ABSTRACT
Background: Recent findings suggest that disruptions of sleep-related memory processing are involved in the development of posttraumatic stress symptoms. More specifically, exposure to an analogue traumatic event resulted in fewer intrusive memories, when it was followed by sleep instead of continued wakefulness. However, competing evidence suggests that sleep deprivation may reduce intrusive re-experiencing. To address these conflicting accounts, we examined how sleep – as opposed to partial sleep deprivation – modulates explicit and implicit trauma memory using an analogue procedure.
Methods: Healthy participants (N = 41) were assigned to a Sleep or Partial sleep deprivation group. Prior to nocturnal sleep, both groups were exposed to “traumatic“ picture stories. After sleep or partial sleep deprivation, participants were subjected to tests of explicit and implicit memory for potential trauma reminders. Thereafter, participants completed an intrusion triggering task that was embedded in a distractor task.
Results: Analyses revealed higher explicit memory for potential trauma reminders after sleep as compared to partial sleep deprivation. No group differences were found for implicit memory. Participants responded with fewer intrusions after sleep than following partial sleep deprivation.
Conclusions: The current findings support a protective role of sleep in trauma memory processing, which may be evident after the first night of sleep post-trauma. Although more research is needed, our results corroborate the importance of promoting restful sleep in trauma-exposed individuals.
HIGHLIGHTS
• Sleep enhanced voluntary trauma memory.• Sleep did not enhance implicit trauma memory.• Sleep was associated with decreased intrusions.• Future studies should investigate effects of sleep-based interventions on PTSD symptoms.
Antecedentes: hallazgos recientes sugieren que las interrupciones del procesamiento de la memoria relacionada con el sueño están involucradas en el desarrollo de los síntomas de estrés postraumático. Más específicamente, la exposición a un evento traumático análogo resultó en menos recuerdos intrusivos, cuando fue seguido por el sueño en lugar de la vigilia continua. Sin embargo, evidencia contraria sugiere que la falta de sueño puede reducir la reexperimentación intrusiva. Para abordar estos reportes en conflicto, examinamos cómo el sueño, en lugar de la privación parcial del sueño, modula la memoria de trauma explícita e implícita mediante un procedimiento análogo.
Métodos: los participantes sanos (N = 41) se asignaron a un grupo de deprivación de sueño total o parcial. Antes del sueño nocturno, ambos grupos fueron expuestos a historias de imágenes ‘traumáticas’. Después de la privación total o parcial del sueño, los participantes fueron sometidos a pruebas de memoria explícita e implícita para posibles recuerdos traumáticos. A partir de entonces, los participantes completaron una tarea activadora de recuerdos intrusivos, que se integró en una tarea distractora.
Resultados: los análisis revelaron una mayor memoria explícita para recuerdos potencialmente traumáticos después del sueño en comparación con la privación parcial del sueño. No se encontraron diferencias de grupo para la memoria implícita. Los participantes respondieron con menos intrusiones después del sueño que después de la privación parcial del sueño.
Conclusiones: Los hallazgos actuales apoyan un papel protector del sueño en el procesamiento de la memoria del trauma, que puede ser evidente después de la primera noche de sueño después del trauma. Aunque se necesita más investigación, nuestros resultados corroboran la importancia de promover el sueño reparador en personas expuestas a traumas.
背景:最近的研究结果表明,与睡眠相关的记忆加工过程的中断,与创伤后应激症状的发展有关。更具体地说,暴露于某一模拟创伤事件后进入睡眠相较一直保持清醒的状态,产生的闯入性记忆更少。然而,也有竞争性的证据表明,睡眠剥夺可以减少侵入性的再体验。为了解决这些相互矛盾的解释,我们用一个模拟程序,以部分睡眠剥夺作为对照,研究了睡眠对外显和内隐创伤记忆的调控作用。
方法:41名健康的参与者被分配到睡眠组或部分睡眠剥夺组。在夜间睡眠之前,两组都先接触了‘创伤性’的图片故事。在经历睡眠或部分睡眠剥夺条件之后,参与者接受了对于潜在创伤提醒的外显记忆和内隐记忆的测试。随后,参与者完成了嵌入在分心任务中的闯入触发任务。
结果:分析显示,与部分睡眠剥夺组相比,睡眠组对潜在创伤提醒的外显记忆更好,而内隐记忆无组间差异。睡眠组的闯入反应少于部分睡眠剥夺组。
结论:当前的研究结果支持睡眠在创伤记忆加工过程中起到的保护作用,且这种作用可能在创伤后第一晚睡眠后出现。尽管还需要更多的研究,我们的结果证实了在创伤暴露的个体中促进安稳睡眠的重要性。
1. Introduction
Posttraumatic stress disorder (PTSD) has been conceptualized as a disorder of memory (Elzinga & Bremner, Citation2002). This conceptualization is based on the importance of memory-related symptoms in the development and maintenance of PTSD (Ehlers, Hackmann, & Michael, Citation2004). Correspondingly, studies highlight the relevance of distressing, intrusive memories in predicting persistent PTSD (Clohessy & Ehlers, Citation1999; Michael, Ehlers, Halligan, & Clark, Citation2005a). Intrusive memories consist of brief sensory fragments of the traumatic event that intrude into consciousness (Hackmann, Ehlers, Speckens, & Clark, Citation2004) and may trigger intense bodily symptoms of anxiety (Pfaltz, Michael, Grossman, Margraf, & Wilhelm, Citation2010). In contrast to voluntary memories, intrusions lack autonoetic awareness, i.e., awareness that the current sensory experiences originate from a past event (Tulving, Citation1985). Thus, intrusive memories often coincide with a sense of ‘nowness’ that perpetuates perceptions of ongoing threat and causes high levels of distress (Ehlers & Clark, Citation2000). Accordingly, studies find that individuals who develop persistent PTSD differ from those showing transient symptoms after trauma with respect to their initial intrusion characteristics (i.e., distress, ‘nowness’, and lack of context; Kleim, Graham, Bryant, & Ehlers, Citation2013; Michael et al., Citation2005a).
Cognitive models assume that intrusions arise from enhanced implicit learning and/or enhanced sensory encoding during trauma (Brewin, Citation2014; Brewin, Gregory, Lipton, & Burgess, Citation2010; Ehlers & Clark, Citation2000). This assumption is based on clinical observations suggesting that intrusions are often triggered by environmental stimuli that are perceptually similar to those related to the trauma (Ehlers et al., Citation2004). To account for this observation, theories posit that strong implicit encoding of trauma-associated stimuli subsequently reduces the perceptual threshold of similar, but unrelated environmental stimuli (Ehlers, Michael, Chen, Payne, & Shan, Citation2006; Michael & Ehlers, Citation2007; Sündermann, Hauschildt, & Ehlers, Citation2013). Thereby, implicit learning may conceivably yield a broad range of potential triggers for intrusive memories. In line with this assumption, studies show that a specific type of implicit memory, i.e., priming, is enhanced for trauma-associated material and that this enhancement is linked to persistent PTSD (Ehring & Ehlers, Citation2011; Kleim, Ehring, & Ehlers, Citation2012; Michael, Ehlers, & Halligan, Citation2005b). Theories further propose that another type of implicit learning, i.e., associative learning, is amplified in PTSD patients (Ehlers & Clark, Citation2000). This amplification is assumed to result in strong conditioned responses to trauma-associated stimuli, which are likely to trigger intrusive memories. Experimental evidence supports this assumption by showing that conditioned trauma cues can indeed trigger intrusive memories and anxiety (Streb, Conway, & Michael, Citation2017; Wegerer, Blechert, Kerschbaum, & Wilhelm, Citation2013).
