontoparietal brain regions, including the frontal eye field (FEF), the supplementary eye field (SEF) and the intraparietal sulcus. In conjunction with other sensory modalities, including auditory, vestibular, and tactile stimuli, eye movements are a key component of the dorsal attentional network, and are critical for probing extrapersonal space to inform one’s internal perspective of the world (Corbetta & Shulman, Citation2002). Sensory information obtained from oculomotion travels to the superior colliculus in the midbrain (Vernet, Quentin, Chanes, Mitsumasu, & Valero-Cabré, Citation2014). The superior colliculus is then responsible for projections to the FEF in the lateral frontal lobe, which aid in visuospatial attentional processes and visuomotor movements (see for details) (Grosbras & Paus, Citation2002; Vernet et al., Citation2014). The FEF, in turn, projects to the lateral posterior parietal cortex, which is involved in perceiving spatial information pertaining to one’s viewer-centred egocentric space, and thus helps to identify self-location and mental navigation through one’s surroundings () (Burgess, Citation2006; Szczepanski, Pinsk, Douglas, Kastner, & Saalmann, Citation2013). In addition, the FEFs aid in the evaluation of the environment from a spatial perspective through interactions with the SEF, which projects to both the lateral and medial posterior parietal cortices to inform both one’s viewer (egocentric) and observer (allocentric) perspective () (Szczepanski et al., Citation2013).
Figure 1. Oculomotor network. Visuospatial sensory information obtained from oculomotion travels to the superior colliculus in the midbrain via cranial nerves III, IV, and VI. The superior colliculus can project visuospatial afferents to the frontal eye field (FEF) to engage the dorsal visual stream, which is a functional component of the dorsal attentional network that helps guide one’s visuospatial processing of the external environment. The FEF functionally connects with the lateral posterior parietal cortex (Par), where one can process visuospatial details related to one’s viewer-centred egocentric perspective (i.e. identifying one’s self-location). The FEF also interacts with the supplementary eye field (SEF), which maintains connections with both the lateral and medial parietal cortices. The SEF, through its connections with the parietal cortex, can process visuospatial details from both an egocentric and observer-centred allocentric perspective, as it can identify one’s self-location based on identifying objects or external locations in the environment. The eye clipart image was retrieved and adapted from a free public domain (clker.com, Rolera LLC).
Although eye movements are critical to gathering current visuospatial information required for the optimal functioning of attentional processes that guide working memory (Beck & Hollingworth, Citation2017; Pearson & Sahraie, Citation2003; Shipstead, Harrison, & Engle, Citation2012), short-term working memory interacts further with long-term episodic memory such that previous experiences provide context to salient stimuli (Baddeley & Hitch, Citation1974; Eriksson, Vogel, Lansner, Bergström, & Nyberg, Citation2015; Souza & Oberauer, Citation2017; Uncapher & Wagner, Citation2009). Taken together, these findings suggest that salient visuospatial sensory information, guided, in part, by oculomotion, informs perspective on the relevance of incoming sensory input. Previous studies have indicated that eye movements performed simultaneously with episodic memory retrieval tax working memory resources; such interference may reduce the capacity to engage in other higher order tasks reliant upon executive functioning (Maxfield, Melnyk, & Hayman, Citation2008; Op den Kelder, Van den Akker, Geurts, Lindauer, & Overbeek, Citation2018).
1.2. Frontoparietal executive control network
This dynamic relationship between working memory and long-term episodic memory depends critically on the ability to use salient sensory information to guide retrieval of episodic autobiographical memories (Baddeley, Citation2010; Burianova, McIntosh, & Grady, Citation2010). Dixon et al. (Citation2018) describe a frontoparietal executive control network comprised of two functional subdivisions involved in sensorimotor and introspective processes, respectively. Here, the sensorimotor frontoparietal subdivision is thought to orient, via oculomotor movements, to salient multisensory cues in the external environment (Corbetta & Shulman, Citation2002), thus assisting in mapping sensory information in the environment through visual search. This subdivision overlaps with neural regions implicated in the dorsal attentional network, including the FEF, SEF, and the right inferior parietal lobule. By contrast, the introspective frontoparietal subdivision is thought to mediate internally based mental thoughts and emotion processing and overlaps with areas involved in autobiographical memory and self-referential processing, including the medial prefrontal cortex. These functional subdivisions of a larger frontoparietal cognitive control network are thought to work in tandem to carry out higher order cognitive tasks, including emotion regulation.
1.3. The role of oculomotion in integration of autobiographical memories
Commonly, autobiographical memories are appraised on a continuum of positive to negative valence, a process associated with changes in physiological homeostasis in response to internal and/or external reminders of the memory (Morawetz, Bode, Derntl, & Heekeren, Citation2017; Picó-Pérez, Radua, Steward, Menchón, & Soriano-Mas, Citation2017). Here, individuals may modulate emotional appraisal of a negative memory by introducing emotion regulation strategies, where one attempts to adjust the internal affective representation of a subjective memory (Morawetz et al., Citation2017; Picó-Pérez et al., Citation2017; Zilverstand, Parvaz, & Goldstein, Citation2017). Critically, in traumatic memory, reappraisal strategies target the down-regulation of negative affective representations associated with the memory in an attempt to reduce its emotional impact. This conscious top–down emotion regulation is thought to engage a frontoparietal network involving brain regions similar to those implicated in oculomotion and in autobiographical memory, including the right dorsolateral and ventrolateral prefrontal cortex, which may work in tandem to attenuate the intense negative affect underlying traumatic memories (Zilverstand et al., Citation2017).
Previous studies have demonstrated that the vividness of traumatic memories is reduced when memory retrieval is performed simultaneous to horizontal eye movements (Andrade et al., Citation1997; Barrowcliff et al., Citation2004; Littel, van Schie, & van Den Hout, Citation2017; Thomaes, Engelhard, Sijbrandij, Cath, & Van den Heuvel, Citation2016). However, no study has sought to investigate the neural underpinnings of this effect, where significant overlap is observed in the frontoparietal networks believed to be involved in oculomotion, autobiographical memory, and emotional regulation. Identification of frontal and parietal neural regions common to these processes may assist in delineating the neurobiological mechanisms contributing to traumatic memory reprocessing using eye movements and provide an organizing framework to identify neural targets for EMDR.
