ABSTRACT
Background: Parents of children with cancer are at risk for sleep problems. If these problems persist, an important perpetuating factor might be ongoing parental distress.
Objective: The aim of this study is to assess the prevalence of sleep problems and the concurrence with distress in parents of children treated for cancer, and to identify predictors of this symptom clustering.
Method: Parents completed the Medical Outcomes Study (MOS) Sleep Scale and Distress Thermometer for Parents (DT-P). Clinically relevant sleep problems were defined as a score >1SD above the norm and clinical distress as a thermometer score above the established cut-off of 4. Four parent categories were constructed: neither sleep problems nor distress; no distress but sleep problems; no sleep problems but distress; both sleep problems and distress. Predictive determinants (sociodemographic, medical, psychosocial) for each category were assessed with multilevel multinomial logistic regression.
Results: Parents (202 mothers and 150 fathers) of 231 children with different cancers participated. Mean time since diagnosis was 3.3 ± 1.4 years (90% off-treatment). The prevalence of sleep problems was 37%. Fifty percent of parents reported neither sleep problems nor distress, 9% had only sleep problems, 13% only distress, and 28% reported both. Compared to parents without sleep problems or distress, parents who reported both were more likely to report parenting problems (OR 4.4, [2.2–9.1]), chronic illness (OR 2.8, [1.2–6.5]), insufficient social support (OR 3.7, [1.5–9.1]), pre-existent sleep problems (OR 6.2, [2.0–18.6]) and be female (OR 1.8, [1.1–4.2]).
Conclusions: Sleep problems are common in parents of children treated for cancer, and occur mostly in the presence of clinical distress. Future research must show which interventions are most effective in this group: mainly targeted at sleep improvement or with prominent roles for stress management or trauma processing.
HIGHLIGHTS
• Pediatric cancer diagnoses are traumatic for parents and can precipitate insomnia. If insomnia persists, an important perpetuating factor might be ongoing distress.• This study in 352 parents of children treated for cancer shows a high prevalence (37%) of sleep problems, in the majority of parents occurring in concurrence with clinical distress. Several predictors were identified.• Future research should reveal which interventions are most effective: targeted at sleep improvement, or at stress management and trauma processing.
Antecedentes: Los padres de niños con cáncer corren el riesgo de tener problemas para dormir. Si estos problemas persisten, podría ser un factor perpetuador importante malestar en los padres.
Método: Los padres completaron el Estudio de Resultados Médicos (MOS, sus siglas en inglés), la escala de sueño y el termómetro de distrés para padres (DT-P, sus siglas en inglés). Los problemas de sueño clínicamente relevantes se definieron como una puntuación> 1 SD por encima de la norma y el malestar clínico como una puntuación de termómetro por encima del límite establecido de 4. Se construyeron cuatro categorías de padres: sin problemas de sueño ni malestar; sin malestar y con problemas de sueño; sin problemas de sueño y con malestar, tanto problemas de sueño como de malestar. Los determinantes predictivos (sociodemográficos, médicos, psicosociales) para cada categoría se evaluaron con regresión logística multinomial multinivel.
Resultados: Participaron padres (202 madres y 150 padres) de 231 niños con diferentes tipos de cáncer. El tiempo medio desde el diagnóstico fue de 3,3 ± 1,4 años (90% sin tratamiento). La prevalencia de problemas de sueño fue del 37%. El cincuenta por ciento de los padres no reportaron problemas de sueño ni malestar, el 9% solo tuvo problemas de sueño, el 13% solo sufrió malestar y el 28% informó de ambos. En comparación con los padres sin problemas de sueño o malestar, los padres que informaron ambos problemas, tenían más probabilidades de reportar problemas de crianza (OR 4.4, [2.2-9.1]), enfermedades crónicas (OR 2.8, [1.2-6.5]), apoyo social insuficiente (OR 3.7), [1.5-9.1]), problemas de sueño preexistentes (OR 6.2, [2.0-18.6]) y ser mujer (OR 1.8, [1.1-4.2]).
Conclusiones: Los problemas del sueño son comunes en los padres de niños tratados por cáncer, y ocurren principalmente en presencia de malestar clínico. Las investigaciones futuras deben mostrar qué intervenciones son más efectivas en este grupo: principalmente dirigidas a mejorar el sueño o con roles prominentes para el manejo del estrés o el procesamiento de traumas.
背景: 患有癌症的儿童的父母有出现睡眠问题的风险。如果这些问题持续存在,一个重要的因素可能是父母出现了持续的精神痛苦。
目的: 本研究的目的是评估患有癌症的儿童的父母中的睡眠问题和并发精神痛苦的患病率,并确定这种症状共存的预测因素。
方法: 父母完成了医疗结果研究(MOS)睡眠量表和父母精神痛苦测量(DT-P)。临床相关的睡眠问题被定义为高于常模1SD,精神痛苦则定义测量得分高于划界分4。构建了四个父母类别:无睡眠问题也无精神痛苦;没有精神痛苦但有睡眠问题;没有睡眠问题但是精神痛苦;睡眠问题和痛苦并存。使用多层多项Logistic回归评估每个类别的(社会人口统计学,医学,社会心理学)预测决定因素。
结果: 231名患有不同癌症的儿童的父母(202名母亲和150名父亲)参加了这项活动。距离诊断的平均时间为3.3±1.4年(90%治疗后)。睡眠问题的患病率为37%。50%的父母报告既没有睡眠问题也没有精神痛苦,9%只有睡眠问题,13%只有精神痛苦,28%报告两者都有。与没有睡眠问题或精神痛苦的父母相比,报告两者都有的父母更有可能报告育儿问题(OR 4.4,[2.2-9.1]),慢性病(OR 2.8,[1.2-6.5]),社会支持不足(OR 3.7) ,[1.5-9.1]),预先存在的睡眠问题(OR 6.2,[2.0-18.6])和作为女性(OR 1.8,[1.1-4.2])。
结论: 睡眠问题在接受癌症治疗的儿童的父母中很常见,并且主要发生在出现临床精神痛苦时。未来的研究必须表明哪些干预措施在这一组中最有效:主要针对睡眠改善或压力管理或创伤加工。
1. Introduction
Sleep problems are common in parents of children with cancer and can have a significant impact on the well-being of the family (Matthews, Neu, Cook, & King, Citation2014; Pollock, Litzelman, Wisk, & Witt, Citation2013; Wright, Citation2011; Zupanec, Jones, & Stremler, Citation2010). On the short term, disrupted sleep leads to higher arousal, psychological stress, decreased cognitive and memory functioning, worse performance and lower quality of life (Klassen et al., Citation2008; Medic, Wille, & Hemels, Citation2017). On the long term, sleep problems are linked to obesity, diabetes, cardiovascular diseases, cancer and even lower life expectancy (Medic et al., Citation2017).