Although traumatic stress appears to enhance implicit learning, this enhancement does not generalize to other memory systems (Brewin et al., Citation2010; Elzinga & Bremner, Citation2002; but see Rubin, Berntsen, & Bohni, Citation2008). On the contrary, PTSD patients often show deficits in voluntary (explicit) memory retrieval such that their trauma narratives lack organization and coherence (Filkukova, Jensen, Sofie Hafstad, Torvund Minde, & Dyb, Citation2016; Jelinek, Randjbar, Seifert, Kellner, & Moritz, Citation2009; Kleim, Wallott, & Ehlers, Citation2008). Based on these findings, cognitive models propose that poor memory elaboration may be a conducive factor in intrusion development (Ehlers & Clark, Citation2000). Poor memory elaboration is assumed to reduce the likelihood of explicit memory retrieval during re-experiencing episodes, which could otherwise inhibit implicit memory processing and decrease the level of distress during these episodes (Brewin et al., Citation2010). However, empirical studies investigating the link between voluntary trauma memory and PTSD symptoms have yielded mixed findings. Whereas some studies establish an association between reduced voluntary trauma memory (i.e., disorganized and/or fragmented memories) and PTSD symptoms (Amir, Stafford, Freshman, & Foa, Citation1998; Halligan, Michael, Clark, & Ehlers, Citation2003; Jones, Harvey, & Brewin, Citation2007; Kleim & Ehlers, Citation2008; Michael & Ehlers, Citation2007; Sachschal, Woodward, Wichelmann, Haag, & Ehlers, Citation2019) others report inconsistent findings (for reviews see Brewin, Citation2014; Crespo & Fernández-Lansac, Citation2016).
Building on the importance of memory-related symptoms in PTSD, research efforts have focused on identifying factors that influence peri- and posttraumatic memory processing (Parsons & Ressler, Citation2013). Current research aims to manipulate these factors (e.g., stress hormone levels and visuospatial processing demands; Holz, Lass-Hennemann, Streb, Pfaltz, & Michael, Citation2014; Iyadurai et al., Citation2018) to target post-encoding memory processes that promote the development and persistence of re-experiencing symptoms. In this context, recent studies suggest a potential role of sleep (Pace-Schott, Germain, & Milad, Citation2015). Extensive research has established the critical role of sleep in memory consolidation (Rasch & Born, Citation2013). During sleep, newly formed memory traces are assumed to be transferred from intermediate storage in the hippocampus to long-term storage in neocortical networks (Diekelmann & Born, Citation2010; but see Yonelinas, Ranganath, Ekstrom, & Wiltgen, Citation2019). This process is tied to the unique neurophysiology of sleep that enables offline reactivations of hippocampal memory traces. Throughout multiple cycles of reactivation and redistribution, hippocampal memories are assumed to be integrated into long-term memory networks that support remote memory retrieval (Gais et al., Citation2007). In line with this model of systems consolidation, studies consistently demonstrate enhanced memory performance after post-encoding sleep as compared to sustained wakefulness (for reviews see Diekelmann & Born, Citation2010; Rasch & Born, Citation2013). These effects are particularly evident for episodic memory (Daurat, Terrier, Foret, & Tiberge, Citation2007; Drosopoulos, Wagner, & Born, Citation2005; Sopp, Michael, & Mecklinger, Citation2018). Moreover, sleep is found to selectively preserve emotional episodic memories (see e.g., Sopp, Michael, Weess, & Mecklinger, Citation2017) and may simultaneously downregulate the affective charge of these memories (Pace-Schott et al., Citation2011). These emotion-specific effects of sleep have been associated with rapid eye movement (REM) sleep (Hutchison & Rathore, Citation2015), whereas post-sleep memory performance for neutral events has been linked with non-REM (NREM) sleep physiology (Diekelmann & Born, Citation2010).
Based on the established role of sleep in memory consolidation, previous studies investigated the effects of posttraumatic sleep on intrusive memories. Using the trauma-film paradigm, Porcheret, Holmes, Goodwin, Foster, and Wulff (Citation2015) compared the effects of sleep and sleep deprivation on intrusive memories that were documented for six consecutive days. The authors found that sleep deprivation was associated with a reduced number of intrusions on the first day of ambulatory assessment. In a second study, Kleim, Wysokowsky, Schmid, Seifritz, and Rasch (Citation2016) used a very similar design to examine the effects of sleep compared to sleep deprivation on intrusive memories measured during a seven-day-assessment period. Contrasting previous findings, they found that sleep deprivation was associated with an enhanced number of intrusions. Moreover, they reported that intrusion distress ratings were significantly enhanced in sleep deprivation participants. Finally, Woud et al. (Citation2018) investigated the effects of post-trauma nap sleep on intrusive memories. In line with Kleim et al. (Citation2016), participants in the wake group were found to experience a higher number of intrusions during the seven-day assessment period. Based on current evidence, researchers have developed different conceptions of how sleep may influence intrusive re-experiencing: Porcheret et al. (Citation2015) argue that sleep deprivation may impair implicit trauma memory, thereby reducing intrusions. They base this argument on the assumption that sleep deprivation impairs implicit memory without affecting explicit memory. Conversely, Kleim et al. (Citation2016) argue that sleep enhances explicit memory by promoting the reorganization and integration of traumatic memories into preexisting memory structures. The authors assume that this process of integration may indirectly inhibit the development of intrusive memories.
Whether posttraumatic sleep affects implicit and/or explicit memory can be tested experimentally. However, to date no such study has been conducted, precluding any assumptions on the underlying effects of sleep on intrusive memories. To address this gap, the current study investigated the effects of sleep as opposed to partial sleep deprivation on priming (i.e., implicit memory) and recognition memory (i.e., explicit memory) for potential trauma reminders. In developing hypotheses regarding the effects of sleep, it is important to take into account that recognition memory is supported by two distinct retrieval processes (Yonelinas, Citation2002) referred to as recollection and familiarity (but see Squire, Wixted, & Clark, Citation2007 for a conflicting account). Recollection describes conscious retrieval of an episode that includes its spatiotemporal context. Recollection is further characterized by autonoetic awareness, thus reflecting the core conceptualization of episodic memory (Tulving, Citation1985). Familiarity, on the other hand, describes an automatic process of recognition that is based on a sense of ‘knowing’ without remembering any contextual details (Yonelinas, Citation2002). Although not unequivocal, research suggests that different medial temporal lobe structures support recollection, familiarity, and implicit memory (e.g., priming; Wang, Ranganath, & Yonelinas, Citation2014): Whereas recollection is assumed to reflect hippocampal processes, familiarity and implicit memory may reflect cortical processes (Eichenbaum, Yonelinas, & Ranganath, Citation2007). The active system consolidation account proposes that sleep specifically consolidates hippocampus-dependent episodic memory (Diekelmann & Born, Citation2010; Marshall & Born, Citation2007). Thus, we hypothesize that sleep selectively enhances recollection (Atienza & Cantero, Citation2008; Daurat et al., Citation2007; Drosopoulos et al., Citation2005; but see Groch, Wilhelm, Diekelmann, & Born, Citation2013). As previous findings indicate that familiarity-based recognition and priming do not rely on the hippocampus (Wang, Ranganath, & Yonelinas, Citation2014; but see Mayes et al., Citation2019; Merkow, Burke, & Kahana, Citation2015), we assume that these memory processes (often referred to as ‘non-episodic memory’; see e.g., de Vanssay-Maigne et al., Citation2011; Eldridge, Knowlton, Furmanski, Bookheimer, & Engel, Citation2000; Rasch, Papassotiropoulos, & de Quervain, Citation2010) are not enhanced across sleep. As such, we assume that sleep enhances explicit recollection-based memory without affecting implicit memory (Casey et al., Citation2016; Giganti et al., Citation2014; but see Plihal & Born, Citation1999).