Accordingly, we sought to identify the neural architecture associated with traumatic/stressful autobiographical memory retrieval during simultaneous performance of horizontal smooth pursuit or saccadic eye movements in patients with PTSD and in healthy controls. Specifically, we hypothesized that (1) traumatic/stressful memory retrieval during performance of horizontal eye movements would engage the two functional subdivisions of the larger frontoparietal executive control network proposed by Dixon et al. (Citation2018) and thought to be involved in sensorimotor and introspective processing. We also hypothesized that (2) (a) oculomotor eye movements would activate sensorimotor brain regions in the dorsal attentional network, including the FEF and SEF; (b) activation of the dorsal attention network in conjunction with traumatic/stressful autobiographical memory retrieval would recruit frontal and parietal brain regions involved in introspective processing; and (c) dual sensorimotor and introspective processing would initialize a larger frontoparietal executive control network that recruits areas involved in higher order cognitive demands, including emotion regulation. In keeping with our own work (Lanius et al., Citation2004), we hypothesized further that (3) individuals with PTSD would show group differences during traumatic memory retrieval as compared to those without PTSD. Furthermore, we hypothesized that (4) in individuals with PTSD, as compared to controls, activation of the dorsal attentional network through eye movements would enhance the recruitment of regions involved in self-referential processing and emotion regulation, thus laying a foundation for understanding of the neurobiological mechanisms underlying EMDR.
2. Methods
2.1. Clinical and demographic information
Thirty-nine participants participated in the present study, including 20 patients with PTSD and 19 age- and gender-matched healthy controls. Recruitment for the study took place during 2014–2016, via referrals from family physicians, mental health professionals, psychology/psychiatric clinics, community programs for traumatic stress, and posters/advertisements within the London, Ontario community.
Inclusion criteria for the study included a PTSD diagnosis based on the Clinician-Administered PTSD Scale (CAPS), versions IV (Blake et al., Citation1995) (n = 26, PTSD diagnosis if score > 50) and 5 (Weathers et al., Citation2013) (n = 13, different scoring system with no definitive cut-off). For all participants, a Structured Clinical Interview for DSM-IV Axis I disorders (SCID) (First, Spitzer, Gibbon, & Williams, Citation2002) was administered, along with a battery of questionnaires assessing trait psychological symptoms, including the Beck Depression Inventory (BDI) (Beck, Guth, Steer, & Ball, Citation1997), Child Trauma Questionnaire (CTQ) (Bernstein & Fink, Citation1998) (94% of participants had a history of childhood trauma, i.e. they scored above the ‘none/minimal’ threshold for any trauma category), and the Multiscale Dissociation Inventory (MDI) (Briere, Weathers, & Runtz, Citation2005). In addition, during the scan, the Responses to Script-Driven Imagery Scale (RSDI) (Hopper, Frewen, Sack, Lanius, & van der Kolk, Citation2007), State–Trait Anxiety Inventory (STAI) (Spielberger, Citation2010), and Clinician-Administered Dissociative States Scale (CADSS) (Bremner et al., Citation1998) were used to assess state-based psychological responses. The clinical and demographic characteristics of the study sample are detailed in .
Table 1. Clinical and demographic information.
Participants were excluded if 3.0 T scanner safety regulations were violated, including the presence of metal implants, and/or if participants had experienced previous head trauma associated with a loss of consciousness, significant untreated medical illness, and/or pervasive developmental disorders. Additional exclusion criteria for PTSD patients included a current or past history of bipolar or psychotic disorders, and/or alcohol/substance dependency or abuse for at least 6 months prior to partaking in the study. Control participants were ineligible if lifetime criteria were met for any Axis I psychiatric disorder from the SCID assessment. If eligible, participants provided written informed consent to participate in the study. No eligible participants were subsequently excluded from study nor did any of the participants drop out over the course of the study. All scanning was conducted in London, Ontario at Robarts Research Institute’s Centre for Functional Metabolic Mapping. The study was approved by the Research Ethics Board at Western University of Canada.
2.2. Functional magnetic resonance imaging protocol
Whole-brain functional magnetic resonance imaging (fMRI) data were collected in a 3.0 T scanner (Magnetom Tim Trio; Siemens Medical Solutions, Erlangen, Germany) with a 32-channel phased-array head coil. Blood oxygen level-dependent (BOLD) fMRI data were collected using a manufacturer’s standard gradient-echo planar imaging (EPI) pulse sequence (single-shot, blipped EPI) with an interleaved slice acquisition with the following specifications: time resolution (TR) = 3000 ms, echo time (TE) = 20 ms, voxel size = 2 × 2 × 2 mm3, field of view (FOV) = 192 × 192 × 128 mm3 (94 × 94 matrix, 64 contiguous slices), and flip angle (FA) = 90°. High-resolution T1-weighted anatomical images were also collected (MPRage: 192 slices, voxel size = 1 × 1 × 1 mm3).
2.3. Eye movement scan procedure
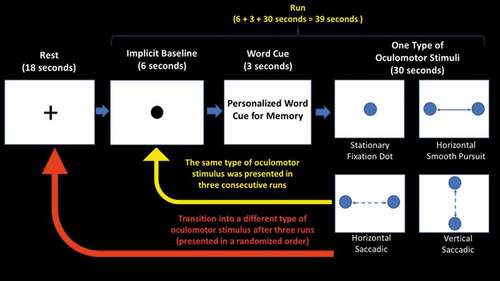
All participants were asked to retrieve both neutral and traumatic/stressful autobiographical memories via a single personalized word cue associated with each memory (chosen by participants before the study; whereas PTSD participants retrieved traumatic memories, controls retrieved their most stressful memories) while following a moving dot to guide eye movements across the screen (). Participants were instructed to select a word representing a traumatic/stressful memory that would be distressing upon retrieval, but not to an extent that a particular memory would inhibit a participant’s capacity to partake in the study. All participants were video recorded throughout the experiment and the recordings were visually inspected to ensure they were performing eye movements while in the scanner. In total, there were three conditions, each lasting 13 minutes, conducted in the following order: no memory retrieval, neutral memory retrieval, and traumatic/stressful memory retrieval. Each condition consisted of 12 runs, separated into four blocks that were presented in a randomized order. For each block, one of four types of oculomotor stimuli (a stationary fixation dot, a horizontal smooth pursuit, a horizontal saccadic pursuit, or a vertical saccadic pursuit) was presented in three consecutive runs. Each run lasted 39 seconds, which included: (i) collection of an implicit baseline measure (6 seconds); (ii) display of a personalized word cue for neutral or traumatic/stressful memory retrieval (e.g. “comb” for a neutral memory, “knife” for a traumatic memory) (3 seconds); and (iii) presentation of an oculomotor stimulus (30 seconds). First, a black central stationary dot was displayed for 6 seconds to obtain an implicit baseline measure (explained below) (Bremner et al., Citation1999; Lanius et al., Citation2004). After the implicit baseline was collected, participants were instructed to retrieve autobiographical memories after reading a personalized word cue displayed on the screen for 3 seconds (replaced with a ‘+’ symbol in the no memory retrieval condition). Subsequently, the oculomotor stimulus, coloured circles to guide eye movements across the screen, was presented for 30 seconds while participants continued to engage with the memory. After three consecutive runs involving the same oculomotor stimulus, participants were asked to rate the severity of PTSD symptoms they experienced during memory retrieval with the specific adjunctive oculomotor stimulus, including emotional intensity, numbing, dissociation, re-experiencing, and vividness of memory. Afterwards, an 18-second rest interval using a black stationary fixation ‘+’ led to a transition into a new block that presented a different oculomotor stimulus. This process was repeated four times to evaluate each type of oculomotor stimulus. Both before the experiment and after each condition (no memory retrieval, neutral memory retrieval, and traumatic/stressful memory retrieval), participants were asked to rate the severity of re-experiencing, avoidance, and dissociative symptoms experienced in the scanner based on the RSDI () (Hopper et al., Citation2007). In addition, participants were asked to report intensity ratings (on a Likert scale of 1 to 6) of different negative emotions experienced after each memory retrieval condition (). After the scan, a brief interview was administered to assess whether participants were successful in retrieving the memories during the scanning protocol.