The estimated prevalence of sleep problems in parents of children with cancer ranges from 48% during (outpatient) treatment to 71% in a hospital setting (Coleman et al., Citation2018; Daniel, Walsh, Meltzer, Barakat, & Kloss, Citation2018; McLoone, Wakefield, Yoong, & Cohn, Citation2013; Zupanec et al., Citation2010). Although the evidence is sparse, there are reports of ongoing parental sleep problems after the end of their child’s cancer treatment (Pollock et al., Citation2013).
Reported sleep problems comprise symptoms of insomnia, such as difficulties with falling asleep and awakening during the night. Insomnia is well studied in the general population and several explanatory models have been established (Perlis, Shaw, Cano, & Espie, Citation2011). The conceptual model of Spielman differentiates predisposing, precipitating and perpetuating factors that contribute to the development and persistence of insomnia (Spielman, Yang, & Glovinsky, Citation2011). Predisposing factors are among others: genetic factors, previous sleeping problems, female gender and chronic (mental or physical) illness (Le Blanc et al., Citation2009; Lind & Gehrman, Citation2016). Insomnia often starts after a highly stressful or traumatic event (Bastien, Vallieres, & Morin, Citation2004). In parents of children with cancer, an important precipitating factor is the child’s cancer diagnosis, while perpetuating factors could be dysfunctional sleep behaviors, treatment-related stressors during the active treatment phase, and ongoing parental distress in the post-treatment phase (Medic et al., Citation2017; Neu, Matthews, & King, Citation2014; Williams & McCarthy, Citation2015; Zupanec et al., Citation2010). The vast majority of parents consider their child’s diagnosis and treatment as their most traumatic event (Phipps et al., Citation2015).
Many parents report elevated levels of distress shortly after diagnosis and during treatment (Sultan, Leclair, Rondeau, Burns, & Abate, Citation2016). As time passes, most parents seem resilient and these levels return to normal (Dunn et al., Citation2012; Kazak, Boeving, Alderfer, Hwang, & Reilly, Citation2005; Ljungman, Hoven, Ljungman, Cernvall, & Von Essen, Citation2015; Muscara et al., Citation2018; Price, Kassam-Adams, Alderfer, Christofferson, & Kazak, Citation2015; Vrijmoet-Wiersma et al., Citation2008). While the prevalence of posttraumatic stress disorder (PTSD) is not increased (Phipps et al., Citation2015), about one fifth to one third of the parents report ongoing distress even years after their child’s diagnosis (Sultan et al., Citation2016; Vrijmoet-Wiersma et al., Citation2008; Wijnberg-Williams, Kamps, Klip, & Hoekstra-Webers, Citation2006). The specific form of distress that parents of children with cancer experience is captured in the Pediatric Medical Traumatic Stress (PMTS) model (Price, Kassam-Adams, Alderfer, Christofferson, & Kazak, Citation2016). PMTS is defined as ‘a set of psychological and physiological responses of children and their families to pain, injury, serious illness, medical procedures, and invasive or frightening treatment experiences’. The PMTS model defines three phases that families go through: (1) the initial potentially traumatic event (a pediatric cancer diagnosis) and related first events; (2) persisting stressors, actions and reactions (during active treatment); (3) prolonged – up to months or years – PMTS after end of treatment (Price et al., Citation2016).
Consistent with the above-described models of insomnia and PMTS, we hypothesize that the prevalence of insomnia will be higher in parents of children treated for cancer compared to the general population and that the underlying perpetuating factor may be prolonged distress (PMTS). However, previous research on sleep problems in pediatric oncology has not addressed the concurrence of sleep problems and distress. This understanding is important, in order to be able to support parents most effectively and inform targeted interventions.
The aims of this study are therefore first to assess the prevalence of sleep problems and second to explore the concurrence of sleep problems and distress in parents of children treated for cancer. For the second aim, parents are categorized based on the presence or absence of sleep problems and/or distress, to identify predictors of this symptom clustering.
2. Patients and methods
2.1. Procedure
This study was conducted as part of a large observational multicenter study in parents of children with cancer (‘The Amsterdam Parent Project’) (Rensen et al., Citation2019). Parents of children diagnosed between 2010 and 2015 with cancer including any type of brain tumour (age at diagnosis <19 years) were eligible if they sufficiently mastered Dutch to fill out questionnaires and their child was not deceased or in palliative treatment. Families’ eligibility was evaluated by medical chart review and consultation of the attending physician. Eligible parents received a written invitation to participate independently from each other. They could choose to complete standardized questionnaires either on paper or online through a secured website and provided additional socio-demographic information (see sections below). Participating centres were the Amsterdam University Medical Centers (Emma Children’s hospital/Academic Medical Center and VU University Medical Center). Parents received at least one written reminder to fill out the questionnaires. If they had not responded to the reminder either, they received a phone call by one of the members of the research team to assess their willingness to participate.
The Institutional Review Board of the VU University Medical Center approved this study.
2.2. Measures
2.2.1. Sociodemographic characteristics
Parents provided the following information through a demographic survey: age, gender, marital status (dichotomized as married/living together versus other), country of birth (Netherlands versus other), educational level (according to Statistics Netherlands (Centraal Bureau voor de Statistiek, Citation2016), dichotomized as low/middle versus high), employment (paid job yes or no), presence (yes or no) and description of chronic illnesses, pre-existent sleep problems (yes or no) and the use of sleep medication (yes or no).