We tested our hypotheses using an established analogue procedure (Holz et al., Citation2014). This procedure allowed us to simultaneously examine memory for potential trauma reminders and intrusive memories in response to trauma-associated stimuli. Healthy participants were exposed to ‘traumatic’ picture stories – consisting of a picture sequence and a running audio commentary – prior to a night of sleep or partial sleep deprivation in the second night half. In the morning, participants were subjected to a blurred picture identification task that measured priming. Thereafter, participants’ explicit memory was tested in a recognition test that differentiated recollection-based from familiarity-based recognition. Finally, participants were subjected to an intrusion triggering task. In order to simulate the occurrence of intrusion triggers in everyday life, participants were exposed to auditory fragments of the picture stories while attending to a distractor task. The rationale of our study design was two-fold: Firstly, we aimed to investigate differences in implicit and explicit ‘trauma’ memory after sleep as opposed to partial sleep deprivation to test whether effects are similar as previously observed for neutral material. Secondly, we aimed to test whether group differences in memory performance co-occur with fewer intrusions in the Sleep group. Although the study was not primarily designed to investigate correlations between intrusions and memory performance, these were examined on an exploratory basis. In line with the aforementioned studies, we predicted that recollection-based recognition memory would be higher in the Sleep group (Atienza & Cantero, Citation2008; Daurat et al., Citation2007; Drosopoulos et al., Citation2005). Moreover, we hypothesized that the Sleep group would show similar priming effects as the Partial sleep deprivation group (Casey et al., Citation2016; Giganti et al., Citation2014). Finally, we predicted that the Sleep group would experience fewer intrusions than the partial sleep deprivation (PSD) group (Kleim et al., Citation2016; Woud et al., Citation2018).
2. Methods
2.1. Participants
Forty-six healthy university students (15 male) took part in the current experiment. Participants were recruited at Saarland University via notices inviting all students aged between 18 and 30 to participate in a laboratory experiment on sleep and social perception in the context of media coverage. This cover story ensured that participants were unaware that they were participating in a memory experiment. All interested students were asked to complete a screening questionnaire comprising measures of sleep quality (PSQI; Buysse et al., Citation1991), circadian preference (rMEQ; Randler, Citation2013), depressive symptoms (PHQ-9; Kroenke, Spitzer, & Williams, Citation2001), handedness (EHI; Oldfield, Citation1971), as well as different health-related questions (e.g., concerning chronic illnesses, long-term medication, and current drug/alcohol use). Participants qualified for further participation if they were good sleepers (PSQI ≤ 5) with an average sleep duration of ≥ 7 hours. They were also required to be in good general health (no chronic illnesses or long-term medication except for oral contraceptives) including normal or corrected-to-normal vision and normal body weight (18 < BMI > 30). Participants were excluded if they met any of the following criteria: Pronounced evening preference (rMEQ > 11), left-handedness (EHI < 60) or indications of drug/alcohol abuse. Participants meeting the preliminary inclusion criteria were further subjected to a semi-structured telephone interview. During the interview, participants were asked about mental disorders, previous psychotherapeutic/psychiatric treatment, and lifetime trauma exposureFootnote1. If participants indicated that they had ever suffered from a mental disorder, sought treatment for mental health problems or experienced a traumatic life event, they were excluded from further participation.
Based on these criteria, we included 46 participants of which one chose to terminate the experiment while being confronted with the ‘traumatic’ picture stories. Three participants had to be excluded because of technical errors during the presentation of picture stories (n = 1) or during polysomnographic assessment (n = 2). One further participant rated the picture stories as non-aversive (Mrating> 50 on a scale from 0 to 100; 0 = extremely negative, 100 = extremely positive) and was consequently excluded from further analyses. Thus, the final sample comprised 20 participants in the partial sleep deprivation (PSD) group (7 male; Mage = 22.15, SDage = 2.16) and 21 participants in the Sleep group (7 male; Mage = 22.71, SDage = 2.83). All participants gave written informed consent in accordance with the Declaration of Helsinki and were paid € 100 for study participation.
2.2. Procedure
The current study used a partial sleep deprivation procedure allowing us to investigate effects of nocturnal sleep while minimizing sleepiness confounds between groups (i.e., partial sleep deprivation does not impact sleepiness and alertness levels to the same extent as total sleep deprivation; see e.g., Philip et al., Citation2012). By depriving participants of sleep in the second night half, we additionally aimed to establish group differences in REM sleepFootnote2, which has been linked to emotional memory processing (Hutchison & Rathore, Citation2015). Prior to the experimental night, participants were asked to maintain a regular sleep-wake schedule for three days and to complete a sleep diary during this time period. They were further asked to refrain from drinking alcohol or caffeinated beverages 24 hours before their laboratory appointment and to wake up by 7:00 a.m. on the morning of the experiment. Group assignment was performed in a pseudo-random fashion to ensure balanced gender ratios across groups. Participants were assigned to individual groups before their arrival at the laboratory. Due to practical reasons, experimenters were not blinded to group assignment. However, participants remained blinded to their assignment until they were awakened after the first night half. Thus, group assignment was single-blind.
Participants were tested in individual sessions. Upon arrival at the laboratory (approx. 7:30 p.m.), they were familiarized with the sleep study room and asked to change into a comfortable tracksuit. Afterwards they were seated in a sound-proof testing booth facing a 27´´ LCD monitor (60 Hz refresh rate) at a viewing distance of about 65 cm. Prior to picture story presentation, participants performed a psychomotor vigilance task (PVT; Roach, Dawson, & Lamond, Citation2006) and completed the Stanford Sleepiness Scale (SSS; Herscovitch & Broughton, Citation1981) which served as measures of baseline alertness. Thereafter, they were prepared for psychophysiological assessment (heart rate and skin conductance levels) followed by a resting state measurement phase of 5 minutes. Subsequently, participants were exposed to five ‘traumatic’ picture stories (for details see 2.3.1.) while psychophysiological recordings were continued. After completion, resting state psychophysiological measures were recorded again for five minutes followed by the preparation of polysomnographic recordings (see 2.4.). During this time, participants were not allowed to speak with the experimenter about the content of the “traumatic“ picture stories or any related topics. If preparation was completed before 11:00 p.m., participants were offered a coloring book or asked to sit and relax for the remaining time. Shortly before 11:00 p.m., participants were accompanied to the sleep study room, where they were instructed to lie down, relax, and fall asleep – whenever possible. The experimenter informed the participant that she/he would briefly enter the room after a few hours to check on the participant. After three hours and 45 minutes, the experimenter entered the room and asked the participant to sit up and complete a brief questionnaire on dream experiences. This phase was identical in both groups to prevent any confounding effects of stress due to nocturnal awakening. Afterwards, participants of the Sleep group were asked to lie down again for another sleep opportunity of three hours and 45 minutes. Participants of the PSD group were accompanied to a different room, where they were kept awake for the identical time period. During this time, they attended to a standardized set of activities that were chosen to limit interference effects (e.g., drawing, crafting, playing non-arousing video games, taking walks in the hallway). Participants were constantly monitored by two experimenters, who were instructed not to talk or interact with the participant. At the end of this phase, participants of the Sleep group were woken up and asked to complete the dream experiences questionnaire again (approx. 06:50 a.m.). Participants of both groups were then seated in the testing booth for a 30-minute rest period, allowing Sleep group participants to recover from sleep inertia. After completing both alertness measures (PVT and SSS), participants performed the blurred picture task (see 2.3.2.) which was followed by a surprise memory test (see 2.3.3.). As it was necessary to preclude test awareness during the blurred picture task, tests were administered in a fixed order. Afterwards participants were prepared for psychophysiological measurements during the intrusion triggering task (ITT; see 2.3.4.). After a 5-minute resting state measurement phase, they completed the ITT, which was followed by another 5-minute resting state measurement phaseFootnote3. Finally, participants underwent debriefing. They were asked whether they had any doubts regarding the cover story before completing the memory test. None of the participants indicated that this was the case.