Figure 2. Experimental paradigm. All participants were asked to retrieve both neutral and traumatic autobiographical memories via a personalized word cue associated with each memory, while following a moving dot to guide eye movements across the screen. In total, there were three conditions, each lasting 13 minutes, conducted in the following order: no memory retrieval, neutral memory retrieval, and traumatic memory retrieval. Each condition consisted of 12 runs, separated into four blocks to present each type of oculomotor stimulus in three consecutive runs (stationary fixation dot, a horizontal smooth pursuit, a horizontal saccadic pursuit, and a vertical saccadic pursuit). Each run lasted (6 + 3 + 30) 39 seconds, where a black central stationary dot was displayed for 6 seconds to obtain an implicit baseline measure, after which participants were instructed to retrieve autobiographical memories while reading a single personalized word cue displayed on the screen for 3 seconds (replaced with a ‘+’ symbol in the no memory retrieval condition). Immediately afterwards, participants were asked to continue retrieving the memory while 30 seconds of one type of oculomotor stimulus was presented using coloured circles to guide eye movements across the screen. After three consecutive runs using the same type of oculomotor stimulus, participants were asked to rate the severity of post-traumatic stress disorder (PTSD) symptoms they experienced during memory retrieval with the specific adjunctive oculomotor stimulus, including emotional intensity, numbing, dissociation, re-experiencing, and vividness of memory. Afterwards, an 18-second rest interval using a black stationary fixation ‘+’ led to a transition into a new type of oculomotion. This process was repeated four times to evaluate the effects of each type of oculomotor stimulus.
2.3.1. Implicit baseline
The implicit baseline measure is a quantitative estimation of a null period during the experiment where the participant is not engaged in task-related activities, and it can also be used as a reset period to obtain a baseline measure between tasks (Bremner et al., Citation1999; Lanius et al., Citation2004). In the present study, the implicit baseline measure was a black stationary fixation dot displayed for 6 seconds before the oculomotor stimulus was presented within each run.
2.4. fMRI preprocessing
Image preprocessing and statistical analyses were performed using Statistical Parametric Mapping software (SPM12; Wellcome Trust Centre for Neuroimaging) within MATLAB 8.6 (R2015b; MathWorks). The functional images collected for each condition were realigned to the first volume of the scan. The images were then normalized to a Montréal Neurological Institute (MNI) anatomical template and spatially smoothed to a Gaussian kernel of 8 mm full-width half-maximum (FWHM).
2.5. fMRI statistical analysis
Voxel-wise general linear models were used to investigate activation patterns during each condition. For each subject, a BOLD contrast map was developed for each type of oculomotor stimulus within each memory retrieval condition (e.g. no memory horizontal smooth pursuit, traumatic/stressful memory horizontal saccadic pursuit). ART (version 2015–10; Gabrieli Lab, McGovern Institute) motion parameters were included as covariates in all within-subject analyses for all statistical analyses (including subtraction analysis and subsequent psychophysiological interactions). A 2 × 3 × 4 full-factorial subtraction analysis was employed to examine interaction effects of group (PTSD, controls), memory retrieval (no memory, neutral memory, and stressful/traumatic memory), and oculomotion (horizontal smooth pursuit, horizontal saccadic pursuit, vertical saccadic pursuit, and stationary dot fixation) versus the implicit baseline as described above; thus, all results obtained from this analysis are based on comparisons to no oculomotor stimuli. Results were reported at a family-wise error (FWE) voxel-wise whole-brain corrected threshold of p < 0.05, with a cluster extent threshold of k = 10, in accordance with Eklund, Nichols, and Knutsson (Citation2016). To examine our primary question concerning the correlation of oculomotion with frontal and parietal neural correlates involved in autobiographical memory retrieval and top–down emotion regulation, the right FEF and SEF were observed as peak areas of activation across all factors and were therefore selected as seed regions in psychophysiological interaction (PPI) analyses to explore their functional connectivity patterns with the whole brain.
A region-of-interest (ROI) approach was used to investigate group comparisons in the PPI analyses between seed regions and four brain regions, identified a priori using coordinates from various meta-analyses employing activation likelihood estimation methodology: (1) the right dorsomedial prefrontal cortex [x: 10, y: 40, z: 52], associated with mentalization of autobiographical memories (Andrews-Hanna, Saxe, & Yarkoni, Citation2014); (2) the right dorsolateral prefrontal cortex [x: 40, y: 23, z: 44] and (3) the right anterior insula [x: 44, y: 16, z: 4], linked to cognitive reappraisal emotion regulation strategies (Morawetz et al., Citation2017); and (4) the right posterior insula [x: –35, y: –13, z: 9], associated with interoception based on insular functional mapping (Kurth, Zilles, Fox, Laird, & Eickhoff, Citation2010). A 10 mm sphere was created around the coordinates listed above using PickAtlas software (Maldjian, Laurienti, Kraft, & Burdette, Citation2003) and was used in an ROI-correction analysis for clusters that did not survive the FWE voxel-wise threshold of p < 0.05, k = 10. Significant clusters identified in the ROI analyses were adjusted for multiple comparisons at a voxel-wise FWE-corrected threshold set at p ≤ 0.0125, k = 10, calculated by dividing the original pFWE < 0.05 threshold by 4 to account for each ROI used. Finally, we correlated neuroimaging data from the PPI analyses with self-reported clinical state symptom scores collected in the scanner (RSDI, STAI, CADSS) and trait symptom scores collected before the experiment (MDI, BDI, CAPS).
