ABSTRACT
Background: Cross-sectional studies have found that a trauma history can be associated with anxious-depressive symptomatology and physiological stress dysregulation in pregnant women.
Methods: This prospective study examines the trajectories of both anxiety and depressive symptoms and salivary cortisol and α-amylase biomarkers from women with (n = 42) and without (n = 59) a trauma history at (i) 38th week of gestation (T1), (ii) 48 hours after birth (T2), and (iii) three months after birth (T3).
Results: The quantile regression model showed that trauma history was associated with higher cortisol levels at T1 and this difference was sustained along T2 and T3. Conversely, there were no significant differences in α-amylase levels between groups across the three time points and both groups showed an increase in α-amylase levels from T2 to T3. The ordinal mixed model showed that trauma history was associated with higher anxiety symptoms at T1 and this remained constant from T1 to T2 but was reversed from T2 to T3. In contrast, both groups showed similar depressive symptoms across the three time points.
Conclusions: Whereas physiological stress dysregulation (in terms of higher cortisol levels) was maintained from pregnancy to postpartum period, pregnancy and childbirth were the most vulnerable stages for developing anxious symptoms in mothers with trauma history.
HIGHLIGHTS
• Follow-up study on pregnant women with a trauma history. •Data analysed by quantile and ordinal regression models.•Trauma history and high cortisol levels from pregnancy to postpartum. • High α-amylase levels during postpartum period, regardless of a trauma history. • Trauma history and high anxious symptoms from late pregnancy to childbirth.
Antecedentes: Los estudios transversales han encontrado que una historia de trauma puede estar asociada con sintomatología ansioso-depresiva y una desregulación del estrés fisiológico en mujeres embarazadas.
Métodos: Este estudio prospectivo examina las trayectorias de los síntomas de ansiedad y depresión y los biomarcadores de estrés en saliva (cortisol y α-amilasa) de mujeres con (n = 42) y sin (n = 59) una historia de trauma a las (i) 38 semanas de gestación (T1), (ii) 48 horas después del nacimiento (T2), y (iii) tres meses después del nacimiento (T3).
Resultados: El modelo de regresión por cuantiles mostró que la historia de trauma se asoció con niveles más altos de cortisol en T1 y esta diferencia se mantuvo a lo largo de T2 y T3. A la inversa, no hubo diferencias significativas en los niveles de α-amilasa entre los grupos en los tres tiempos y ambos grupos mostraron un aumento en los niveles de α-amilasa de T2 a T3. El modelo mixto ordinal mostró que la historia de trauma se asoció con más síntomas de ansiedad en T1 y esta diferencia se mantuvo constante de T1 a T2, pero se invirtió de T2 a T3. En contraste, ambos grupos mostraron síntomas depresivos similares en los tres tiempos.
Conclusiones: Mientras que la desregulación del estrés fisiológico (en términos de niveles más altos de cortisol) se mantuvo desde el embarazo hasta el posparto, el embarazo y el parto fueron las etapas más vulnerables para el desarrollo de síntomas ansiosos en madres con historia de trauma.
背景:横断研究发现,创伤史可能与孕妇的焦虑抑郁症状和生理应激失调有关。
方法:本研究采用前瞻研究设计,在三个时间点检验有创伤史(n = 42)和无创伤史(n = 59) 的女性的焦虑和抑郁症状以及唾液皮质醇和α-淀粉酶生物标志物的轨迹。这三个时间点分别为(i)妊娠第38周(T1) ,(ii)出生后48小时(T2),和(iii)出生后三个月(T3)。
结果:分位数回归模型显示创伤史在T1与较高的皮质醇水平相关,并且这种差异持续至T2和T3。相反,在三个时间点α-淀粉酶组间水平没有显著差异,两组均显示从T2到T3的α-淀粉酶水平增加。序数混合模型(ordinal mixed model)显示创伤史在T1与较高的焦虑症状相关,并且从T1到T2保持不变,但从T2到T3时发生逆转。与之对比,两组在三个时间点都表现出相似的抑郁症状。
结论:尽管生理应激失调(就较高的皮质醇水平而言)从妊娠期持续到产后期,但妊娠和分娩阶段对有创伤史的母亲来说是出现焦虑症状最易感的阶段。
1. Introduction
A history of traumatic events is a potential predictor of mental health problems in pregnant women (Muzik et al., Citation2016; Onoye et al., Citation2013), even in those who did not meet all the criteria for the diagnosis of posttraumatic stress disorder (PTSD) (Habersaat et al., Citation2014). Not only does trauma history potentially have negative effects on the mother, but it can also lead to negative health outcomes for the child, affecting their physical and mental health (Blackmore et al., Citation2016; Pearlstein, Howard, Salisbury, & Zlotnick, Citation2009). Despite having such consequences, studies on the impact of trauma history on the trajectories of perinatal anxious-depressive symptoms (i.e. those that occur during pregnancy and/or the postpartum period) are limited. Additionally, the association between measurements of reported stress and biomarkers is not straightforward in the research of pregnancy (Harville, Savitz, Dole, Herring, & Thorp, Citation2009). In the present follow-up study, we have adopted simultaneous measurements of self-reported symptoms and stress biomarkers from pregnancy to three months postpartum (García-Blanco et al., Citation2018; Laurent et al., Citation2018).