2.3. Tasks
2.3.1. Picture stories
‘Traumatic’ picture stories were taken from Holz et al. (Citation2014). Using a between-subjects design, the authors examined priming for ‘traumatic’ and neutral stories and found that priming was significantly enhanced for ‘traumatic’ stories as compared to neutral stories (see also Sündermann et al., Citation2013). Hence, the validity of the paradigm has been established, allowing us to include only ‘traumatic’ stories in the current study. Participants were presented with five picture stories consisting of seven picture slides (see ). The first three picture slides introduced the main character (e.g., 7-year-old Catherine being picked up by her father for the school holidays.). The following four picture slides described the traumatic event ending with the death of the main character (e.g., Catherine and her father are involved in a road accident. Catherine is heavily wounded and dies on the way to the hospital.). Each picture slide included a running audio commentary spoken by a female voice. In order to measure memory performance, each picture story comprised six neutral objects (primes) that were presented interspersed. Primes were presented in pairs prior to the onset of picture slides # 4, 5, and 6. As these stimuli signaled the onset and course of the traumatic event, they were meant to serve as potential trauma reminders during the subsequent test phase.
Figure 1. Trial sequence of ‘traumatic’ picture stories.
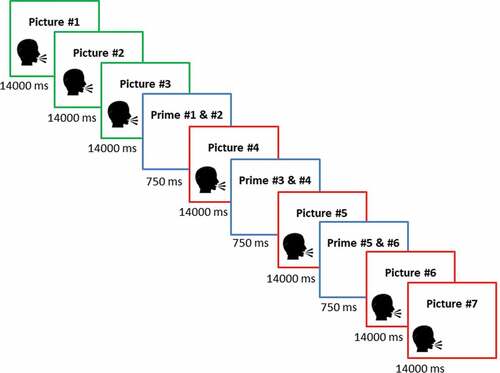
In order to avoid any salience effects, object pairs were selected to match the semantic context of the story (e.g., office setting – stapler, puncher). Pictures # 1, 2, 3, 4, 6, and 7 were selected from documentaries and feature films. Picture # 5 was taken from the IAPS database (Lang, Bradley, & Cuthbert, Citation1997) and displayed a mutilated or heavily injured person that closely resembled the main character of the respective story. To further enhance authenticity, participants were told that the picture stories were based on events that actually happened and had been reenacted for the purpose of the study (except for picture # 5). They were instructed that the purpose of the experiment was to test how the stories influenced their social perception and that they should pay attention to the events while imaging that they were an eyewitness to the scene. In order to avoid interfering with incidental encoding, no instructions were given concerning the presentation of neutral objects.
The order of story presentation was randomized individually for each participant. After each picture story, participants rated the valence and arousal of the picture story on a visual analogue scale (0 to 100; 0 = extremely negative/low arousing, 100 = extremely positive/high arousing). Thereafter, participants determined the start of the subsequent picture story by pressing the space bar. Picture story presentation lasted approximately 20–30 minutes in total.
2.3.2. Blurred picture identification task
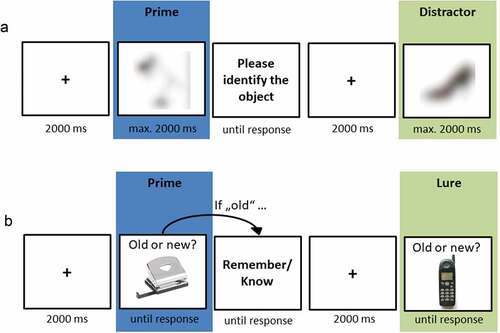
The blurred picture identification task (BPIT), also taken from Holz et al. (Citation2014), was used to measure implicit memory for potential trauma reminders (see )). Prior to the task, participants were instructed that they would be performing a test that was unrelated to the previous procedure and served as a control measure of general identification abilities. During the task, participants were presented with blurred primes and distractor objects. Objects were blurred with a Gaussian filter. Distractor objects were blurred to a lesser extent (see Holz et al., Citation2014 for details), thereby reducing the likelihood that participants noticed that the task measures memory performance for picture story stimuli. To further prevent doubts regarding the cover story, the majority of presented objects were distractor objects (20 distractor objects vs. 15 primes). All 30 priming objects that were originally presented in the picture stories were assigned to two parallel lists of 15 items each. Half of the participants saw the primes of list 1 whereas the other half saw the primes of list 2. Blurred objects were presented in an individual randomized order for each participant. Upon presentation, participants were instructed to label the object as quickly as possible by pressing the space bar and typing in its name. To prevent participants from reconsidering their immediate response, objects disappeared from the screen after the space bar had been pressed. Whenever participants were unsure, they were asked to type in their initial guess. The BPIT took about 7–8 minutes to complete.
Figure 2. Trial sequence of the blurred picture identification task (a) and the recognition test (b).
Objects were scored as correctly identified if participants typed in the correct object name or a close synonym (defined a priori; e.g., handbag, purse, bag). Two independent raters scored each label assigned to primes and distractor objects. If raters disagreed, a third rater was consulted to reach consensus. For each participant, percentages of correctly identified primes and distractors were calculated. These percentages were subtracted to serve as an index of the relative processing advantage of primed objects (priming score = hit rate of primes – hit rate of distractors).
2.3.3. Recognition test
The recognition test was adapted from Holz et al. (Citation2014) and measured explicit memory performance (see )). Participants were instructed to differentiate objects that had been presented in the picture stories from new objects (lures). Whenever they recognized an object, they were additionally asked to indicate whether their recognition judgement was based on remembering details of its previous presentation (‘Remember’) or on a feeling of ‘knowing’ (‘Know’). This procedure allows differentiating between recollection- and familiarity-based recognition (Yonelinas, Citation2002). Prior to the test phase, participants received standardized instructions for Remember and Know judgements (Rajaram, Citation1993). To ensure full comprehension, they were asked to repeat the instructions in their own words.