2.2.2. Child (medical) characteristics
Information on child (medical) variables was collected through chart review. Available characteristics were child’s current age and gender, type of diagnosis (categorized as hematologic malignancy, brain tumor, solid tumor or retinoblastoma), time since diagnosis, recurrence of cancer or a second tumor (yes or no), active treatment at time of study (yes or no) and type of treatment. Type of treatment was categorized as low risk (wait-and-see policy, surgery only, local therapy other than radiotherapy), middle risk (chemotherapy with or without surgery) and high risk (any combination with radiotherapy and/or stem cell transplantation). This is a commonly used categorization in pediatric oncology studies, based on the associated risk of therapy with acute and chronic toxicity (Bhakta et al., Citation2017; Gibson et al., Citation2018).
2.2.3. Sleep
Sleep was measured using the Medical Outcomes Study (MOS) sleep scale (Hays, Martin, Sesti, & Spritzer, Citation2005). The MOS-Sleep is a one-week retrospective, validated and reliable 12-item instrument with six scales. These include: (i) sleep disturbance (problems with falling asleep initially and falling back asleep after nightly awakenings, 4 items); (ii) sleep adequacy (getting enough sleep and feeling rested in the morning, 2 items); (iii) daytime somnolence (daytime naps and feeling somnolent, 3 items); (iv) snoring (1 item); (v) awakening short of breath or with headache (1 item), and (vi) quantity of sleep (1 item). Quantity of sleep is scored as the average hours slept per night, with optimal sleep duration defined as between 7 and 8 h per night. The other scales are scored on a 0–100 possible range, with higher scores indicating more sleep problems on each scale (except for sleep adequacy, where higher scores reflect better sleep). Second, items are scored into a 9-item sleep problem index (SLP-9), ranging from 0 to 100 (Spritzer & Hays, Citation2003). The SLP-9 comprises 9 of 12 items (all but the items on the quantity of sleep, snoring and daytime naps) and mostly represents symptoms consistent with insomnia like troubled initiation or maintenance of sleep, and daytime consequences of poor sleep.
MOS questionnaire (sub)scale scores were constructed following the MOS manual’s guidelines (Spritzer & Hays, Citation2003). The Dutch version of the MOS-Sleep has adequate psychometric properties and Dutch reference values of healthy adults are available (de Weerd et al., Citation2004; Hays et al., Citation2005). Cronbach’s alpha for the (sub)scales in the study sample ranged from 0.74 to 0.88.
2.2.4. Distress
The Distress Thermometer for Parents (DT-P) was used to measure psychosocial functioning (Haverman et al., Citation2013). The DT-P consists of a thermometer on which parents are asked to rate their overall distress regarding physical, emotional, social and practical issues on a scale of 0 to 10, with 4 or higher indicating clinical distress. Additionally, there are specific items on different problem domains (practical, social, emotional, physical, cognitive and parenting), and five single items on the presence of chronic illness in the parent, rating of contact with the hospital personnel, perceived social support, understanding from surroundings, and wish for a referral. Scores on problem domains were calculated if at least 50% of the items within a domain were completed. The parenting problem domain was included in this study (dichotomized as no or at least one problem), but not the other problem domains due to concerns about collinearity with the overall thermometer score. Furthermore, the DT-P single items on perceived social support (sufficient/insufficient) and wish for referral (dichotomized as yes/maybe or no) were included, but not the items on chronic illness (already derived from sociodemographic questionnaire), contact with hospital personnel (since the majority of the child sample had finished treatment) and understanding from surroundings (correlated with social support). The validity and internal consistency of the DT-P are good and Dutch reference values of parents of healthy children are available (Haverman et al., Citation2013; van Oers, Schepers, Grootenhuis, & Haverman, Citation2017).
2.3. Statistical analysis
Independent t-tests and Fisher’s exact tests were used to assess differences in child characteristics between responders and non-responders.
2.3.1. Description of sleep and distress
Since there were no Dutch norm values separately for men and women on the MOS Sleep Scale, MOS subscale values and DT-P scores were described for the entire study population and compared to norm values with one-sided t-tests (continuous variables) or chi-square tests (dichotomous items). For the differences on the MOS subscales, effect sizes (Cohen’s d) were calculated. The effect sizes were interpreted as follows: 0.2–0.5 small, 0.5–0.8 moderate, ≥0.8 large (Cohen, Citation1988). Moreover, the percentages of parents with clinically relevant sleep problems and clinical distress levels were described. For this purpose, the SLP-9 sum scores were dichotomized into scores ≤1SD and >1SD above the Dutch population’s mean (de Weerd et al., Citation2004). Parents with SLP-9 scores >1SD above the population’s mean were considered to have clinically relevant sleep problems. Additionally, the percentage of parents with severe sleep problems was described, defined as an SLP-9 score >2SD above the population’s mean. These SD cut-offs have been widely used for psychological scales in the previous literature (1SD for moderate/subclinical problems, 2SD for severe/clinical problems), including pediatric oncology studies (Litsenburg et al., Citation2014; Sung et al., Citation2009, Citation2011). For distress, the thermometer scores were dichotomized into clinical versus non-clinical according to the pre-established cut-off value of 4.
2.3.2. Concurrence of sleep problems and distress
Four categories of parents were explored, according to the presence or absence of clinical sleep problems and/or distress: 1) parents with neither sleep problems nor distress; 2) parents with sleep problems but without distress; 3) parents without sleep problems but with distress; and 4) parents with both sleep problems and distress. The prevalence of each group was determined. Per group, descriptive statistics were performed on sociodemographic and child (medical) factors, sleep characteristics, distress thermometer score, and psychosocial factors as measured with the DT-P. Since parents of children that were still receiving treatment at the time of the study were expected to experience more (acute) distress and sleep problems than parents of children that finished treatment, the prevalence of each category was additionally determined separately for parents of children in the post-treatment phase. Furthermore, the prevalence of each category was additionally determined for parents with severe sleep problems (cut-off of >2SD above the Dutch population’s SLP-9 score), to explore if conclusions would be different if a different cut-off value was chosen for sleep problems.