Mental health status in women with a trauma history has been usually assessed in terms of self-reported anxious-depressive symptoms at a certain point in time during the perinatal period in cross-sectional studies (see Álvarez-Segura et al., Citation2014, for a review). Few studies have assessed how a trauma history contributes to the trajectories of depression and anxiety examined simultaneously over time. Nevertheless, these few studies addressing anxiety and depression in women with a trauma history have shown inconclusive results. The reported inconclusive results could be due to potential confounding variables, such as age (García-Blanco et al., Citation2017), parity (Dipietro, Costigan, & Sipsma, Citation2008), time of testing (Gourounti, Citation2016), and other stress-generating factors like medical status and risk of social exclusion (Álvarez-Segura et al., Citation2014).
Recent follow-up studies across community samples have found that whereas depressive symptoms decrease from pregnancy to postpartum, anxiety symptoms fluctuate over time with no specific trajectory (García-Blanco et al., Citation2018, Citation2017; Underwood, Waldie, D’Souza, Peterson, & Morton, Citation2017; Whisman, Davila, & Goodman, Citation2011). With respect to the impact of traumatic events on depressive symptoms, mothers of infants admitted to a neonatal intensive care unit (NICU) and who had experienced an accumulation of maternal, infant, and family stressful events before the NICU discharge show a less sharp decline of depressive symptoms from the NICU discharge to two-years period than mothers who experienced few stressful factors (Poehlmann, Schwichtenberg, Bolt, & Dilworth-Bart, Citation2009). Similarly, a recent study with women from low socioeconomic status found that, although depressive symptoms decrease from pregnancy to 24-months postpartum, women with a trauma history show higher depressive symptoms from pregnancy to postpartum period with a rise at 24-months postpartum (Grekin, Brock, & O’Hara, Citation2017). However, Lang, Rodgers, and Lebeck (Citation2006) found that whereas a trauma history during childhood is associated with increased depression at pregnancy, no significant relationship is found at one-year postpartum. Concerning anxiety symptoms, Lang et al. (Citation2006) found that a trauma history was associated with higher anxiety symptoms at one-year postpartum but not at pregnancy. Nevertheless, a recent meta-analysis (Howard, Oram, Galley, Trevillion, & Feder, Citation2013) concludes that, although there are few studies on women with a trauma history, women with anxiety during pregnancy and postpartum periods reported more traumatic experiences.
Regarding physiological stress, a trauma history has been associated with a persistent and abnormal adaptation of neurobiological systems to stress, such as the hypothalamic-pituitary-adrenal axis (HPA) and the sympathetic-adrenal-medullary axis (SAM) (Vreeburg et al., Citation2010). As for HPA axis, cortisol is considered the main biomarker since this hormone regulates the HPA axis activation through negative feedback (Smith & Vale, Citation2006). When stress is maintained for a long time, excessive secretion of cortisol impairs the HPA axis regulation and, subsequently, the immune response (Kuhlman, Vargas, Geiss, & Lopez-Duran, Citation2015). Stress also elicits the SAM axis activation, releasing catecholamines and preparing the body for action (e.g. heart rate increase, breath rate increase, pupil dilation) (Thoma, Kirschbaum, Wolf, & Rohleder, Citation2012b). To assess SAM activity, α-amylase is a useful and non-invasive biomarker since it is associated with catecholamines levels (Bosch, Veerman, de Geus, & Proctor, Citation2011; Nater & Rohleder, Citation2009; Thoma et al., Citation2012b). The scarce number of follow-up studies across community samples have found that cortisol basal levels increase from late pregnancy to childbirth and decrease from childbirth to postpartum period (García-Blanco et al., Citation2018), being even lower at the postpartum period than at late pregnancy (García-Blanco et al., Citation2017). As opposed to α-amylase basal levels, where an increase from childbirth to postpartum period has been found (García-Blanco et al., Citation2017, Citation2018), it is unclear if α-amylase levels from pregnancy to childbirth decrease (García-Blanco et al., Citation2018) or maintain the same levels (García-Blanco et al., Citation2017).
Regarding the association between a trauma history and stress biomarkers under basal conditions, several meta-analyses on cortisol levels have yielded mixed results depending on the presence of PTSD. That is, whereas higher cortisol levels have been found in individuals with a trauma history, but not suffering from PTSD (Miller, Chen, & Zhou, Citation2007), lower cortisol levels have been found in PTSD patients (Meewisse, Reitsma, De Vries, Gersons, & Olff, Citation2007; Morris, Compas, & Garber, Citation2012). As for α-amylase levels, the scarce literature in small groups of patients suggests that further investigations are required. For instance, although a positive association have been found between PTSD symptoms and α-amylase levels, no differences have been found between non-exposed individuals and those with a trauma history, regardless of the presence of PTSD (see Keeshin, Strawn, Out, Granger, & Putnam, Citation2015, for those without PTSD; Thoma, Joksimovic, Kirschbaum, Wolf, & Rohleder, Citation2012a, for those with PTSD).