The self-paced recognition test comprised 30 old (primes) and 30 new (lures) objects. All primes were presented during this test phase in order to enhance the signal-to-noise-ratio by including as many trials as possible. However, primes with and without prior exposure (during the BPIT) were aggregated separately for statistical analyses (see 2.5.3.). To ensure medium task difficulty, we selected a weakly associated lure for each prime (e.g., prime: stapler, lure: ruler). Objects were presented in an individual randomized order for each participant. Upon presentation, participants were instructed to respond as quickly as possible by categorizing the object as ‘old’ or ‘new’. Whenever they identified an object as ‘old’, they were asked to indicate whether their response was based on ‘remembering’ or ‘knowing’. The recognition task took about 8–9 minutes to complete.
We calculated d’ (sensitivity) as an index of recognition memory (Holz et al., Citation2014). For each participant, hit and false alarm rates were calculated. Extreme proportions of zero or one were replaced with values of ‘1/(2N)’ and ‘1–1/(2N)’, respectively. N equals the number of trials upon which the proportion is based (Macmillan & Kaplan, Citation1985). Hit and false alarm rates were then transformed into Z-scores and subtracted to compute d’. To account for effects of prior exposure, we calculated d’ separately for objects that had been presented during the BPIT and for objects that had not been presented during the BPIT. Moreover, to examine differential effects of sleep on recollection-based recognition, we calculated d’ separately for Remember and Know responses (for details see Yonelinas & Jacoby, Citation1994).
2.3.4. Intrusion triggering task
Based on previous research (Streb et al., Citation2017; Wegerer et al., Citation2013), we used an intrusion triggering task to model intrusions in the laboratory. In order to simulate the occurrence of trauma cues in daily life, auditory fragments of the picture stories were presented while participants were engaged in an ongoing task. Prior to the task, participants were instructed that they would be performing a face rating test that measures different qualities of social perception. Each trial would entail the presentation of a face that they would be asked to rate afterwards. They were informed that they might hear different fragments of sentences through their headphones while performing the task. Participants were instructed not to pay attention to these fragments and to attend to the rating task. In addition, they received instructions on the characteristics of intrusive memories and were asked to press a specific key whenever they experienced an intrusion. Intrusions were defined as spontaneous, non-initiated memories of the picture stories. It was emphasized that intrusions do not include attempts at actively retrieving the source of the fragments or conscious and deliberate thinking about the picture stories.
The task consisted of 45 trials in total. During 15 trials, auditory fragments of the picture stories were presented simultaneously with the face. The unexpected exposure to the speaker’s voice was intended to trigger intrusive memories of the picture stories. Trauma-associated fragments did not contain any emotional words or any mentions of the main characters/setting of the stories. The other 30 trials followed the same structure, but face presentation was coupled with neutral auditory fragments spoken by either a female (15 trials) or male (15 trials) voice. Each trial started with a fixation cross (4000 ms) that was followed by the face+fragment presentation (4000 ms). Afterwards, the rating question (10,000 ms) was presented (e.g., how likely is it that this person owns a dog?) with a corresponding rating scale ranging from 1 (very unlikely) to 4 (very likely). The next trial started after the participant had made a response. The trial order was pseudo-randomized to ensure that no more than two trials of each category (female trauma-associated voice, female neutral voice, male neutral voice) occurred consecutively. The intrusion triggering task lasted approximately 15–20 minutes in total.
Key presses were considered as intrusions if they occurred after the trauma-associated fragment had ended and before the onset of the next neutral auditory fragment. The intrusion frequency variable was skewed and square root transformed to approximate normality (skewness: 0.66; kurtosis: 0.64).
2.4. Polysomnographic measures
During both night halves standard polysomnography measures were recorded (AASM, Citation2012). Polysomnographic recordings included EEG at frontal, central, and occipital sites (F3, F4, C3, C4, O1, O2), submental electromyography (EMG), and horizontal electrooculography (EOG; lower right and higher left canthi). Signals were digitized at a sampling rate of 512 Hz (first-order high-pass and second-order butterworth low-pass filter: 0.3 and 75 Hz respectively, notch filter: 50 Hz) and amplified by SOMNOscreen amplifiers (SOMNOmedics GmbH, Randersacker, Germany). All electrodes were recorded referenced to Cz and were re-referenced offline to the contralateral mastoid for sleep stage scoring. A 0.3–35 Hz bandpass filter was applied offline.
Visual sleep stage scoring was performed by two trained raters independently in accordance with the AASM criteria (2012). Each epoch (20 s) was scored visually as NREM stages N1, N2, and N3 (corresponding to SWS), REM sleep (stage R), and wake (stage W). PSG recordings during the sleep deprivation phase were also double scored to confirm continued wakefulness. The absolute amount of minutes spent in each sleep stage and the relative amount with reference to total sleep time (% TST) were determined for further analyses.
2.5. Data analyses
All statistical analyses were calculated using IBM SPSS Statistics 22 (IBM Corp., Armonk, NY, USA). Based on our directional hypotheses supported by prior work, we conducted one-tailed tests (α = .05) to examine whether sleep enhances recollection and reduces intrusions. Two-tailed tests were conducted for all other hypotheses (α = .05). Data was checked for normality by visual inspection and using the Kolmogorov-Smirnov test. All variables were normally distributed except for intrusion frequency (see 2.3.4.). Data was also checked for influential cases on all measures.Footnote4
To ensure that groups were comparable in baseline characteristics, we calculated independent t-tests including age, sleep quality, circadian preference, sleepiness, and depressive symptoms as dependent variables. We also examined potential group differences in emotional responses to the picture stories. To this end, we calculated mean valence and arousal ratings of all picture stories and subjected these to independent t-tests. Finally, we examined potential group differences in subjective and objective alertness levels. For this purpose, we subjected mean PVT and SSS scores to separate ANOVAs including the between-subject factor ‘Group’ (Sleep group vs. PSD group) and the within-subject factor ‘Time’ (pre- vs. post-sleep/PSD). Absolute and relative sleep stage durations were subjected to separate MANOVAs including the factor ‘Group’ (Sleep group vs. PSD group). The priming index was analyzed in an ANOVA including the between subject factors ‘Group’ (Sleep group vs. PSD group) and ‘List’ (List 1 vs. List 2). The factor ‘List’ was included in all analyses to account for potential effects driven by individual items of either list condition (see Holz et al., Citation2014). Recognition performance was analyzed in ANOVAs including the between subject factors ‘Group’ (Sleep group vs. PSD group) and ‘List’ (List 1 vs. List 2) and the within subject factor ‘Testing’ (previously tested vs. untested). Significant main or interaction effects of ANOVAs were further explored using post hoc t-tests. Intrusion frequency was analyzed by subjecting the transformed intrusion frequency variable to an independent t-test. The Two One-Sided Tests procedure was used to test for equivalence between the Sleep and PSD group (Lakens, Scheel, & Isager, Citation2018). For exploratory correlation analyses, non-parametric correlation coefficients (Spearman’s ρ) were computed between implicit memory (priming scores, primed % correct, unprimed % correct), explicit memory (recollection, familiarity, % hits, % FAs), and intrusion frequency. Due to a technical error, recognition memory data of one PSD participant was not recorded. Thus, degrees of freedom vary across analyses.
3. Results
3.1. Baseline characteristics
Participants of the Sleep and PSD group demonstrated comparable demographic characteristics (see ). Moreover, groups did not differ in average sleep duration, sleep quality, daytime sleepiness, circadian preference, and depressive symptoms.
Table 1. Sample characteristics.