2.3.3. Determinants of sleep problems and distress
To assess predictive determinants of each group membership, multilevel multinomial logistic regression was performed, with a random intercept on child level to correct for the dependency of caregivers. The group of parents without sleep problems or distress was chosen as the reference category. The above-mentioned parent sociodemographic, psychosocial and child variables were tested as predictors, except for active treatment and use of sleep medication because of low prevalence, and wish for a referral. A forward selection approach was performed. First, all variables were tested for their relationship with the outcome in univariate regression. The variable with the lowest p-value was added to the model first, and all other variables were tested again in this new model, with the now strongest predictor added to the model as the second. This procedure was repeated until there were no more significant variables to add, defined as having a p-value of <0.05 in the final model. A p-value of <0.05 was preferred to the commonly used cut-off of 0.10 in these kind of analyses, considering the multiple determinants that were tested. The effect of each variable was shown as Odds ratio (OR) with a 95% confidence interval (C.I.).
3. Results
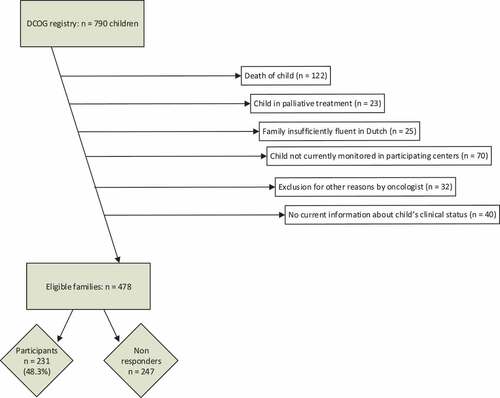
shows the establishment of the study population (flowchart) (Rensen et al., Citation2019). Of the 790 families assessed for eligibility, 312 were excluded according to the predefined criteria. The remaining 478 families were invited and 231 of them responded (response rate 48%). Respondents were 121 parental couples and 110 individual parents, in total 202 mothers and 150 fathers. Respondents’ child characteristics did not differ from non-respondents, besides a small but statistically significant difference in time since diagnosis (respectively, 39 months vs 42 months, p = 0.03). The participants had a child that was most often post-treatment (90%) and on average 3.3 ± 1.4 years from diagnosis. Sixty-four parents self-reported that they had one or more chronic diseases, mostly psychological/psychiatric (11 times), endocrine (10 times), musculoskeletal (8 times), pulmonary (7 times), neurological (7 times) or gastrointestinal (5 times).
3.1. Description of sleep and distress
All parents combined () reported significantly more sleep problems than people from the Dutch reference population on the scales sleep disturbance, somnolence, sleep adequacy and the SLP-9 sum scale. Effect sizes were small to moderate (0.2–0.6). The prevalence of overall clinically relevant sleep problems was 37%, compared to 16% (1SD) in the general population. Severe sleep problems (cut-off of 2SD) occurred in 18%, compared to 2.5% in the general population (not shown in table). There were no significant differences on the scales snoring and waking up short of breath/with headache. Regarding distress (), parents of children with cancer scored on average below the clinical threshold of 4 and reported values similar to the reference sample of parents of healthy children (mean thermometer score 3.3 vs 3.2). Prevalence of insufficient social support, wishes for referral and parenting problems were significantly higher than in the reference sample.
Table 1. Mean MOS scale scores, standard deviations and effect sizes of parents of children with cancer and the reference sample (de Weerd et al., Citation2004).
Table 2. Distress thermometer score, perceived social support, parenting problems and wish for referral of parents of children with cancer and the reference sample. (Van Oers et al., Citation2017).
3.2. Concurrence of sleep problems and distress
Data on both sleep and distress were available from 332 parents. Half of them (50%) reported neither relevant sleep problems nor elevated distress levels. On the other hand, more than one fourth (28%) reported both; 17% of the fathers and 35% of the mothers fell in this category, and 50% of the parents in this group stated a wish for a referral. The prevalence of sleep problems without distress (9%) or clinical distress levels without sleep problems (13%) was lower. summarizes parent and child characteristics per group, and shows sleep and psychosocial characteristics. Exclusion of the parents (n = 40) of children still in active treatment (n = 24) did not importantly change the prevalence of parent categories: 52% of the parents reported neither clinical sleep problems nor elevated distress levels; 26% reported both; 10% reported only sleep problems; and 11% only distress. Using severe sleep problems as cut-off, the prevalence of parent categories changed into: no sleep problems or distress (56%); both sleep problems and distress (15%); only sleep problems (2%); only distress (27%).
Table 3. Sociodemographic and child characteristics for each group of parents, classified by sleep and distress (n = 332).
Table 4. Sleep and psychosocial characteristics for each group of parents, classified by sleep and distress (n = 332).
3.3. Determinants of sleep problems and distress
shows the final multinomial multilevel logistic regression model. Parents with sleep problems and distress were more likely to experience parenting problems (OR 4.4, 95% C.I. [2.2, 9.1]), report a chronic illness (OR 2.8, 95% C.I. [1.2, 6.5]), experience insufficient social support (OR 3.7, 95% C.I. [1.5, 9.1]), have pre-existent sleep problems (OR 6.2, 95% C.I. [2.0, 18.6]) and be female (OR 2.1, 95% C.I. [1.1, 4.2]) compared to parents that had no sleep problems or distress. Parents with sleep problems only were likely to have pre-existent sleep problems (OR 12.4, 95% C.I. [3.6, 43.4]) and to have a younger child (OR 0.9 (per one year increase), 95% C.I. [0.8, 1.0]) compared to parents without sleep problems or distress. Parents with clinically elevated distress levels but without sleep problems were more likely to have a child with (a history of) high-risk treatment (OR 3.9, 95% C.I. [1.1, 13.7]) and to report parenting problems (OR 3.3, 95% C.I. [1.5, 7.5]). The final multinomial multilevel logistic regression model was significant (p < 0.001).
Table 5. Multilevel multinomial logistic regression: predictors of sleep problems and distress per group.