Based on this disparity in the stress biomarkers levels, a useful statistical method is the quantile regression over the entire range of an outcome. This method detects potential heterogeneity in exposure-outcome associations according to individual outcome levels (Bind et al., Citation2016), and it does not require any assumption about the distribution of the biomarkers levels. Hence, the use of quantile regression will allow for estimating associations between a trauma history in pregnant women and stress biomarkers levels that are not normally distributed.
To our knowledge, this is the first follow-up study that evaluates simultaneously stress biomarkers levels (i.e. cortisol and α-amylase) and anxious-depressive symptoms comparing a large sample of pregnant women with a trauma history and those without one from pregnancy to postpartum. According to Miller et al. (Citation2007) and Keeshin et al. (Citation2015), who studied cortisol and α-amylase levels in people with a trauma history but not current PTSD, we hypothesized that pregnant women with a trauma history would have higher cortisol and similar α-amylase levels from pregnancy to postpartum. According to Poehlmann et al. (Citation2009) and Howard et al. (Citation2013), who studied women that reported stressful events during pregnancy, we hypothesized that women with a trauma history would show higher anxiety and less sharp decline of depressive symptoms from pregnancy to postpartum periods.
2. Material and methods
2.1. Study design and participants
This is a prospective cohort study performed in the Division of Obstetrics at a tertiary referral hospital during a 12-month period in 2015. The ethics committee of the hospital approved the study and an informed consent was signed by each participant. The follow-up was carried out at three different assessment times: (i) time#1 (T1) at 38th week of gestation; (ii) time#2 (T2) at 48 hours after birth; and (iii) time#3 (T3) at three months after birth.
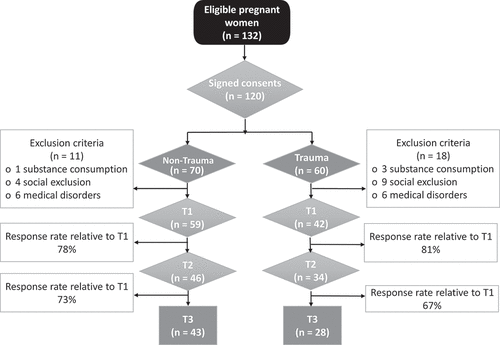
Eligible participants were women >18 years old at 38th week of gestation admitted to the hospital. Pregnancy should be desired. Pregnant women were classified into two groups: pregnant with a trauma history (n = 60), and pregnant without a trauma history (n = 68). Participants who fulfilled the eligibility criteria were interviewed by a clinical psychologist at T1, at the Maternity Inpatient Unit at T2, and at the Maternity Outpatient Unit at T3.
The exclusion criteria were chosen to control additional stress-generating factors and to guarantee that stress is due to trauma history rather than medical or social conditions. Participants should not be affected by pre-existing major medical disorders that require close supervision during pregnancy (e.g. heart disease, high blood pressure, diabetes type I, severe mental illness). Additionally, the exclusion criteria included substance consumption during pregnancy since they could affect the HPA and SAM axis. As for social conditions, participants should not be at risk of social exclusion in terms of: (i) risk-of poverty, (ii) severe material deprivation, or (iii) jobless households (Middlemiss, Citation2003). Finally, xerostomia, periodontitis, and gingivitis were additional exclusion criteria. shows the flow diagram of recruitment.
2.2. Procedure
Participants were recruited at 38th week of gestation during their routine prenatal visit to the obstetric unit. Once the obstetrician confirmed the inclusion criteria based on a case note review, a clinical psychologist carried out a lengthy clinical interview at T1 in order to determine the trauma history by means of Trauma Questionnaire (TQ; Escalona, Tupler, Saur, Krishnan, & Davidson, Citation1997). Additionally, participants were instructed to report whether a traumatic event occurred during the study (no events were reported). The Trait form of the State-Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, Citation1983) was also applied at T1. Saliva samples and depressive and anxiety symptoms were determined at T1, T2, and T3, using the Beck Depression Inventory Short Form (BDI/SF; Beck, Rial, & Rickels, Citation1974) and the State form of the State-Trait Anxiety Inventory (STAI; Spielberger et al., Citation1983), respectively.
2.3. Psychological assessment
The TQ (Escalona et al., Citation1997) is an instrument to screen PTSD. The TQ contains 19 items that assess the presence of a personal history of exposure to traumatic events according to criteria A of DSM-IV. That is, a traumatic event which elicited intense fear, helplessness, or horror when a person experienced, witnessed, or was confronted with an event or events that involved actual or threatened death or serious injury, or a threat to the physical integrity of self or others (e.g. physical and sexual trauma, accidents, natural disasters). Age at the time of most relevant traumatic event is also registered. Additionally, the TQ includes nine items on the event characteristics and a list of 18 symptoms according to criteria B, C, and D of DSM-IV for PTSD. The presence or absence of each PTSD symptom is quantified as 1 or 0, respectively. Higher score on this list of 18 symptoms indicates higher severity of PTSD symptomatology. The internal consistency coefficient for the symptoms scale was 0.88 in our sample.