Analyses of picture story ratings across both groups confirmed that mean valence ratings significantly fell below the scale mean of 50 [t(40) = 12.95, p < .001] indicating that participants experienced negative emotions while attending to the picture stories. Mean arousal ratings significantly exceeded the scale mean of 50 [t(40) = 3.77, p = .001] indicating that arousal levels were moderately enhanced during picture story presentation. Analyses of ratings between groups revealed comparable valence and arousal ratingsFootnote5 (see ).
Analyses of PVT performance revealed a significant main effect of Time [F(1,39) = 20.97, p < .001, η2p = .35] and no significant group-related effects [Group: F(1,39) = 3.77, p = .059, η2p = .09; Group × Time: F(1,39) = 0.97, p = .331, η2p = .02]Footnote6. Analyses of subjective sleepiness levels similarly yielded a significant main effect of Time [F(1,39) = 4.28, p = .045, η2p = .10] in the absence of any significant group-related effects [Group: F(1,39) = 1.34, p = .255, η2p = .03; Group×Time: F(1,39) = 0.86, p = .359, η2p = .02]. Objective (pre: M = 291.67 ms, SD = 30.98 ms; post: M = 304.08 ms, SD = 30.36 ms) and subjective (pre: M = 2.76, SD = 1.14; post: M = 3.27, SD = 1.25) sleepiness levels were found to increase from pre- to post-sleep across both groups.
3.2. Sleep physiology
Sleep stage durations in both groups are reported in . To preclude that both groups differed in their sleep characteristics during the first night half, we conducted a MANOVA including all absolute sleep parameters of the first night half as dependent variables. The analysis did not yield an effect of Group [F(5,35) = 0.69, p = .638, η2p = .09] indicating that sleep stage durations were comparable between groups (see ). However, groups did differ in sleep stage durations across both night halves [F(5,35) = 9977.70, p < .001, η2p = .99]. As to be expected, all sleep stage durations were significantly higher in the Sleep group (see ). In order to examine whether the relative amount of REM sleep was significantly higher in the Sleep group, we conducted a MANOVA of relative sleep stage durations across both night halves. Analyses revealed significant differences between groups [F(5,35) = 18.31, p < .001, η2p = .60]. Relative REM sleep duration was significantly higher in the Sleep group and relative SWS duration was significantly higher in the PSD group (see ). This pattern of results is in line with models of homeostatic sleep regulation.
Table 2. Sleep stage data.
3.3. Priming
Analyses of priming scores did not reveal any significant main or interaction effects [Group: F(1,37) = 0.03, p = .871, η2p = .01; List: F(1,37) = 3.99, p = .054, η2p = .10; Group×List: F(1,37) = 0.06, p = .811, η2p = .01]. Thus, Sleep and PSD groups demonstrated similar performance levels on the BPIT (see ) and ). As these results do not allow us to infer statistical equivalence, we used the Two One-Sided Tests (TOST) procedure to test for equivalence (Lakens et al., Citation2018). Equivalence bounds were based on the lowest effect size (d = 0.80) that we had sufficient power to detect. The TOST procedure indicated that the observed effect size (d = 0.06) was significantly within the equivalent bounds of d = −0.80 and d = 0.80 [t(39) = 2.36, p = .012].
Table 3. Memory performance and intrusion frequency.
3.4. Recognition memory
3.4.1. Recollection
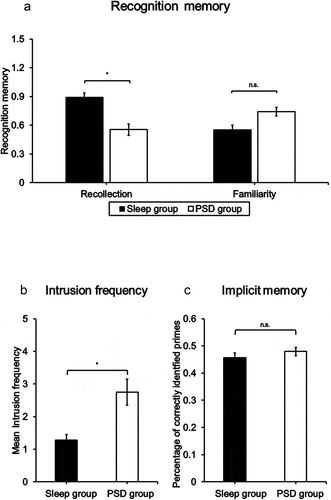
Analyses of recollection-based recognition memory revealed significant main effects of Testing [F(1,36) = 7.47, p = .010, η2p = .17] and Group [F(1,36) = 6.44, p = .016, η2p = .15]. The main effect of Testing indicated that participants were generally more successful in recognizing objects that had been included in the BPIT. Importantly, there were no significant interaction effects involving the Group factor and the Testing or List factor [Group×Testing: F(1,36) = 0.28, p = .601, η2p = .01; Group×List: F(1,36) = 2.40, p = .811, η2p = .06; Group×Testing×List: F(1,36) = 0.33, p = .570, η2p = .01]. Hence, group-related effects can be interpreted independently of previous presentation. As depicted in ), recollection-based recognition memory was significantly higher [t(38) = 2.51, p = .008, d = 0.80] in participants of the Sleep group than in participants of the PSD group. No further main or interaction effects reached significance [List: F(1,36) = 0.07, p = .800, η2p = .01; Testing×List: F(1,36) = 2.59, p = .117; η2p = .07].
3.4.2. Familiarity
Analyses of familiarity-based recognition memory revealed a significant main effect of Testing [F(1,36) = 7.79, p = .008, η2p = .18]. In line with recollection-based memory, performance levels were higher for objects that had been presented in the BPIT than for those without previous presentation. No further main or interaction effects reached significance [Group: F(1,36) = 1.62, p = .212, η2p = .04; List: F(1,36) = 0.04, p = .847, η2p = .01; Group×Testing: F(1,36) = 0.97, p = .331, η2p = .03; Group×List: F(1,36) = 2.43, p = .128, η2p = .06; Testing×List: F(1,36) = 0.03, p = .861, η2p = .01; Group×Testing×List: F(1,36) = 1.34, p = .255, η2p = .04]. Thus, in contrast to recollection, Sleep and PSD participants did not differ in familiarity-based recognition memory (see )).
3.5. Intrusion frequency
Analyses of intrusions across both groups confirmed that the mean intrusion frequency significantly exceeded zero [t(40) = 4.54, p < .001]. Analyses of intrusions between groups revealed a significant Group effect [t(39) = 1.82, p = .038, d = 0.57]. As depicted in ), PSD participants responded with a higher number of intrusions to auditory trauma cues than participants of the Sleep group.
3.6. Exploratory correlation analyses
Correlations are reported in Supplementary Tables 1 and 2. Analyses did not yield any significant associations between explicit and implicit memory (p > .05). Concerning intrusion frequency, analyses revealed a significant correlation between % FAs and intrusion frequency, which was only evident in the PSD group (ρ = .58, p = .009). None of the other correlations reached significance (p > .05).
4. Discussion
The current study investigated the effects of sleep (as opposed to partial sleep deprivation) on memory for trauma reminders and intrusive memories using an established analogue procedure. Participants were exposed to ‘traumatic’ picture stories prior to nocturnal sleep or partial sleep deprivation in the second night half. In the morning, participants performed an implicit memory task, a recognition memory test, and an intrusion triggering task. Our results demonstrate a clear enhancement of recollection for potential trauma reminders after sleep as compared to partial sleep deprivation. By contrast, groups did not differ in familiarity and implicit memory. Moreover, participants responded with fewer intrusions after sleep than following partial sleep deprivation.