3. Results
3.1. fMRI statistical analyses
The peak coordinates of activation in the omnibus analysis of variance (ANOVA) test included the right FEF [x: 46, y: 0, z: 56] and the right SEF [x: 2, y: 2, z: 62], which were subsequently used as seed regions for PPIs to explore their functional connectivity with the frontal and parietal brain regions listed as ROIs above (right dorsomedial prefrontal cortex, right dorsolateral prefrontal cortex, right anterior insula, and right posterior insula). Additional cortical regions that were activated in the ANOVA effect, interactions, and main factor effects are listed in the supplementary material. Although we present inclusively all post-hoc results from the ANOVA in the supplementary material, including all measures from each factor, we discuss here horizontal saccadic and smooth pursuit eye movements in the context of traumatic/stressful memory only, as these are of primary interest for studying the underpinnings of emotion regulation using horizontal eye movements during EMDR. All results are based on comparisons to the implicit baseline measure (without oculomotor stimuli).
3.2. Psychophysiological interactions
3.2.1. Right FEF
Significant regions in the PPI omnibus ANOVA test are listed in the supplementary material. There were no significant two-way or three-way interactions observed between the memory, oculomotion, or participant group factors, and no significant clusters within the main effect for each factor. Post-hoc one-sample t-tests within each variable did not yield significant connectivity with the frontal and parietal brain regions studied.
Between-group comparisons
(i) Between participant group, within motion, between memory
As compared to the PTSD group, the healthy control group showed increased right FEF connectivity with the right posterior insula during horizontal smooth pursuit eye movements in the traumatic/stressful memory retrieval versus neutral memory retrieval condition ( (A), )).
(ii)Between participant group, between motion, between memory
Table 2. Right frontal (FEF) and supplementary eye field (SEF) psychophysiological interaction post-hoc two-sample t-tests and correlations with clinical dissociative symptoms.
Figure 3. Explorative functional connectivity analyses (psychophysiological interaction) of (A) the right frontal eye field [FEF (x: 46, y: 0, z: 56)] and (B) the right supplementary eye field [SEF (x: 2, y: 2, z: 62)] seed regions during the traumatic memory retrieval condition. (A) During retrieval of a traumatic/stressful memory, as compared to the post-traumatic stress disorder (PTSD) patient group, healthy controls demonstrated increased right FEF connectivity with the right posterior insula with simultaneous smooth pursuit eye movements. In contrast, as compared to healthy controls, PTSD patients demonstrated increased right FEF connectivity with the right dorsolateral prefrontal cortex during retrieval of a traumatic memory with simultaneous smooth pursuit eye movements. (B) During retrieval of a traumatic/stressful memory with smooth pursuit eye movements, as compared to healthy controls, PTSD patients showed increased right SEF connectivity with the right dorsomedial and the right dorsolateral prefrontal cortices. In addition, as compared to controls, PTSD patients showed increased right SEF connectivity with the right anterior insula during retrieval of a traumatic memory with concurrent saccadic eye movements. All results are shown at pFWE ≤ 0.0125, k = 10, to correct for multiple comparisons; however, the precuneus is pFWE whole-brain corrected at p < 0.05, k = 10.
![Figure 3. Explorative functional connectivity analyses (psychophysiological interaction) of (A) the right frontal eye field [FEF (x: 46, y: 0, z: 56)] and (B) the right supplementary eye field [SEF (x: 2, y: 2, z: 62)] seed regions during the traumatic memory retrieval condition. (A) During retrieval of a traumatic/stressful memory, as compared to the post-traumatic stress disorder (PTSD) patient group, healthy controls demonstrated increased right FEF connectivity with the right posterior insula with simultaneous smooth pursuit eye movements. In contrast, as compared to healthy controls, PTSD patients demonstrated increased right FEF connectivity with the right dorsolateral prefrontal cortex during retrieval of a traumatic memory with simultaneous smooth pursuit eye movements. (B) During retrieval of a traumatic/stressful memory with smooth pursuit eye movements, as compared to healthy controls, PTSD patients showed increased right SEF connectivity with the right dorsomedial and the right dorsolateral prefrontal cortices. In addition, as compared to controls, PTSD patients showed increased right SEF connectivity with the right anterior insula during retrieval of a traumatic memory with concurrent saccadic eye movements. All results are shown at pFWE ≤ 0.0125, k = 10, to correct for multiple comparisons; however, the precuneus is pFWE whole-brain corrected at p < 0.05, k = 10.](/cms/asset/321e8a5c-4fa6-4677-abc4-3011f35ea171/zept_a_1586265_f0003_oc.jpg)
As compared to the healthy control group, the PTSD group showed increased right FEF connectivity with the right dorsolateral prefrontal cortex during horizontal smooth pursuit versus horizontal saccadic eye movements in the traumatic/stressful memory retrieval versus no memory retrieval condition (, ).
3.2.2. Right SEF
Significant regions in the PPI omnibus ANOVA test, interactions, and main factor effects are listed in the supplementary material. Post-hoc one-sample t-tests within each variable did not yield significant connectivity with the frontal and parietal brain regions studied.
Between-group comparisons
(i) Between participant group, within motion, within memory
As compared to the healthy control group, the PTSD group showed increased right SEF connectivity with the right dorsomedial prefrontal cortex during horizontal smooth pursuit eye movements in the traumatic/stressful memory retrieval condition. By contrast, as compared to the PTSD group, the healthy control group showed increased right SEF connectivity with the medial precuneus during horizontal saccadic eye movements in the traumatic/stressful memory retrieval condition (, ).
(ii) Between participant group, within motion, between memory
As compared to the PTSD group, the healthy control group showed increased right SEF connectivity with the medial precuneus during horizontal saccadic eye movements in the traumatic/stressful memory retrieval versus no memory retrieval condition (, ).
(iii) Between participant group, between motion, within memory
As compared to the healthy control group, the PTSD group showed increased right SEF connectivity with the right dorsolateral prefrontal cortex during horizontal smooth pursuit eye movements as compared to both the stationary central fixation dot stimulus and the horizontal saccadic eye movements in the traumatic/stressful memory retrieval condition. In addition, as compared to the healthy control group, the PTSD group showed increased right SEF connectivity with the right dorsomedial prefrontal cortex during horizontal smooth pursuit as compared to horizontal saccadic stressful memory retrieval condition/stressful memory retrieval condition (, ).
(vi) Between participant group, between motion, between memory
As compared to the healthy control group, the PTSD group showed increased right SEF connectivity with the right anterior insula during horizontal saccadic eye movements versus the stationary central fixation dot stimulus in the traumatic/stressful versus neutral memory retrieval condition (, ).
(v) Clinical correlations
Trait dissociative symptoms, measured by the self-reported MDI scale prior to the study, correlated negatively with right SEF connectivity with the right dorsolateral prefrontal cortex in the PTSD group during traumatic memory retrieval while performing concurrent horizontal smooth pursuit eye movements. Moreover, dissociative symptoms reported by the PTSD group in the scanner just prior to the experiment and measured by the RSDI correlated negatively with right SEF connectivity with the right dorsolateral prefrontal cortex during traumatic memory retrieval with concurrent horizontal smooth pursuit eye movements (, ). No significant correlations emerged between dissociative symptoms and right FEF connectivity patterns.