The Trait and State forms of the STAI, form Y (Spielberger et al., Citation1983), was used to measure patient’s anxiety trait and current anxiety state, respectively. Each form comprised 20 items assessing tension, nervousness, and concerns, on a 4-point Likert scale (i.e. from ‘Almost Never’ to ‘Almost Always’). Scores ranged from 0 to 60, with higher scores indicating greater anxiety symptoms. The threshold for clinical anxiety is a total score of 19. State forms can be used in clinical settings to diagnose anxiety and to distinguish anxiety symptoms from depressive symptoms. The internal consistency coefficient for the scale was 0.89 in our sample population.
The BDI/SF (Beck et al., Citation1974) was used to measure the intensity of attitudes and depressive symptoms and to detect depression in the general population. Because of its high correlation with the standard form, the short form is considered a reliable and valid brief screening measure of depression (Beck et al., Citation1974). It contains 13 items rated on a 4-point Likert scale, from 0 (low) to 3 (high). Scores ranged from 0 to 39, with higher scores indicating higher depressive symptoms. The threshold for clinical depression is a total score of 4 (Beck et al., Citation1974). Our sample population demonstrated an internal consistency for the BDI of 0.73.
2.4. Analytical determinations
Saliva samples were collected by spitting into sterile bottles (between 10 and 12 a.m.). Participants were instructed to rest during the night, to eat breakfast, and to rinse their mouth a minimum one hour before saliva collection. Additionally, they were instructed to avoid coffee, food, or cigarettes intake before sample collection. Then, the samples were aliquoted into 1.5 mL tubes, and those with visible blood contamination were excluded from the study. Finally, samples were stored at −80ºC until analysis.
Saliva samples were thawed on ice and homogenized. The sample treatment to determine cortisol was based on a previous work (García-Blanco, Vento, Diago, & Cháfer-Pericás, Citation2016). Briefly, 25 µL of sample were subjected to liquid-liquid extraction to extract cortisol, then the organic layer was evaporated to dryness and the residues were reconstituted in water: methanol (85:15 v/v) solution. Finally, they were injected in the chromatographic system.
For the α-amylase determination, samples were vortexed and centrifuged. Then, they were diluted with the α-amylase diluent. Finally, they were subjected to the kinetic enzyme assay as described by the assay kit manufacturer (Alpha-Amylase Assay Kit – Enzymatic, Saliva – Salimetrics Assays, 1–1902).
2.5. Statistical analysis
Data were summarized using mean (standard deviation) and median (1st and 3rd quartile) in the case of continuous variables and relative and absolute frequencies in the case of categorical variables. Correlations among stress biomarkers and anxious-depressive symptoms along T1, T2, and T3 were assessed with Spearman’s correlation. Association of the different explicative variables with cortisol, α-amylase, BDI/SF, and STAI-State was assessed using Bayesian hierarchical modelling. Models included an interaction between time and trauma to assess differences in evolution between the trauma group and the non-trauma group. Firstly, since an interaction has been included in the models, the differences at T1 between both groups were examined. Subsequently, the differences in slopes between both groups from T1 to T2 and from T2 to T3 were analysed. All models included also age and parity as covariates. Additionally, models including only the trauma group were adjusted to assess the influence of years since trauma, TQ score, and trauma type. In the case of cortisol and α-amylase, quantile regression to the median was performed. The use of quantile regression was motivated by the presence of values under the limit of detection in the case of cortisol (19%). In the case of STAI-State and BDI/SF, cumulative link ordinal regression was used. All models included a random intercept in order to take dependency among observations from the same individual into account. Weakly informative priors for the coefficients of the fixed effects were used in all models. In the quantile regression models they were set as N(0, 50) and in the cumulative regression models as N(0, 10). A sensitivity analysis using less informative priors was performed to assess the influence of the chosen priors in the final estimates of the models. 95% Credibility Intervals (95% CI) were provided for all estimates and marginal effect plots were performed to ease interpretation of the models. All statistical analyses were performed using R (version 3.4.1) and brms package (version 1.8.0.1).
3. Results
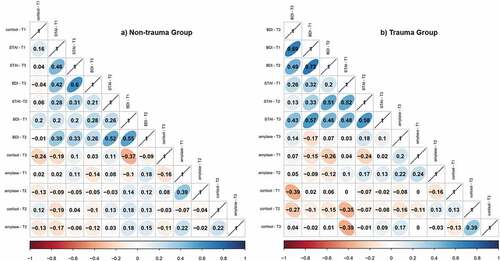
In this study, 101 healthy pregnant women were followed from the 38th week of gestation until three months after labour. shows the demographic information, clinical variables, range of cortisol concentrations, and range of α-amylase concentrations, as well as the STAI and BDI scores in each group. Prior to modelling, an exploratory data analysis was performed by examining the correlations between the different studied variables in both groups. Correlation plots are depicted in . Interestingly, the trauma group showed stronger correlations between the different STAI-State and BDI/SF measures compared to the non-trauma group. Specifically, BDI/SF and STAI-State scores were sizeably correlated along the different time points. Remarkably, BDI/SF and STAI-State scores were also moderately correlated to each other. The quantile regression models for cortisol and α-amylase concentrations are summarized in . The ordinal regression models for STAI-State and BDI/SF scores are summarized in . The models that included only the trauma group in order to examine the influence of years since trauma, TQ score, and trauma type are shown as supplementary material (see Table S1 for cortisol and α-amylase levels and Table S2 for anxiety and depressive symptoms).