Previous analogue studies (Kleim et al., Citation2016; Porcheret et al., Citation2015) investigating the effects of sleep on intrusive memories based their hypotheses on basic sleep-memory research. However, no study to date examined whether these basic research findings also apply in the context of traumatic memories. Our results show several correspondences with basic research: In line with findings from Giganti et al. (Citation2014) and Casey et al. (Citation2016), we found that explicit – but not implicit – ‘trauma’ memory was higher after sleep than after partial sleep deprivation. Moreover, group differences were selectively evident for recollection-based recognition memory, which is in agreement with previous findings from Drosopoulos et al. (Citation2005) and others (Atienza & Cantero, Citation2008; Daurat et al., Citation2007). As such, our findings add to the current state of research by suggesting that effects of sleep on memory consolidation may also be evident for potential reminders of an analogue stressor. Moreover, our overall pattern of results is in line with the notion that sleep selectively consolidates hippocampus-dependent episodic memory – as evident in enhanced recollection – without affecting familiarity and priming.
Group differences indicate that sleep may strengthen episodic memory of trauma reminders, thus resulting in deliberate and organized recollection of traumatic events. This finding is in line with the position brought forward by Kleim et al. (Citation2016) and others (Pace-Schott et al., Citation2015; Stickgold & Manoach, Citation2017) that sleep may actively support integrative processing of memories after trauma yielding integrated, consciously accessible memories of the traumatic event. Although we did not directly observe an association between this putative process of integration and intrusive re-experiencing, our findings suggest that both enhanced recollection and decreased intrusive re-experiencing seem to emerge after sleep. As such, we found that participants of the Sleep group responded with fewer intrusions than participants of the PSD group. Exploratory correlation analyses did not reveal any significant associations between recollection-based recognition memory after sleep and intrusions. However, we did find that higher false alarm rates were associated with more intrusions in the PSD group suggesting that controlled retrieval might be inversely related to intrusion development after restricted sleep (for related findings see e.g., Hayes et al., Citation2011; Hayes, VanElzakker, & Shin, Citation2012). Given that the current study was not primarily designed to investigate correlational patterns, future studies using more suitable designs are needed to readdress these associations. Importantly, our main results are in line with previous studies showing that long-term intrusion development is reduced if sleep (rather than wakefulness) follows analogue trauma exposure (Kleim et al., Citation2016; Woud et al., Citation2018). In extension of these studies, our results suggest that sleep after trauma may also reduce immediate intrusion development in response to trauma-associated stimuli. However, given the sample size of our analyses (N = 41) and the modest effect observed for intrusion frequency (d = 0.57), this finding should be interpreted with caution. Although specific features of our design (e.g., mild analogue stressor, laboratory-based intrusion assessment) may account for the moderate effect size, further studies need to replicate our findings in a larger sample.
Taken together, the current findings support an effect of sleep on recollection of potential trauma reminders and provide indications that sleep may be associated with reduced intrusive re-experiencing. Future research should focus on how both of these effects may be associated and how this association could influence symptom development across time. In this regard, studies should look further into qualitative – rather than quantitative – changes of trauma memories during sleep. Kleim et al. (Citation2016) argue that sleep-related processing may foster integration of trauma memories into pre-existing memory networks in a similar way as during trauma-focused cognitive behavioral therapy. This process of integration may simultaneously prevent the occurrence of fragmented, involuntary intrusions, thus facilitating natural recovery from trauma. To address this hypothesis, future studies should measure ‘trauma’ memory immediately after exposure to an analogue stressor and at multiple time points during the subsequent week. Such longitudinal approaches will enable the investigation of changes in ‘trauma’ memory across multiple nights and the examination of links between these changes, intrusive memories, and sleep physiology. Moreover, studies examining associations between sleep and intrusive re-experiencing after real-life traumatic events are urgently needed. Such studies may shed light on potential moderating effects of trauma type (e.g., interpersonal vs. accidental) and onset/course of traumatization (e.g., acute vs. chronic). Indeed, cross-sectional studies indicate that sleep disturbances are particularly pronounced after chronic interpersonal trauma (Belleville, Dubé-Frenette, & Rousseau, Citation2019; Brown, Akeeb, & Mellman, Citation2015; Grossman et al., Citation2019; Wamser-Nanney & Chesher, Citation2018), which could be associated with a greater impact on memory processing and intrusive re-experiencing.
The current pattern of results is in agreement with cognitive models of PTSD in suggesting that enhancements of explicit memory for potential trauma reminders co-occur with decreased intrusive re-experiencing. However, the lack of significant correlations between memory performance and intrusive memories deviates from the predictions made by these models. Specifically, intrusive memories would be assumed to correlate with enhanced implicit memory and reduced explicit memory. The Ehlers and Clark (Citation2000) model proposes that traumatic memories of PTSD patients are poorly elaborated and inadequately integrated into autobiographical memory structures enhancing the ease of unintentional, implicit memory. In a similar vein, Brewin et al. (Citation2010) assume that traumatic stress reduces the formation of hippocampus-dependent contextual representations resulting in a lack of top-down control on sensory-based representations that drive intrusions. Strengthening of hippocampus-dependent representations is thus assumed to result in a decrease of intrusions (Bisby & Burgess, Citation2017). In line with this assumption, experimental studies have established correlations between contextual memory/learning and intrusive memories (Bisby, King, Brewin, Burgess, & Curran, Citation2010; Meyer, Krans, van Ast, & Smeets, Citation2017). That is, strong contextual memory/learning was correlated with fewer intrusive memories. As such, it seems plausible that selective sleep-dependent strengthening of hippocampus-dependent episodic memory should be associated with fewer intrusive memories. Although we did not find correlations between explicit memory and intrusions, the direction of group differences aligns with this assumption. Moreover, it is important to consider our sample size in light of the strength of correlations found using the current paradigm (e.g., r = −.28, Sündermann et al., Citation2013). Our study was thus not sufficiently powered to examine correlations between explicit/implicit memory performance and intrusions.
Both cognitive models assume that explicit memory reduces intrusive re-experiencing by inhibiting implicit memory retrieval. Based on this assumption, one could argue that sleep’s impact on recollection should result in reduced implicit retrieval of trauma reminders (i.e., via putative inhibitory effects). By contrast, the current study did not yield evidence of lower implicit memory after sleep. However, it is important to consider that behavioral outcomes of inhibitory effects could be more complex: Inhibitory effects may modulate implicit retrieval such that effects are evident in instances of excessive implicit retrieval without decreasing overall implicit memory. In line with this assumption, autobiographical memory elaboration is found to reduce intrusions of ‘traumatic’ stories (Ehlers, Mauchnik, & Handley, Citation2012; Krans, Näring, Holmes, & Becker, Citation2009) without reducing priming for story reminders (Ehlers et al., Citation2012). Further research is required to address the dynamics between explicit and implicit trauma memory both in general as well as in the context of sleep-related consolidation.