4. Discussion and conclusion
This study aimed to explore the prevalence and concurrence of sleep and distress in parents of children with cancer within the first few years from diagnosis and to identify predictive determinants. Importantly, 50% of the parents did not report sleep problems nor distress, which suggests that these parents are resilient. However, the prevalence of clinically relevant sleep problems was 37% in our population (18% if only severe sleep problems were taken into account), compared to 16% (or 2.5%) in the general population. Although on average our sample did not experience significantly more distress than parents of healthy children, the majority (75%) of the parents with sleep problems reported clinical distress levels as well. In parents with severe sleep problems, almost 90% of these parents also reported clinical distress. Compared to those with neither sleep problems nor distress, parents who reported both were more likely to experience parenting problems, have a chronic illness, perceive little social support, have pre-existent sleep problems and be female (OR 2.1–6.2). Besides these two groups, we identified two smaller groups of parents with either sleep problems or distress. The group with sleep problems only were more likely to report pre-existent sleep problems and to have a younger child. The group of distressed parents without sleep problems was more likely to perceive parenting problems and have a child with (a history of) high-risk treatment.
This study adds new and important information on parental functioning in childhood cancer.
According to the insomnia and PMTS models (Price et al., Citation2016; Spielman et al., Citation2011), we hypothesized that the prevalence of insomnia would be higher in parents of children with cancer and that a perpetuating factor could be ongoing distress. In previous studies distress is mostly seen as a predictive factor of sleep problems (Lee, Yiin, Lu, & Chao, Citation2015; Meltzer & Moore, Citation2007; Seixas et al., Citation2015), although the relation could also be reversed (Doane & Thurston, Citation2014; Pollock et al., Citation2013). Sleep disruption can lead to higher distress through increased sympathetic activity with the release of catecholamines ((nor)adrenalin) and enhanced cortisol secretion (Medic et al., Citation2017) – which in turn affects sleep. Our study does not answer questions on causal mechanisms of distress and sleep in parents of children with cancer. It could be that these parents primarily suffer from ongoing distress due to the traumatic life event of having their child diagnosed with a life-threatening disease and the subsequent worries about prognosis, long-term effects of treatment or relapses, following the phases of the PMTS model. Moreover, after treatment, parents need to process an intense period of hospital visits and concerns, and some experience ongoing PMTS. However, given the fact that distress levels return to normal in most parents as time passes (Vrijmoet-Wiersma et al., Citation2008), it might also be that parents first develop sleep problems, which form the basis of increased distress. For example because of nighttime caregiving to their ill child or troubled nights in the hospital with (sometimes) poor sleeping facilities for parents, leading to dysfunctional sleep habits (Bevan, Grantham-Hill, & Bowen et al., Citation2019; Stremler et al., Citation2014).
Furthermore, it is possible that some parents in this study were experiencing posttraumatic stress symptoms (PTSS) or even PTSD (Kazak, Citation2004). However, no formal PTSS or PTSD assessment was performed in this study, and other PTSS such as re-experience, avoidance, negative thoughts or feelings and signs of increased arousal (besides sleep problems) were not assessed. Moreover, the MOS sleep scale that we used in this study is not developed for assessing causes of PTSS-related sleep disturbance, e.g. due to nightmares. This should be addressed in future research.
The following clinical implications can be derived. First, it is important to incorporate psychosocial screening and assistance in clinical practice, also after completion of the child’s cancer treatment, to recognize parents in need. This recommendation has already been made in the Standards of Psychosocial Care for Parents of Children with Cancer (Kearney, Salley, & Muriel, Citation2015) and the European Standards of Care for Children with Cancer (Kowalczyk et al., Citation2014), but is still not routinely available in many hospitals (Jones et al., Citation2018; Kowalczyk et al., Citation2016). We argue, based on the results of this study that screening should include a sleep assessment as well. Special attention should be paid to vulnerable parents, i.e. mothers, parents who are chronically ill themselves, and parents who perceive parenting difficulties and little social support. Interestingly, none of these factors are specifically treatment-related. The finding that medical factors are not majorly important in predicting parental well-being, especially after a child’s cancer treatment (which was true for 90% of our sample), is in line with findings from previous pediatric oncology studies (Ljungman et al., Citation2014; Rensen et al., Citation2019; Vrijmoet-Wiersma et al., Citation2008). For example, it is known from the literature that parent-reported prognostic estimates often mismatch physician’s estimates, with parents being far more optimistic; hence Klassen and colleagues included ‘parent-rated prognosis’ in their multivariate models of parental quality of life (QoL), and did not identify worse prognosis as a significant predictor of QoL impairment (Klassen et al., Citation2008).
Second, we should aim at designing effective interventions that target both sleep problems and distress, especially since more than 50% of the parents in this group stated a wish for a referral. First-line treatment of insomnia is cognitive behavioural therapy for insomnia (CBT-i) (Sateia & Buysse, Citation2011). CBT-i usually contains the following components: education on sleep hygiene, instructions on stimulus control, sleep restriction, restructuring cognitions and thoughts on sleep, and relaxation techniques (Morin, Citation2011). It has been shown to be effective for improving several sleep outcomes in multiple populations (Morin, Citation2011). As some parts of CBT-i focus on relaxation (Morin, Citation2011), it may simultaneously have a positive effect on distress. As such, previous research has shown positive effects of CBT-i on comorbid depressive disorder and on sleep disorders related to PTSD (Ho, Chan, & Tang, Citation2016; Manber et al., Citation2008). To our knowledge, only one pilot study tested a form of CBT-i (including relaxation) to improve sleep in family caregivers of adult patients with cancer. The study had minor favourable results but was limited by the small sample size (Carter, Citation2006). No sleep interventions have been tested in parents of children with cancer. In this population, it might be that regular CBT-i is insufficient and should be adapted to specific needs of these parents, e.g. with a more prominent role for stress-management or trauma processing in the context of childhood cancer. Some stress-focused cognitive behavioural therapy (CBT) and acceptance and commitment therapy (ACT) interventions reported in literature are proven effective or seem promising in relieving parental distress in childhood cancer, but their effects on sleep have not been investigated yet (Kazak et al., Citation2004; Ljungman et al., Citation2018; Rayner et al., Citation2016; Sahler et al., Citation2013; Wakefield et al., Citation2016). Additionally, other therapies such as Eye Movement Desensitization and Reprocessing (EMDR) might also be useful in co-treating sleep disturbances and post traumatic symptoms, an effect that was shown in several (small) PTSD populations (Brownlow, Harb, & Ross, Citation2015; Raboni, Alonso, Tufik, & Suchecki, Citation2014; Raboni, Tufik, & Suchecki, Citation2006). Yet this should be investigated in future studies.