Figure 2. Correlations among stress biomarkers and anxious-depressive symptoms along T1, T2, and T3.
3.1. Cortisol levels
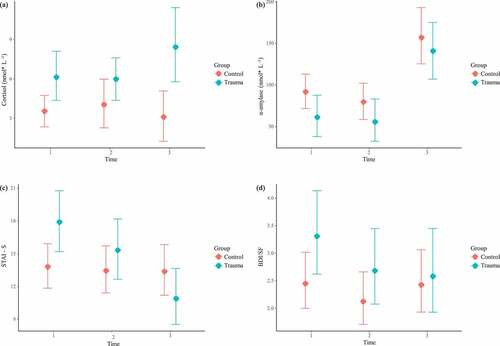
As we can see in , results for the cortisol model show that the trauma group have higher cortisol levels compared to the non-trauma group at T1 (Estimate = 2.6, 95% CI [0.52, 4.85]), which is sustained along T2 and T3 (see Figure 3(a)). When assessing the influence of years since trauma, TQ score, and trauma type in the trauma group, no association was found.
Table 1. Characteristics of studied population.
Table 2. Statistical parameters corresponding to the quantile regression models obtained for the cortisol and α-amylase variables.
Table 3. Statistical parameters corresponding to the ordinal regression models obtained for the anxiety and depressive symptoms.
3.2. α-amylase levels
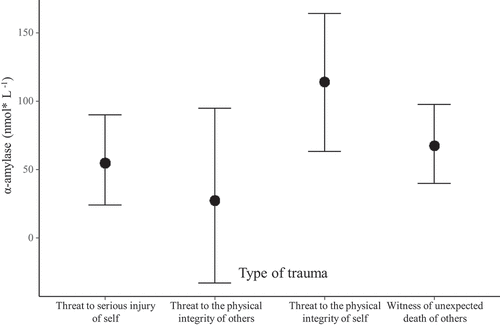
Regarding α-amylase concentrations, there were no significant differences in α-amylase levels between the trauma and non-trauma groups across the three time points (see ). Nevertheless, the model showed a steep increase in α-amylase levels in T3 in both groups (Estimate = 65.65, 95% CI [31.14, 101.80]). When assessing the influence of years since trauma, TQ score, and trauma type in the trauma group, an association was found between trauma type and α-amylase concentrations (see ). That is, women who suffered from a threat to the physical integrity of self showed higher α-amylase levels than other types of trauma (Estimate = 58.82, 95% CI [2.57, 11.96]).
3.3. STAI-State scores
Results of the mixed effects ordinal model for the STAI-State levels at T1 are similar to those of cortisol. As we can see in ), there is an initial difference between both groups (OR = 3.61, 95% CI [1.24, 10.56]). That is, the trauma group showed higher anxiety symptoms at T1. However, this effect was reverted at T3, where the trauma group had lower STAI-State values than the non-trauma group (OR = 0.11, 95% CI [0.03, 0.40]). When assessing the influence of years since trauma, TQ score, and trauma type in the trauma group, no association was found.
3.4. BDI-SF scores
In the case of BDI-SF scores, there were no significant differences in depressive symptoms between the trauma and non-trauma groups across the three time points (see )). When assessing the influence of years since trauma, TQ score, and trauma type in the trauma group, no association was found.
4. Discussion
Women with a trauma history showed higher cortisol levels at the third trimester of pregnancy than women without one and this difference was sustained until three months after birth. In contrast, women with a trauma history showed similar α-amylase levels to women without one. Furthermore, both groups showed an increase in α-amylase levels at three months after birth. In the case of anxious-depressive symptoms, women with a trauma history showed higher anxiety symptoms from the third trimester of pregnancy to 48 hours after birth but this difference was reversed during postpartum period. Nevertheless, despite women with a trauma history having higher scores in anxiety symptoms, these scores did not reach the cut-off to be considered clinically significant. Conversely, women with a trauma history showed similar depressive symptoms to women without one. Therefore, women with a trauma history showed high stress levels in terms of both cortisol concentrations and anxiety symptoms. However, whereas anxiety symptoms become reversed during postpartum period, there was a constant difference in cortisol levels from late pregnancy until postpartum period.