Figure 4. Explorative negative functional connectivity correlations with clinical dissociative measures in the right supplementary eye field (SEF) psychophysiological interaction during the traumatic memory retrieval condition. During retrieval of a traumatic memory with horizontal smooth pursuit eye movements, trait dissociation [Multiscale Dissociation Inventory (MDI)] symptoms and state dissociation symptoms [Responses to Script-Driven Imagery Scale (RSDI)] measures collected just prior to the scan correlated negatively with right SEF connectivity with the right dorsolateral prefrontal cortex. Pos, posterior; Ant, anterior. Results are shown at pFWE ≤ 0.0125, k = 10, corrected for multiple comparisons.
![Figure 4. Explorative negative functional connectivity correlations with clinical dissociative measures in the right supplementary eye field (SEF) psychophysiological interaction during the traumatic memory retrieval condition. During retrieval of a traumatic memory with horizontal smooth pursuit eye movements, trait dissociation [Multiscale Dissociation Inventory (MDI)] symptoms and state dissociation symptoms [Responses to Script-Driven Imagery Scale (RSDI)] measures collected just prior to the scan correlated negatively with right SEF connectivity with the right dorsolateral prefrontal cortex. Pos, posterior; Ant, anterior. Results are shown at pFWE ≤ 0.0125, k = 10, corrected for multiple comparisons.](/cms/asset/336149bf-b987-4a8c-a5e0-fba085d7aee1/zept_a_1586265_f0004_oc.jpg)
4. Discussion
In a pilot study aimed at enhancing our current understanding of the neurobiological mechanisms underlying EMDR therapy, we examined how oculomotion influences the neural circuitry engaged during retrieval of traumatic/stressful autobiographical memories in PTSD patients and healthy controls. We hypothesized initially that eye movements would activate the dorsal attentional network at the FEF and SEF. In turn, this network was expected to interact with frontoparietal brain regions involved in autobiographical memory retrieval, thus initializing a larger frontoparietal executive control network that recruits areas involved in higher order cognitive demands, including emotion regulation. Overall, our results supported these hypotheses, demonstrating that frontoparietal regions involved in autobiographical memory retrieval and emotion regulation show connectivity with the right FEF and SEF during the retrieval of a traumatic/stressful memory while performing concurrent horizontal saccadic and smooth pursuit eye movements. A full summary of the study results can be found in the supplementary material. In keeping with previous studies (Andrade et al., Citation1997; Barrowcliff et al., Citation2004), however, we discuss here only those results pertaining to the implementation of horizontal eye movements during retrieval of traumatic/stressful memories, as these findings have direct relevance to identifying the neural mechanisms underlying EMDR. In addition, we highlight below the influence of simultaneous oculomotion during traumatic/stressful autobiographical memory retrieval on the recruitment of a frontoparietal executive control network that has the potential to facilitate top–down emotion regulation.
4.1. Top–down emotion regulation
The findings of the present study point towards co-activation of the two functional subdivisions of the frontoparietal executive control network (Dixon et al., Citation2018), where ocular sensorimotor processing and introspection during traumatic/stressful autobiographical memory retrieval are thought to work in tandem to facilitate higher order cognitive processes such as emotion regulation. Specifically, during traumatic/stressful memory retrieval with simultaneous horizontal smooth pursuit eye movements, as compared to controls, the PTSD group showed increased right FEF and SEF connectivity with the right dorsolateral prefrontal cortex, as well as increased right SEF connectivity with the right dorsomedial prefrontal cortex.
Pagani et al. (Citation2012), in trying to elucidate the role of eye movements in cognitive processing of traumatic memories during EMDR, suggest that the prefrontal cortex is central to this processing due to its involvement in self-referential processing of the emotional content underlying a memory. Indeed, self-referential processing is thought to be critical for event processing, as it aids in introspective reflection on a memory by providing context through interpretation of the emotion it evokes (St. Jacques, Kragel, & Rubin, Citation2011; Svoboda, McKinnon, & Levine, Citation2006). Here, the dorsolateral and dorsomedial prefrontal cortices are critical not only to the mediation of emotion regulation strategies to dampen negative emotions, but also for initiating the retrieval of an episodic memory (Andrews-Hanna et al., Citation2014; Frewen, Thornley, Rabellino, & Lanius, Citation2017; Steinvorth, Corkin, & Halgren, Citation2006). Although individuals tend to integrate negative memories during rapid eye movement (REM) sleep, when the frontal lobe is largely inhibited (Hobson, Stickgold, & Pace-Schott, Citation1998; Marshall, Helgadóttir, Mölle, & Born, Citation2006; Nishida, Pearsall, Buckner, & Walker, Citation2008), Stickgold (Citation2002) suggests that eye movements may, conversely, engage the frontal lobe during the retrieval of episodic memories, thus enhancing the capacity for top–down emotion regulation. Critically, individuals with PTSD have been shown to have a decreased capacity for top–down emotion regulation (Frewen, Dozois, Neufeld, & Lanius, Citation2011), and, thus, may require greater effort to recruit brain regions necessary for top–down emotion regulation as compared to healthy individuals. Accordingly, we suggest that engagement of the oculomotor frontoparietal network observed here among individuals with PTSD may represent a compensatory neurobiological mechanism that facilitates downstream recruitment of regions impacted by emotion regulation, including the insula, in an effort to reduce the intense negative affect associated with a traumatic memory.
Brain regions involved in top–down emotion reappraisal, such as the dorsal prefrontal cortex, act on downstream structures, including the anterior and posterior regions of the insula (Goldin, McRae, Ramel, & Gross, Citation2008). Here, in the PTSD group as compared to controls, during horizontal saccadic eye movements, the right SEF showed increased connectivity with the right anterior insula, a region thought to be central to identifying emotional feeling states. As compared to controls, the PTSD group reported more intense negative emotions following retrieval of a traumatic memory (). Hence, increased connectivity between the right SEF and the right insula may represent an increased attempt at regulation of intense emotion associated with traumatic memory retrieval in PTSD.
The anterior insula is thought to maintain one’s sense of time; however, sensory overload from emotionally salient events may consume neural resources at the expense of the ability to assess the chronology of these events (Craig, Citation2009). This disruption may impact negatively memory processing, where the anterior insula is believed to be critical to the creation of a coherent emotional narrative of a memory with respect to time (Craig, Citation2009). Individuals with PTSD have been shown to suffer from a compromised ability to produce a coherent narrative of traumatic memories (Ehlers et al., Citation1998; Gray & Lombardo, Citation2001; van der Kolk & Fisler, Citation1995), and, accordingly, may show reduced higher order processing of its affective and sensory elements.