Finally, future research should longitudinally study parental sleep and distress. We identified two smaller subgroups of parents reporting either distress or sleep problems. It might be that parents in one of these groups transform to the group without any problems or progress to having both sleep problems and clinical distress. Longitudinal research is necessary to assess the course of problems and determine optimal timing for interventions.
4.1. Limitations
We are aware that the cross-sectional design used in this study only provides information on one specific moment in time. Second, there are limitations in comparing our sample to reference data. In particular, we had no age- or gender-specific reference data on sleep. However, distribution of age (mean 43 years in both populations) and gender (43% male in study population versus 41% in MOS reference sample) did not differ, and these variables are known to be associated with sleep (Le Blanc et al., Citation2009). Third, the relatively low response rate (48%) might indicate some participation bias, since we have no information on the characteristics of non-participating parents. Yet we did compare responders and non-responders in terms of child’s (clinical) characteristics and only found a minimal difference in time since diagnosis (with children in the study population being on average 3 months closer to diagnosis). Fourth, it might be that the DT-P is not specific enough for really measuring clinical distress. The DT-P is validated against the Hospital Anxiety and Depression Scale (HADS) and has a specificity of 67%; hence people with a clinical thermometer score may not directly have a clinical HADS score as well. Besides, we did not include a formal PTSS/PTSD instrument, as mentioned before. Finally, it would have been interesting to have information on parental pre-existing psychopathology and include questionnaires on personality traits, sleep behaviours and coping styles, as well as to include child’s sleep, since we know from previous research that these aspects influence parental sleep and functioning as well (Cousino & Hazen, Citation2013; Daniel et al., Citation2018; Matthews et al., Citation2014; Price et al., Citation2016).
4.2. Conclusion
Sleep problems in combination with clinically elevated levels of distress are common in parents of children with cancer, even years after their child’s diagnosis. Particularly at risk are mothers who are chronically ill themselves, had pre-existent sleep problems, and report insufficient social support and parenting difficulties. This study stresses the need for psychosocial screening in clinical practice. Future research must show which type of interventions are most effective in this group: mainly targeted at sleep improvement, or with prominent roles for stress management or trauma processing, or both.
Data availability statement
The data of this study are available from the corresponding author (RvL), upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bastien, C. H., Vallieres, A., & Morin, C. M. (2004). Precipitating factors of insomnia. Behavioral Sleep Medicine, 2, 50–12.
- Bevan, R., Grantham-Hill, S., Bowen, R.,Clayton, E., Grice, H., Venditti, H. C., … Hill, C. M. (2019). Sleep quality and noise: Comparisons between hospital and home settings. Archives of Disease in Childhood, 104, 147–151.
- Bhakta, N., Liu, Q., Ness, K. K., Baassiri, M., Eissa, H., Yeo, F., … Robison, L. L. (2017). The cumulative burden of surviving childhood cancer: An initial report from the St Jude lifetime cohort study (SJLIFE). The Lancet, 390, 2569–2582.
- Brownlow, J. A., Harb, G. C., & Ross, R. J. (2015). Treatment of sleep disturbances in post-traumatic stress disorder: A review of the literature. Current Psychiatry Reports, 17, 41.
- Carter, P. A. (2006). A brief behavioral sleep intervention for family caregivers of persons with cancer. Cancer Nursing, 29, 95–103.
- Centraal Bureau voor de Statistiek. (2016). Standaard Onderwijsindeling 2016 [Standard educational classification]. Den Haag/Heerlen: Centraal Bureau voor de Statistiek.
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. New York: Academy Press.
- Coleman, K., Flesch, L., Petiniot, L., Pate, A., Lin, L., Crosby, L., … Dandoy, C. E. (2018). Sleep disruption in caregivers of pediatric stem cell recipients. Pediatric Blood & Cancer, 65, e26965.
- Cousino, M. K., & Hazen, R. A. (2013). Parenting stress among caregivers of children with chronic illness: A systematic review. Journal of Pediatric Psychology, 38, 809–828.
- Daniel, L. C., Walsh, C. M., Meltzer, L. J., Barakat, L. P., & Kloss, J. D. (2018). The relationship between child and caregiver sleep in acute lymphoblastic leukemia maintenance. Supportive Care in Cancer, 26, 1123–1132.
- de Weerd, A., de Haas, S., Otte, A., Trenité, D. K., van Erp, G., Cohen, A., … van Gerven, J. (2004). Subjective sleep disturbance in patients with partial epilepsy: A questionnaire-based study on prevalence and impact on quality of life. Epilepsia, 45, 1397–1404.
- Doane, L. D., & Thurston, E. C. (2014). Associations among sleep, daily experiences, and loneliness in adolescence: Evidence of moderating and bidirectional pathways. Journal of Adolescence, 37, 145–154.
- Dunn, M. J., Rodriguez, E. M., Barnwell, A. S., Grossenbacher, J. C., Vannatta, K., Gerhardt, C. A., & Compas, B. E. (2012). Posttraumatic stress symptoms in parents of children with cancer within six months of diagnosis. Health Psychology, 31, 176–185.
- Gibson, T. M., Mostoufi-Moab, S., Stratton, K. L., Leisenring, W. M., Barnea, D., Chow, E. J., … Oeffinger, K. C. (2018). Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: A report from the childhood cancer survivor study cohort. The Lancet Oncology, 19, 1590–1601.
- Haverman, L., van Oers, H. A., Limperg, P. F., Houtzager, B. A., Huisman, J., Darlington, A.-S., … Grootenhuis, M. A. (2013). Development and validation of the distress thermometer for parents of a chronically ill child. The Journal of Pediatrics, 163, 1140–1146 e1142.
- Hays, R. D., Martin, S. A., Sesti, A. M., & Spritzer, K. L. (2005). Psychometric properties of the medical outcomes study sleep measure. Sleep Medicine, 6, 41–44.
- Ho, F. Y., Chan, C. S., & Tang, K. N. (2016). Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: A meta-analysis of randomized controlled trials. Clinical Psychology Review, 43, 90–102.