With regard to stress biomarkers, trauma history was associated with higher cortisol levels from late pregnancy to postpartum period compared to the non-trauma group. On the other hand, the trauma and non-trauma groups were similar in α-amylase levels throughout the studied time points. These findings support that a trauma history can elicit higher activity in the HPA-axis. Therefore, as cortisol levels indicate, a trauma history is associated with a maladaptive course of stress-related metabolism in women with a trauma history after a pregnancy without complications. Similarly, previous research on individuals with a trauma history but non suffering from PTSD has found an increase of cortisol levels (Miller et al., Citation2007). Conversely, other studies have found lower cortisol levels but only when it triggered a PTSD (Meewisse et al., Citation2007; Morris et al., Citation2012). Of note, our patients had a history of traumatic events and subsyndromic PTSD, but it was not diagnosed (Habersaat et al., Citation2014). Thus, high cortisol levels may be an indicator of high chronic stress in traumatized women over time (Kuhlman et al., Citation2015). Nevertheless, both groups showed an increase in α-amylase levels at three months after birth, which indicates that when women have to face motherhood, SAM axis may be over-activated, triggering an acute physiological response to stress (Giesbrecht, Granger, Campbell, & Kaplan, Citation2013). Furthermore, women who suffered from a threat to the physical integrity of self showed higher α-amylase levels than other types of trauma (see Feldman, Vengrober, Eidelman-Rothman, & Zagoory-Sharon, Citation2013, for a similar finding with war-exposed children). This association may indicate a hypervigilance symptom due to the fact that their body is permanently prepared for the action (Thoma et al., Citation2012b).
Regarding psychological symptoms, our findings showed that the differences between women with and without a trauma history depend on the time of testing: a trauma history was associated with higher anxiety symptoms from late pregnancy to childbirth, but this difference was reversed during postpartum period. Previous research has found that women with anxiety during pregnancy and postpartum periods reported higher traumatic experiences (Howard et al., Citation2013), which may be associated with a higher difficulty to adapt to new stressors such as pregnancy, childbirth, or breeding. Our findings add to previous literature that, when women with a trauma history are compared with women with non-trauma history, the difference in anxiety symptoms at late pregnancy is reversed from childbirth. Keeping in mind that childbirth is a time of intense stress for some mothers who develop a fear of subsequent childbirth (Räisänen et al., Citation2014), women with a trauma history did not feel higher anxiety. Therefore, a trauma history may not constitute a vulnerable factor for the fear of subsequent childbirth. Moreover, anxiety symptoms were lower in women with trauma history at three months after birth. This finding may indicate that, when anxiety related to the concern for the security of pregnancy, foetal health and delivery, was resolved, women with a trauma history may perceive a good adaptation to motherhood (Gourounti, Anagnostopoulos, & Lykeridou, Citation2013). According to depressive symptoms, women who reported a trauma history showed similar depressive symptoms from late pregnancy to postpartum period. Some studies have indicated that a trauma history is associated with a less sharp decline from pregnancy to postpartum (Grekin et al., Citation2017; Poehlmann et al., Citation2009). This apparent discrepancy may be explained by the control of potential confounding factors that may affect depressive symptoms after birth: age (García-Blanco et al., Citation2017), parity (Dipietro et al., Citation2008), time of testing (Gourounti, Citation2016), and additional stress-generating factors such as medical status and risk of social exclusion (Álvarez-Segura et al., Citation2014).
Finally, concerning the relationship between self-reported symptoms and stress biomarkers, women with a trauma history showed stronger associations than non-exposed women. Anxiety symptoms showed the highest associations across time. Finally, anxiety symptoms and depressive symptoms were the highest correlated measures. These findings support the lack of association between stress biomarkers and self-reported stress symptoms showed by previous studies (Harville et al., Citation2009), even in women with a trauma history.
Our study is strengthened because we examined both physiological and psychological stress at different points of time considering other potential confounding factors (age, parity, gestational week at T1, and additional stress-generating factors). Regarding the limitations of our study, the lack of measures taken before pregnancy make it impossible to conclude if anxious-depressive symptoms and stress biomarkers reach a similar profile prior to pregnancy. Furthermore, although participants were instructed to rest during the night and the sampling time was controlled (minimum one hour after breakfast and between 10 and 12 a.m.), the time of awakening was not registered. Additionally, our results could not be generalized to women with medical complications or at risk of social exclusion. The very strict selection criteria of participants limited the size of our sample. Finally, although additional models with the trauma group examined the influence of years since trauma, PTSD symptoms, and trauma type, the size of our sample does not allow reaching conclusive findings. Therefore, further research should consolidate these preliminary results.
To sum up, trauma history is associated with an increase in cortisol levels in pregnant women from late pregnancy to postpartum period. That is, the HPA axis activity is increased in women with a trauma history until postpartum period. Contrarily, if delivery has been achieved without major medical complications, a trauma history does not seem to have a negative effect on anxiety symptoms during postpartum period. In fact, anxiety symptoms displayed a significant decrease at three months after birth in women with a trauma history in comparison with women without one. Therefore, whereas a maternal trauma history may not be a risk for mother–child bonding due to the absence of anxious-depressive symptoms during postpartum period, it can increase the risk of newborn neurodevelopment impairment, which is associated with maternal HPA axis dysregulation (Zijlmans, Riksen-Walraven, & de Weerth, Citation2015) and prenatal anxiety symptoms (Tuovinen et al., Citation2018). In clinical practice, psychological therapy to improve cognitive-behavioral coping strategies should be applied during pregnancy in women with a trauma history.