The increased right SEF recruitment of the right anterior insula in the PTSD group as compared to controls suggests a potential role of eye movements in strengthening the internal sense of time during retrieval of a traumatic memory. In turn, this enhanced chronological awareness may facilitate being able to more accurately retrieve a traumatic memory as an experience belonging to the past. Notably, these findings align with the concept of ‘neuroentrainment’ in EMDR (Coubard, Citation2015), which postulates that rhythmic eye movements engage attentional processes to synchronize both affective and temporal components of traumatic memories.
Thus, among individuals with PTSD, the right supplementary eye field may: i) recruit the right anterior insula to assist in identifying a temporally coherent emotional narrative associated with the retrieval of a traumatic memory; and ii) recruit other cortical midline structures (e.g., dorsal prefrontal cortex) to assist in processing its intense negative emotional content.
As noted, as compared to the PTSD group, controls reported significantly less intense negative emotions following retrieval of a stressful memory while engaged in oculomotor movements (). We suggest that, among those who are not traumatized by a stressful experience, it may not be necessary to recruit additional cortical regions in an effort to engage top–down emotion regulation processes. As compared to individuals with PTSD, the healthy control group showed increased right FEF connectivity with the right posterior insula only during retrieval of a stressful memory with simultaneous horizontal smooth pursuit eye movements. Pagani, Högberg, Fernandez, and Siracusano (Citation2013) emphasize the importance of EMDR in facilitating explicit cortical emotional processing of a traumatic memory over subcortical structures that carry implicit affective components of a memory. Similarly, Corrigan and Grand (Citation2013) suggest that top–down cortical integration of the episodic and the emotional components of a traumatic memory through EMDR may aid in memory reprocessing at the level of midbrain subcortical structures that help generate basic autonomic and instinctual responses to sensory input from the memory, such as the superior colliculus and the periaqueductal grey, where the latter may relay the implicit affective component of the memory through functional connections with the insula (Harricharan et al., Citation2016). Hence, during retrieval of a traumatic memory with simultaneous horizontal smooth pursuit eye movements, controls may require cortical control of the implicit negative affective intensity experienced at the level of the posterior insula only. In contrast, individuals with PTSD may require additional recruitment of higher order emotion regulation brain regions (e.g. dorsolateral prefrontal cortex) to cope with the heightened emotional intensity experienced during retrieval.
On balance, we suggest that in individuals with PTSD, as compared to controls, horizontal eye movements may activate the right FEF and SEF as an alternative mechanism to engage prefrontal regions involved in emotion regulation. These neural operations, in turn, are likely to assist in top–down reappraisal of a traumatic memory, thus reducing the negative affective intensity experienced upon its retrieval.
4.2. Dissociative symptoms impede emotion regulation
PTSD patients with symptoms of depersonalization and derealization often experience an altered perception of the self and its surroundings. In the present study, among individuals with PTSD, dissociative symptoms (MDI and RSDI) correlated negatively with right SEF connectivity with the right dorsolateral prefrontal cortex during traumatic memory retrieval involving simultaneous horizontal smooth pursuit eye movements (). A previous study (Bae, Kim, and Park, Citation2016) revealed poor treatment outcomes in patients with high scores on the Dissociative Experiences Scale (Bernstein & Putnam, Citation1986) when undergoing EMDR therapy. Taken together, we suggest that decreased ability of the oculomotor brain regions (i.e. FEF and SEF) to engage regions involved in top–down emotion regulation, including the right dorsolateral prefrontal cortex, during traumatic memory retrieval may limit the efficacy of EMDR therapy in PTSD patients with significant dissociative symptoms.
4.3. Limitations and future directions
Several limitations of the current study need to be considered, including prominently its small sample size. Given that this was a pilot study, replication of the present study with a larger sample is warranted. A larger sample size will also be necessary to delineate any gender differences in activation of the oculomotor frontoparietal network during traumatic/stressful memory retrieval. Inclusion of a larger sample may also render it more feasible to include a trauma-exposed control group; however, it is often difficult to generate a comparably sized, lifetime trauma-matched sample group of traumatized controls who do not meet the lifetime criteria for one or more psychiatric disorders. Future studies are also required to elucidate the impact of each type of eye movement (i.e. horizontal versus vertical, smooth versus saccadic eye movements) in a larger sample. Notably, other types of bilateral stimulation, including tactile or auditory alternating bilateral stimulation, have been used in clinical practice with EMDR (González, Del Río-Casanova, & Justo-Alonso, Citation2017; Nieuwenhuis et al., Citation2013). Additional research is therefore necessary to determine whether alternative bilateral stimulation methods show similar or different patterns of neural activation and of connectivity. Finally, given the potential of the present paradigm to identify the neural mechanisms underlying EMDR, it will be crucial to assess further the frontoparietal neural correlates of oculomotion, autobiographical memory, and emotion regulation pre- and post-treatment among PTSD patients undergoing multiple sessions of EMDR (Power et al., Citation2002; Rothbaum, Astin, & Marsteller, Citation2005).
5. Conclusions
The present study represents an important first step in identifying the role of the frontoparietal executive control network in the reprocessing of traumatic/stressful memories using eye movements. Here, we describe the influence of oculomotion on the recruitment of frontoparietal brain regions that impact top–down emotion regulatory processes during traumatic memory retrieval. In addition, we suggest that top–down emotion reappraisal strategies that occur in association with eye movements in PTSD may enhance self-referential processing to assist in reducing the negative emotional context associated with a memory. These processes may, in turn, facilitate integration of the exteroceptive and interoceptive details underlying traumatic memories, thus reducing what is often their time-independent and fragmentary nature. Overall, these findings begin to shed light on the potential neurobiological mechanisms underlying the use of EMDR as a treatment for PTSD.
Supplemental Material
Download MS Word (60.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Andrade, J., Kavanagh, D., & Baddeley, A. (1997). Eye-movements and visual imagery: A working memory approach to the treatment of post-traumatic stress disorder. British Journal of Clinical Psychology, 36, 209–15.
- Andrews-Hanna, J. R., Saxe, R., & Yarkoni, T. (2014). Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. NeuroImage, 91, 324–335.
- Baddeley, A. (2010). Working memory. Current Biology, 20(4), R136–R140.
- Baddeley, A., & Hitch, G. (1974). Working memory. Psychology of Learning and Motivation, 8, 47–89.
- Bae, H., Kim, D., & Park, Y. C. (2016). Dissociation predicts treatment response in eye-movement desensitization and reprocessing for posttraumatic stress disorder. Journal of Trauma & Dissociation, 17(1), 112–130.