- Jones, B., Currin-Mcculloch, J., Pelletier, W., Sardi-Brown, V., Brown, P., & Wiener, L. (2018). Psychosocial standards of care for children with cancer and their families: A national survey of pediatric oncology social workers. Social Work in Health Care, 57, 221–249.
- Kazak, A. E. (2004). Posttraumatic stress disorder (PTSD) and posttraumatic stress symptoms (PTSS) in families of adolescent childhood cancer survivors. Journal of Pediatric Psychology, 29, 211–219.
- Kazak, A. E., Alderfer, M. A., Streisand, R., Simms, S., Rourke, M. T., Barakat, L. P., … Cnaan, A. (2004). Treatment of posttraumatic stress symptoms in adolescent survivors of childhood cancer and their families: A randomized clinical trial. Journal of Family Psychology, 18, 493–504.
- Kazak, A. E., Boeving, A., Alderfer, M. A., Hwang, W., & Reilly, A. (2005). Posttraumatic stress symptoms during treatment in parents of children with cancer. Journal of Clinical Oncology, 23, 7405–7410.
- Kearney, J. A., Salley, C. G., & Muriel, A. C. (2015). Standards of psychosocial care for parents of children with cancer. Pediatric Blood & Cancer, 62(Suppl 5), S632–S683.
- Klassen, A. F., Klaassen, R., Dix, D., Pritchard, S., Yanofsky, R., O’Donnell, M., … Sung, L. (2008). Impact of caring for a child with cancer on parents’ health-related quality of life. Journal of Clinical Oncology, 26, 5884–5889.
- Kowalczyk, J. R., Samardakiewicz, M., Fitzgerald, E., Essiaf, S., Ladenstein, R., Vassal, G., … Pritchard-Jones, K. (2014). Towards reducing inequalities: European standards of care for children with cancer. European Journal of Cancer, 50, 481–485.
- Kowalczyk, J. R., Samardakiewicz, M., Pritchard-Jones, K., Ladenstein, R., Essiaf, S., Fitzgerald, E., … Vassal, G. (2016). European survey on standards of care in paediatric oncology centres. European Journal of Cancer, 61, 11–19.
- Le Blanc, M., Mérette, C., Savard, J., Ivers, H., Baillargeon, L., & Morin, C. M. (2009). Incidence and risk factors of insomnia in a population-based sample. Sleep, 32, 1027–1037.
- Lee, K. C., Yiin, J. J., Lu, S. H., & Chao, Y. F. (2015). The burden of caregiving and sleep disturbance among family caregivers of advanced cancer patients. Cancer Nursing, 38, E10–E18.
- Lind, M. J., & Gehrman, P. R. (2016). Genetic pathways to insomnia. Brain Sciences, 6, E64.
- Litsenburg, R. R. L., Huisman, J., Pieters, R., Verhaak, C. M., Kaspers, G. J. L., & Gemke, R. J. B. J. (2014). Determinants of quality of life during induction therapy in pediatric acute lymphoblastic leukemia. Supportive Care in Cancer, 22, 3235–3242.
- Ljungman, L., Cernvall, M., Ghaderi, A., Ljungman, G., von Essen, L., & Ljotsson, B. (2018). An open trial of individualized face-to-face cognitive behavior therapy for psychological distress in parents of children after end of treatment for childhood cancer including a cognitive behavioral conceptualization. Peer J, 6, e4570.
- Ljungman, L., Cernvall, M., Gronqvist, H., Ljotsson, B., Ljungman, G., & von Essen, L. (2014). Long-term positive and negative psychological late effects for parents of childhood cancer survivors: A systematic review. PLoS One, 9, e103340.
- Ljungman, L., Hoven, E., Ljungman, G., Cernvall, M., & Von Essen, L. (2015). Does time heal all wounds? A longitudinal study of the development of posttraumatic stress symptoms in parents of survivors of childhood cancer and bereaved parents. Psycho-Oncology, 24, 1792–1798.
- Manber, R., Edinger, J. D., Gress, J. L., San Pedro-Salcedo, M. G., Kuo, T. F., & Kalista, T. (2008). Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep, 31, 489–495.
- Matthews, E. E., Neu, M., Cook, P. F., & King, N. (2014). Sleep in mother and child dyads during treatment for pediatric acute lymphoblastic leukemia. Oncology Nursing Forum, 41, 599–610.
- McLoone, J. K., Wakefield, C. E., Yoong, S. L., & Cohn, R. J. (2013). Parental sleep experiences on the pediatric oncology ward. Supportive Care in Cancer, 21, 557–564.
- Medic, G., Wille, M., & Hemels, M. E. (2017). Short- and long-term health consequences of sleep disruption. Nature and Science of Sleep, 9, 151–161.
- Meltzer, L. J., & Moore, M. (2007). Sleep disruptions in parents of children and adolescents with chronic illnesses: Prevalence, causes, and consequences. Journal of Pediatric Psychology, 33, 279–291.
- Morin, C. M. (2011). Psychological and behavioral treatments for insomnia. In M. H. Kryger, T. Roth, & W. C. Dement (Eds.), Principles and practice of sleep medicine (5th ed., chap. 79, pp. 866–883). Elsevier Saunders.
- Muscara, F., McCarthy, M. C., Hearps, S. J. C., Nicholson, J. M., Burke, K., Dimovski, A., … Anderson, V. A. (2018). Trajectories of posttraumatic stress symptoms in parents of children with a serious childhood illness or injury. Journal of Pediatric Psychology, 43, 1072–1082.
- Neu, M., Matthews, E., & King, N. A. (2014). Exploring sleep-wake experiences of mothers during maintenance therapy for their child’s acute lymphoblastic leukemia. The Journal of Pediatric Nursing, 29, 410–421.
- Perlis, M., Shaw, P. J., Cano, G., & Espie, C. A. (2011). Models of insomnia. In M. H. Kryger, T. Roth, & W. C. Dement (Eds.), Principles and practice of sleep medicine (5th ed., chap. 78, pp. 850–865). Elsevier Saunders.
- Phipps, S., Long, A., Willard, V. W., Okado, Y., Hudson, M., Huang, Q., … Noll, R. (2015). Parents of children with cancer: At-risk or resilient? Journal of Pediatric Psychology, 40, 914–925.