Supplemental Material
Download Zip (23.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Álvarez-Segura, M., García-Esteve, L., Torres, A., Plaza, A., Imaz, M. L., Hermida-Barros, L., … Burtchen, N. (2014). Are women with a history of abuse more vulnerable to perinatal depressive symptoms? A systematic review. Archives of Women‘S Mental Health, 17, 343–11.
- Beck, A. T., Rial, W. Y., & Rickels, K. (1974). Short form of depression inventory: Cross-validation. Psychological Reports, 34, 1184–1186.
- Bind, M. A., Peters, A., Koutrakis, P., Coull, B., Vokonas, P., & Schwartz, J. (2016). Quantile regression analysis of the distributional effects of air pollution on blood pressure, heart rate variability, blood lipids, and biomarkers of inflammation in elderly American men: The normative aging study. Environmental Health Perspectives, 124, 1189–1198.
- Blackmore, E. R., Putnam, F. W., Pressman, E. K., Rubinow, D. R., Putnam, K. T., Matthieu, M. M., … O‘Connor, T. G. (2016). The effects of trauma history and prenatal affective symptoms on obstetric outcomes. Journal of Traumatic Stress, 29, 245–252.
- Bosch, J. A., Veerman, E. C. I., de Geus, E. J., & Proctor, G. B. (2011). α-Amylase as a reliable and convenient measure of sympathetic activity: Don’t start salivating just yet! Psychoneuroendocrinology, 36, 449–453.
- Dipietro, J. A., Costigan, K. A., & Sipsma, H. L. (2008). Continuity in self-report measures of maternal anxiety, stress, and depressive symptoms from pregnancy through two years postpartum. Journal of Psychosomatic Obstetrics & Gynecology, 29, 115–124.
- Escalona, R., Tupler, L. A., Saur, C. D., Krishnan, K. R. R., & Davidson, J. R. (1997). Screening for trauma history on an inpatient affective‐disorders unit: A pilot study. Journal of Traumatic Stress, 10, 299–305.
- Feldman, R., Vengrober, A., Eidelman-Rothman, M., & Zagoory-Sharon, O. (2013). Stress reactivity in war-exposed young children with and without posttraumatic stress disorder: Relations to maternal stress hormones, parenting, and child emotionality and regulation. Development and Psychopathology, 25, 943–955.
- García-Blanco, A., Diago, V., Hervás, D., Ghosn, F., Vento, M., & Cháfer-Pericás, C. (2018). Anxiety and depressive symptoms, and stress biomarkers in pregnant women after in vitro fertilization: A prospective cohort study. Human Reproduction, 33, 1237–1246.
- García-Blanco, A., Monferrer, A., Grimaldos, J., Hervás, D., Balanzá-Martínez, V., Diago, V., … Cháfer-Pericás, C. (2017). A preliminary study to assess the impact of maternal age on stress-related variables in healthy nulliparous women. Psychoneuroendocrinology, 78, 97–104.
- García-Blanco, A., Vento, M., Diago, V., & Cháfer-Pericás, C. (2016). Reference ranges for cortisol and α-amylase in mother and newborn saliva samples at different perinatal and postnatal periods. Journal of Chromatography B, 1022, 249–255.
- Giesbrecht, G. F., Granger, D. A., Campbell, T., & Kaplan, B. (2013). Salivary alpha-amylase during pregnancy: Diurnal course and associations with obstetric history, maternal demographics, and mood. Developmental Psychobiology, 55, 156–167.
- Gourounti, K. (2016). Psychological stress and adjustment in pregnancy following assisted reproductive technology and spontaneous conception: A systematic review. Women Health, 56, 98–118.
- Gourounti, K., Anagnostopoulos, F., & Lykeridou, K. (2013). Coping strategies as psychological risk factor for antenatal anxiety, worries, and depression among Greek women. Archives of Women‘S Mental Health, 16, 353–361.
- Grekin, R., Brock, R. L., & O’Hara, M. W. (2017). The effects of trauma on perinatal depression: Examining trajectories of depression from pregnancy through 24 months postpartum in an at-risk population. Journal of Affective Disorders, 218, 269–276.
- Habersaat, S., Borghini, A., Nessi, J., Pierrehumbert, B., Forcada-Guex, M., Ansermet, F., & Müller-Nix, C. (2014). Posttraumatic stress symptoms and cortisol regulation in mothers of very preterm infants. Stress & Health, 30, 134–141.
- Harville, E. W., Savitz, D. A., Dole, N., Herring, A. H., & Thorp, J. M. (2009). Stress questionnaires and stress biomarkers during pregnancy. Journal of Women‘S Health, 18, 1425–1433.
- Howard, L. M., Oram, S., Galley, H., Trevillion, K., & Feder, G. (2013). Domestic violence and perinatal mental disorders: A systematic review and meta-analysis. PLoS Medicine, 10, e1001452.