- Barrowcliff, A. L., Gray, N. S., Freeman, T. C. A., & MacCulloch, M. J. (2004). Eye-movements reduce the vividness, emotional valence and electrodermal arousal associated with negative autobiographical memories. The Journal of Forensic Psychiatry and Psychology, 15(2), 325–345.
- Beck, A. T., Guth, D., Steer, R. A., & Ball, R. (1997). Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behaviour Research and Therapy, 35(8), 785–791.
- Beck, V. M., & Hollingworth, A. (2017). Competition in saccade target selection reveals attentional guidance by simultaneously active working memory representations.Journal of Experimental Psychology: Human Perception and Performance, 43(2), 225–231.
- Bernstein, D., & Fink, L. (1998). Childhood trauma questionnaire: A retrospective self-report: Manual. San Antonio, TX: The Psychological Corporation.
- Bernstein, E. M., & Putnam, F. W. (1986). Development, reliability, and validity of a dissociation scale. The Journal of Nervous and Mental Disease, 174(12), 727–735.
- Blake, D. D., Weathers, F. W., Nagy, L. M., Kaloupek, D. G., Gusman, F. D., Charney, D. S., & Keane, T. M. (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90.
- Bremner, J. D., Krystal, J. H., Putnam, F. W., Southwick, S. M., Marmar, C., Charney, D. S., & Mazure, C. M. (1998). Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). Journal of Traumatic Stress, 11(1), 125–136.
- Bremner, J. D., Narayan, M., Staib, L. H., Southwick, S. M., McGlashan, T., & Charney, D. S. (1999). Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry, 156(11), 1787–1795.
- Brewin, C. R., Huntley, Z., & Whalley, M. G. (2012). Source memory errors associated with reports of posttraumatic flashbacks: A proof of concept study. Cognition, 124(2), 234–238.
- Briere, J., Weathers, F. W., & Runtz, M. (2005). Is dissociation a multidimensional construct? Data from the Multiscale Dissociation Inventory. Journal of Traumatic Stress, 18(3), 221–231.
- Burgess, N. (2006). Spatial memory: How egocentric and allocentric combine. Trends in Cognitive Sciences, 10(12), 551–557.
- Burianova, H., McIntosh, A. R., & Grady, C. L. (2010). A common functional brain network for autobiographical, episodic, and semantic memory retrieval. NeuroImage, 49(1), 865–874.
- Corbetta, M., & Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215.
- Corrigan, F., & Grand, D. (2013). Brainspotting: Recruiting the midbrain for accessing and healing sensorimotor memories of traumatic activation. Medical Hypotheses, 80(6), 759–766.
- Cotter, P., Meysner, L., & Lee, C. W. (2017). Participant experiences of eye movement desensitisation and reprocessing vs. cognitive behavioural therapy for grief: Similarities and differences. European Journal of Psychotraumatology, 8(1), 1375838.
- Coubard, O. A. (2015). Eye Movement Desensitization and Reprocessing (EMDR) re-examined as cognitive and emotional neuroentrainment. Frontiers in Human Neuroscience, 8, 1035.
- Craig, A. D. B. (2009). How do you feel – Now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1), 59–70.
- Dixon, M. L., De La Vega, A., Mills, C., Andrews-Hanna, J., Spreng, R. N., Cole, M. W., & Christoff, K. (2018). Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proceedings of the National Academy of Sciences, 115(7), E1598–E1607.
- Ehlers, A., Clarke, D. M., Dunmore, E., Jaycox, L., Meadows, E., & Foa, E. B. (1998). Predicting response to exposure treatment in posttraumatic stress disorder: Role of mental defeat and alienation. Journal of Traumatic Stress, 11(3), 457–471.
- Ehlers, A., Hackmann, A., & Michael, T. (2004). Intrusive re-experiencing in post-traumatic stress disorder: Phenomenology, theory, and therapy. Memory, 12(4), 403–415.
- Eklund, A., Nichols, T. E., & Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113(28), 7900–7905.
- Eriksson, J., Vogel, E. K., Lansner, A., Bergström, F., & Nyberg, L. (2015). Neurocognitive architecture of working memory. Neuron, 88(1), 33–46.
- First, M., Spitzer, R., Gibbon, M., & Williams, J. (2002). Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition. New York, NY: Biometrics Research, New York Psychiatric Institute.
- Frewen, P., Thornley, E., Rabellino, D., & Lanius, R. (2017). Neuroimaging the traumatized self: FMRI reveals altered response in cortical midline structures and occipital cortex during visual and verbal self-and other-referential processing in women with PTSD. European Journal of Psychotraumatology, 8(1), 1314164.
- Frewen, P. A., Dozois, D. J. A., Neufeld, R. W. J., & Lanius, R. A. (2011). Disturbances of emotional awareness and expression in posttraumatic stress disorder: Meta-mood, emotion regulation, mindfulness, and interference of emotional expressiveness. Psychological Trauma: Theory, Research, Practice, and Policy, 4(2), 152–161.
- Goldin, P. R., McRae, K., Ramel, W., & Gross, J. J. (2008). The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry, 63(6), 577–586.
- González, A., Del Río-Casanova, L., & Justo-Alonso, A. (2017). Integrating neurobiology of emotion regulation and trauma therapy: Reflections on EMDR therapy. Reviews in the Neurosciences, 28(4), 431–440.
- Gray, M. J., & Lombardo, T. W. (2001). Complexity of trauma narratives as an index of fragmented memory in PTSD: A critical analysis. Applied Cognitive Psychology, 15(7), S171–186.
- Grosbras, M.-H., & Paus, T. (2002). Transcranial magnetic stimulation of the human frontal eye field: Effects on visual perception and attention. Journal of Cognitive Neuroscience, 14(7), 1109–1120.
- Harricharan, S., Rabellino, D., Frewen, P. A., Densmore, M., Théberge, J., McKinnon, M. C., … Lanius, R. A. (2016). fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain and Behavior, 6(12), e00579.
- Hobson, J. A., Stickgold, R., & Pace-Schott, E. F. (1998). The neuropsychology of REM sleep dreaming. Neuroreport, 9(3): R1–R14. PMID: 9512371.
- Hopper, J. W., Frewen, P. A., Sack, M., Lanius, R. A., & van der Kolk, B. A. (2007). The responses to script-driven imagery scale (RSDI): Assessment of state posttraumatic symptoms for psychobiological and treatment research. Journal of Psychopathology and Behavioral Assessment, 29(4), 249–268.
- Kurth, F., Zilles, K., Fox, P. T., Laird, A. R., & Eickhoff, S. B. (2010). A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function, 214(5–6), 519–534.
- Lanius, R. A., Williamson, P. C., Densmore, M., Boksman, K., Neufeld, R. W., Gati, J. S., & Menon, R. S. (2004). The nature of traumatic memories : A 4-T fMRI functional connectivity analysis. American Journal of Psychiatry, 161, 36–44.