- Pollock, E. A., Litzelman, K., Wisk, L. E., & Witt, W. P. (2013). Correlates of physiological and psychological stress among parents of childhood cancer and brain tumor survivors. Academic Pediatrics, 13, 105–112.
- Price, J., Kassam-Adams, N., Alderfer, M. A., Christofferson, J., & Kazak, A. E. (2015). Systematic review: A reevaluation and update of the integrative (Trajectory) model of pediatric medical traumatic stress. Journal of Pediatric Psychology, 41, 86–97.
- Price, J., Kassam-Adams, N., Alderfer, M. A., Christofferson, J., & Kazak, A. E. (2016). Systematic review: A reevaluation and update of the integrative (Trajectory) model of pediatric medical traumatic stress. Journal of Pediatric Psychology, 41, 86–97.
- Raboni, M. R., Alonso, F. F., Tufik, S., & Suchecki, D. (2014). Improvement of mood and sleep alterations in posttraumatic stress disorder patients by eye movement desensitization and reprocessing. Frontiers in Behavioral Neuroscience, 8, 209.
- Raboni, M. R., Tufik, S., & Suchecki, D. (2006). Treatment of PTSD by eye movement desensitization reprocessing (EMDR) improves sleep quality, quality of life, and perception of stress. Annals of the New York Academy of Sciences, 1071, 508–513.
- Rayner, M., Dimovski, A., Muscara, F., Yamada, J., Burke, K., McCarthy, M., … Nicholson, J. M. (2016). Participating from the comfort of your living room: Feasibility of a group videoconferencing intervention to reduce distress in parents of children with a serious illness or injury. Child & Family Behavior Therapy, 38, 209–224.
- Rensen, N., Steur, L. M., Schepers, S. A., Merks, J. H., Moll, A. C., Kaspers, G. J., … Litsenburg, R. R. (2019). Gender-specific differences in parental health-related quality of life in childhood cancer. Pediatric Blood & Cancer, e27728.
- Sahler, O. J., Dolgin, M. J., Phipps, S., Fairclough, D. L., Askins, M. A., Katz, E. R., … Butler, R. W. (2013). Specificity of problem-solving skills training in mothers of children newly diagnosed with cancer: Results of a multisite randomized clinical trial. Journal of Clinical Oncology, 31, 1329–1335.
- Sateia, M. J., & Buysse, D. J. (2011). Treatment guidelines for insomnia. In M. H. Kryger, T. Roth, & W. C. Dement (Eds.), Principles and practice of sleep medicine (5th ed., chap. 83, pp. 931–937). Elsevier Saunders.
- Seixas, A. A., Nunes, J. V., Airhihenbuwa, C. O., Williams, N. J., Pandi-Perumal, S. R., James, C. C., & Jean-Louis, G. (2015). Linking emotional distress to unhealthy sleep duration: Analysis of the 2009 national health interview survey. Neuropsychiatric Disease and Treatment, 11, 2425–2430.
- Spielman, A. J., Yang, C.-M., & Glovinsky, P. B. (2011). Assessment techniques for insomnia. In M. H. Kryger, T. Roth, & W. C. Dement (Eds.), Principles and practice of sleep medicine (5th ed., chap. 144, pp. 1632–1645). Elsevier Saunders.
- Spritzer, K. L., & Hays, R. D. (2003). MOS sleep scale: A manual for use and scoring, Version 1.0. Los Angeles: RAND.
- Stremler, R., Dhukai, Z., Pullenayegum, E., Weston, J., Wong, L., & Parshuram, C. (2014). Sleep, sleepiness, and fatigue outcomes for parents of critically ill children. Pediatric Critical Care Medicine, 15, e56–e65.
- Sultan, S., Leclair, T., Rondeau, E., Burns, W., & Abate, C. (2016). A systematic review on factors and consequences of parental distress as related to childhood cancer. European Journal of Cancer Care, 25, 616–637.
- Sung, L., Klaassen, R. J., Dix, D., Pritchard, S., Yanofsky, R., Dzolganovski, B., … Klassen, A. (2009). Identification of paediatric cancer patients with poor quality of life. British Journal of Cancer, 100, 82–88.
- Sung, L., Yanofsky, R., Klaassen, R. J., Dix, D., Pritchard, S., Winick, N., … Klassen, A. (2011). Quality of life during active treatment for pediatric acute lymphoblastic leukemia. International Journal of Cancer, 128, 1213–1220.
- van Oers, H. A., Schepers, S. A., Grootenhuis, M. A., & Haverman, L. (2017). Dutch normative data and psychometric properties for the distress thermometer for parents. Quality of Life Research, 26, 177–182.
- Vrijmoet-Wiersma, C. M., van Klink, J. M., Kolk, A. M., Koopman, H. M., Ball, L. M., & Maarten Egeler, R. (2008). Assessment of parental psychological stress in pediatric cancer: A review. Journal of Pediatric Psychology, 33, 694–706.
- Wakefield, C. E., Sansom-Daly, U. M., McGill, B. C., Ellis, S. J., Doolan, E. L., Robertson, E. G., … Cohn, R. J. (2016). Acceptability and feasibility of an e-mental health intervention for parents of childhood cancer survivors: “Cascade”. Supportive Care in Cancer, 24, 2685–2694.
- Wijnberg-Williams, B. J., Kamps, W. A., Klip, E. C., & Hoekstra-Webers, J. E. H. M. (2006). Psychological adjustment of parents of pediatric cancer patients revisited: Five years later. Psycho-Oncology, 15, 1–8.
- Williams, L. K., & McCarthy, M. C. (2015). Parent perceptions of managing child behavioural side-effects of cancer treatment: A qualitative study. Child: Care, Health and Development, 41, 611–619.
- Wright, M. (2011). Children receiving treatment for cancer and their caregivers: A mixed methods study of their sleep characteristics. Pediatric Blood & Cancer, 56, 638–645.
- Zupanec, S., Jones, H., & Stremler, R. (2010). Sleep habits and fatigue of children receiving maintenance chemotherapy for ALL and their parents. Journal of Pediatric Oncology Nursing, 27, 217–228.