- Keeshin, B. R., Strawn, J. R., Out, D., Granger, D. A., & Putnam, F. W. (2015). Elevated salivary alpha amylase in adolescent sexual abuse survivors with posttraumatic stress disorder symptoms. Journal of Child and Adolescent Psychopharmacology, 25, 344–350.
- Kuhlman, K. R., Vargas, I., Geiss, E. G., & Lopez-Duran, N. L. (2015). Age of trauma onset and HPA axis dysregulation among trauma-exposed youth. Journal of Traumatic Stress, 28, 572–579.
- Lang, A. J., Rodgers, C. S., & Lebeck, M. M. (2006). Associations between maternal childhood maltreatment and psychopathology and aggression during pregnancy and postpartum. Child Abuse & Neglect, 30, 17–25.
- Laurent, H., Goodman, S. H., Stowe, Z. N., Halperin, M., Khan, F., Wright, D., … Knight, B. (2018). Course of ante-and postnatal depressive symptoms related to mothers’ HPA axis regulation. Journal of Abnormal Psychology, 127(4), 404–416.
- Meewisse, M. L., Reitsma, J. B., De Vries, G. J., Gersons, B. P. R., & Olff, M. (2007). Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. The British Journal of Psychiatry, 191, 387–392.
- Middlemiss, W. (2003). Poverty, stress, and support: Patterns of parenting behaviour among lower income black and lower income white mothers. Infant and Child Development, 12, 293–300.
- Miller, G. E., Chen, E., & Zhou, E. S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45.
- Morris, M. C., Compas, B. E., & Garber, J. (2012). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clinical Psychology Review, 32, 301–315.
- Muzik, M., McGinnis, E. W., Bocknek, E., Morelen, D., Rosenblum, K. L., Liberzon, I., … Abelson, J. L. (2016). PTSD symptoms across pregnancy and early postpartum among women with lifetime PTSD diagnosis. Depression and Anxiety, 33, 584–591.
- Nater, U. M., & Rohleder, N. (2009). Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology, 34, 486–496.
- Onoye, J. M., Shafer, L. A., Goebert, D. A., Morland, L. A., Matsu, C. R., & Hamagami, F. (2013). Changes in PTSD symptomatology and mental health during pregnancy and postpartum. Archives of Women‘S Mental Health, 16, 453–463.
- Pearlstein, T., Howard, M., Salisbury, A., & Zlotnick, C. (2009). Postpartum depression. American Journal of Obstetrics and Gynecology, 200, 357–364.
- Poehlmann, J., Schwichtenberg, A. J., Bolt, D., & Dilworth-Bart, J. (2009). Predictors of depressive symptom trajectories in mothers of preterm or low birth weight infants. Journal of Family Psychology, 23, 690–704.
- Räisänen, S., Lehto, S. M., Nielsen, H. S., Gissler, M., Kramer, M. R., & Heinonen, S. (2014). Fear of childbirth in nulliparous and multiparous women: A population‐based analysis of all singleton births in Finland in 1997–2010. BJOG: an International Journal of Obstetrics & Gynaecology, 121(8), 965–970.
- Smith, S. M., & Vale, W. W. (2006). The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience, 8, 383–395.
- Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R., & Jacobs, G. A. (1983). Manual for the state-trait anxiety scale. Palo Alto: Consulting Psychologists Press.
- Thoma, M. V., Joksimovic, L., Kirschbaum, C., Wolf, J. M., & Rohleder, N. (2012a). Altered salivary alpha-amylase awakening response in Bosnian War refugees with posttraumatic stress disorder. Psychoneuroendocrinology, 37, 810–817.
- Thoma, M. V., Kirschbaum, C., Wolf, J. M., & Rohleder, N. (2012b). Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biological Psychology, 91, 342–348.
- Tuovinen, S., Lahti-Pulkkinen, M., Girchenko, P., Lipsanen, J., Lahti, J., Heinonen, K., … Räikkönen, K. (2018). Maternal depressive symptoms during and after pregnancy and child developmental milestones. Depression and Anxiety, 35, 732–741.
- Underwood, L., Waldie, K. E., D’Souza, S., Peterson, E. R., & Morton, S. M. (2017). A longitudinal study of pre-pregnancy and pregnancy risk factors associated with antenatal and postnatal symptoms of depression: Evidence from growing up in New Zealand. Maternal and Child Health Journal, 21, 915–931.
- Vreeburg, S. A., Hartman, C. A., Hoogendijk, W. J., van Dyck, R., Zitman, F. G., Ormel, J., & Penninx, B. W. (2010). Parental history of depression or anxiety and the cortisol awakening response. The British Journal of Psychiatry, 197, 180–185.
- Whisman, M. A., Davila, J., & Goodman, S. H. (2011). Relationship adjustment, depression, and anxiety during pregnancy and the postpartum period. Journal of Family Psychology, 25, 375–383.
- Zijlmans, M. A., Riksen-Walraven, J. M., & de Weerth, C. (2015). Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neuroscience & Biobehavioral Reviews, 53, 1–24.