- Littel, M., van Schie, K., & van den Hout, M. A. (2017). Exploring expectation effects in EMDR: Does prior treatment knowledge affect the degrading effects of eye movements on memories? European Journal of Psychotraumatology, 8(sup1), 1328954.
- Maldjian, J. A., Laurienti, P. J., Kraft, R. A., & Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239.
- Marshall, L., Helgadóttir, H., Mölle, M., & Born, J. (2006). Boosting slow oscillations during sleep potentiates memory. Nature, 444(7119), 610.
- Maxfield, L., Melnyk, W. T., & Hayman, G. C. (2008). A working memory exlanation for the effects of eye movements in EMDR. Journal of EMDR Practice and Research, 2(4), 247–261.
- Morawetz, C., Bode, S., Derntl, B., & Heekeren, H. R. (2017). The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neuroscience and Biobehavioral Reviews, 72, 111–128.
- Nieuwenhuis, S., Elzinga, B. M., Ras, P. H., Berends, F., Duijs, P., Samara, Z., & Slagter, H. A. (2013). Bilateral saccadic eye movements and tactile stimulation, but not auditory stimulation, enhance memory retrieval. Brain and Cognition, 81(1), 52–56.
- Nishida, M., Pearsall, J., Buckner, R. L., & Walker, M. P. (2008). REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebral Cortex, 19(5), 1158–1166.
- Op Den Kelder, R., Van Den Akker, A. L., Geurts, H. M., Lindauer, R. J., & Overbeek, G. (2018). Executive functions in trauma-exposed youth: A meta-analysis. European Journal of Psychotraumatology, 9(1), 1450595.
- Pagani, M., Di Lorenzo, G., Verardo, A. R., Nicolais, G., Monaco, L., Lauretti, G., … Siracusano, A. (2012). Neurobiological correlates of EMDR monitoring - An EEG study. PloS One, 7(9), e45753.
- Pagani, M., Högberg, G., Fernandez, I., & Siracusano, A. (2013). Correlates of EMDR therapy in functional and structural neuroimaging: A critical summary of recent findings. Journal of EMDR Practice and Research, 7(1), 29–38.
- Pearson, D., & Sahraie, A. (2003). Oculomotor control and the maintenance of spatially and temporally distributed events in visuo-spatial working memory. The Quarterly Journal of Experimental Psychology Section A, 56(7), 1089–1111.
- Picó-Pérez, M., Radua, J., Steward, T., Menchón, J. M., & Soriano-Mas, C. (2017). Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Progress in Neuropsychopharmacology and Biological Psychiatry, 79, 96–104.
- Power, K., McGoldrick, T., Brown, K., Buchanan, R., Sharp, D., Swanson, V., & Karatzias, A. (2002). A controlled comparison of eye movement desensitization and reprocessing versus exposure plus cognitive restructuring versus waiting list in the treatment of post-traumatic stress disorder. Clinical Psychology and Psychotherapy, 9, 299–318.
- Rothbaum, B. O., Astin, M. C., & Marsteller, F. (2005). Prolonged exposure versus eye movement desensitization and reprocessing (EMDR) for PTSD rape victims. Journal of Traumatic Stress, 18(6), 607–616.
- Shapiro, F. (1989). Eye movement desensitization: A new treatment for post-traumatic stress disorder. Journal of Behavior Therapy and Experimental Psychiatry, 20(3), 211–217.
- Shipstead, Z., Harrison, T. L., & Engle, R. W. (2012). Working memory capacity and visual attention: Top-down and bottom-up guidance. The Quarterly Journal of Experimental Psychology, 65(3), 401–407.
- Souza, A. S., & Oberauer, K. (2017). Time to process information in working memory improves episodic memory. Journal of Memory and Language, 96, 155–167.
- Spielberger, C. D. (2010). State-Trait Anxiety Inventory. In The corsini encyclopedia of psychology. Hoboken, NJ: John Wiley & Sons. doi:10.1002/9780470479216.corpsy0943
- St. Jacques, P. L., Kragel, P. A., & Rubin, D. C. (2011). Dynamic neural networks supporting memory retrieval. Neuroimage, 57(2), 608–616.
- Steinvorth, S., Corkin, S., & Halgren, E. (2006). Ecphory of autobiographical memories: An fMRI study of recent and remote memory retrieval. NeuroImage, 30(1), 285–298.
- Stickgold, R. (2002). EMDR: A putative neurobiological mechanism of action. Journal of Clinical Psychology, 58(1), 61–75.
- Svoboda, E., McKinnon, M. C., & Levine, B. (2006). The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia, 44(12), 2189–2208.
- Szczepanski, S. M., Pinsk, M. A., Douglas, M. M., Kastner, S., & Saalmann, Y. B. (2013). Functional and structural architecture of the human dorsal frontoparietal attention network. Proceedings of the National Academy of Sciences, 110, 15806–15811.
- Thomaes, K., Engelhard, I. M., Sijbrandij, M., Cath, D. C., & Van den Heuvel, O. A. (2016). Degrading traumatic memories with eye movements: A pilot functional MRI study in PTSD. European Journal of Psychotraumatology, 7(1), 31371.
- Uncapher, M. R., & Wagner, A. D. (2009). Posterior parietal cortex and episodic encoding: Insights from fMRI subsequent memory effects and dual-attention theory. Neurobiology of Learning and Memory, 91(2), 139–154.
- van der Kolk, B. A., & Fisler, R. (1995). Dissociation and the fragmentary nature of traumatic memories : Overview and exploratory study. Journal of Traumatic Stress, 8(4), 505–525. PMID: 8564271
- van der Kolk, B. A., Spinazzola, J., Blaustein, M. E., Hopper, J. W., Hopper, E. K., Korn, D. L., & Simpson, W. B. (2007). A Randomized clinical trial of eye movement desensitization and reprocessing (EMDR), fluoxetine, and pill placebo in the treatment of posttraumatic stress disorder: Treatment effects and long-term maintenance. Journal of Clinical Psychiatry, 68(1), 37–46.
- Vernet, M., Quentin, R., Chanes, L., Mitsumasu, A., & Valero-cabré, A. (2014). Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Frontiers in Integrative Neuroscience, 8, 66.
- Weathers, F. W., Blake, D. D., Schnurr, P. P., Kaloupek, D. G., Marx, B. P., & Keane, T. M. (2013). The clinician-administered PTSD scale for DSM-5 (CAPS-5). Interview available from the National Center for PTSD. Retrieved from www.ptsd.va.gov.
- Zilverstand, A., Parvaz, M. A., & Goldstein, R. Z. (2017). Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. NeuroImage, 151(June 2016), 105–116.