Although the dynamics between explicit and implicit memory require further study, it is important to note that we did not find any indications that implicit memory was higher after sleep. Thus, our findings do not support the assumption that sleep deprivation after trauma differentially impairs implicit – compared to explicit – trauma memory (Porcheret et al., Citation2015). Indeed, a comparison of group differences on implicit (d = 0.06) and explicit (d = 0.80) memory measures strongly opposes this assumption. As such, our findings challenge a potential beneficial effect of sleep deprivation immediately after trauma on subsequent intrusive memories (Porcheret et al., Citation2015). Based on the TOST procedure, we were able to demonstrate a lack of group differences for implicit memory assuming a large effect size. Thus, further studies with greater sample sizes are needed to test a lack of small- or medium-sized effects. However, even if confirmed, sleep deprivation effects are likely disproportionately larger for explicit than for implicit memory (dexplicit = 0.80 vs. dimplicit < 0.80) rendering the preventive value of clinical sleep deprivation questionable. Yet, interpreting these findings in terms of a dissociation of explicit and implicit memory is complicated by the fact that explicit and implicit tests differed not only in retrieval modes but also in their degree of overlap between initial presentation and testing conditions (i.e., pictures were intact in the recognition task, whereas they were blurred in the BPIT). According to the transfer-appropriate processing theory, priming should be stronger if the test format matches previous presentation conditions (see e.g., Rajaram & Roediger, Citation1993; Roediger & Blaxton, Citation1987). Sleep may thus enhance performance for such test formats rather than recollection per se (but see Sopp et al., Citation2017, Citation2018). Although extensively used in previous research, the current paradigm should be further adapted to exclude this confound. Moreover, the current design does not enable a strict dissociation of perceptual and conceptual memory. This distinction should be considered in designing future experiments due to its relevance in cognitive models (Ehlers & Clark, Citation2000) and context accounts of PTSD (Hoffman, Shrira, Bodner, & Ben-Ezra, Citation2017).
Despite the lack of group differences in priming, sleep deprivation could affect other types of implicit memory (Lissek & van Meurs, Citation2015). The current study investigated effects of partial sleep deprivation on priming, as this aspect of implicit memory is strongly linked to intrusion development in healthy participants and PTSD patients (Ehlers et al., Citation2006; Michael & Ehlers, Citation2007; Sündermann et al., Citation2013). Critically, longitudinal studies indicate that priming predicts the course of symptom development in PTSD (Ehring & Ehlers, Citation2011; Kleim et al., Citation2012; Michael et al., Citation2005b). However, cognitive models (Ehlers & Clark, Citation2000) also assume that strong associative learning during trauma contributes to intrusion development and, as a consequence, to persistent PTSD. Due to the requirements of our implicit learning paradigm (e.g., presentation times), it was not possible to measure retention of conditioned fear responses. Future studies thus need to investigate how sleep deprivation affects conditioned responses to ‘traumatic’ stimuli.
Some further limitations need to be addressed. First of all, we examined memory processes in response to an analogue stressor, limiting the application of our findings to clinical populations. The current paradigm has been validated extensively in previous analogue studies (see e.g., Ehlers et al., Citation2012; Holz et al., Citation2014; Sündermann et al., Citation2013). Moreover, an adapted version of the blurred picture task has been shown to reliably measure implicit trauma memory in PTSD patients (Kleim et al., Citation2012). Nevertheless, further research in clinical samples needs to verify whether the current findings generalize to explicit and implicit memory of real-life traumatic events (Ehring, Kleim, & Ehlers, Citation2011). Secondly, our partial sleep deprivation design holds certain limitations. Although groups did not differ in their increase in sleepiness across the night (see 3.1.), PSD participants showed a trendwise decrease in vigilance levels (PVT score) that may have affected retrieval performance on the memory tasks. However, follow-up analyses did not verify that differences in PVT scores contributed to group differences in memory performance. Moreover, previous research did not find any marked vigilance decrements after one night of partial sleep deprivation (Drummond, Anderson, Straus, Vogel, & Perez, Citation2012; Philip et al., Citation2012). Nevertheless, future studies should include further control measures (e.g., working memory and independent long-term memory tasks after sleep/sleep deprivation) to address this alternative hypothesis. Such measures as well as other cognitive measures (e.g., intelligence and executive function tests) should also be assessed at baseline to examine preexisting differences between experimental groups. This is particularly important as implicit memory assessment precludes any pre-sleep measurement of memory performance. Assessment of these measures would further enable the investigation of moderating effects of baseline performance levels. Finally, the current sample size (N = 41) and composition (i.e., mostly female university students) bears certain limitations. Although sufficiently powered to detect differences in recollection-based recognition memory, our sample size limits the investigation of smaller group differences in implicit memory. Thus, further studies are needed to confirm our findings in a larger and more representative sample of participants.
Overall, our findings are in line with growing evidence that sleep-related processes may reduce the occurrence of intrusive memories after trauma (Kleim et al., Citation2016; Woud et al., Citation2018). These sleep-related processes, in turn, may be compromised by sleep disturbances in the aftermath of trauma, resulting in enhanced intrusive re-experiencing. Critically, sleep disturbances are highly prevalent after trauma (70–91%; Maher, Rego, & Asnis, Citation2006) and have been identified as a risk factor for persistent PTSD (Babson & Feldner, Citation2010; Marcks, Weisberg, Edelen, & Keller, Citation2010). Experimental evidence (Ackermann, Cordi, La Marca, Seifritz, & Rasch, Citation2019; Sopp, Brueckner, Schäfer, Lass-Hennemann, & Michael, Citation2019) further indicates that sleep disturbances can arise as a direct result of (‘traumatic’) stress. Thus, sleep could act as an early modulator of intrusive re-experiencing. Accordingly, interventions promoting restful sleep in the immediate aftermath of trauma may bear the potential to reduce the risk for PTSD. Although promising, sleep-based interventions such as cognitive behavioral therapy for insomnia have rarely been investigated as preventive treatment after trauma (Colvonen et al., Citation2018). The current state of research underscores that such investigations are both timely and warranted. Given that our study revealed an enhanced number of intrusions and reduced explicit memory after just one night of restricted sleep, the potential consequences of chronic sleep disturbances may be dire and long lasting.
Supplemental Material
Download MS Word (14.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data for this article can be accessed here.
Additional information
Funding
Notes
1. The interview consisted of open questions, which participants were instructed to answer to the best of their knowledge. If answers were insufficient to determine eligibility, the interviewer asked further (non-specified) questions. First, participants were asked whether they had ever sought treatment for mental health problems. If this was not the case, the interviewer named the most common mental disorders. Participants were asked to indicate whether they had ever suffered from one of these or any other mental disorder. If this was not confirmed, participants were provided with a definition of traumatic events (according to DSM-5) and a list of examples. If participants indicated that they had never experienced a traumatic event, they were admitted to the study. As such, the study did not include any structured assessment of psychopathological symptoms except for depressive symptoms (measured by the PHQ-9).
2. Models of homeostatic sleep regulation posit that slow wave sleep (SWS) mainly occurs in the first night half whereas REM sleep dominates the second night half (Ekstrand, Barrett, West, & Maier, Citation1977). SWS in the first night half has further been linked to the recovery of alertness across sleep (Åkerstedt, Gillberg, & Folkard, Citation1992).
3. Results of psychophysiological analyses (during the picture stories and the ITT) will be reported elsewhere.
4. Two participants scored more than 1.5 times the interquartile range above the upper quartile for recollection (n = 1) and intrusion frequency (n = 1). However, neither of these scores was found to influence the pattern of results in respective analyses. Thus, both participants were retained in the data set and analyses were conducted using unchanged scores.
5. Inspecting all study variables for potential gender effects revealed a significant effect of Gender on arousal and valence ratings. Women responded with greater arousal [t(39) = 2.29, p = .028] and lower valence ratings [t(39) = 2.58, p = .013] than men. However, when Gender was introduced as an additional between-subjects factor into respective ANOVAs, no significant Group×Gender interactions or Group main effects emerged. Hence, gender differences did not influence group-related findings.
6. Given that the main effect of Group trended towards significance, we repeated our analyses of memory performance introducing PVT T2 scores as a mean-centered covariate. All analyses yielded the same pattern of results indicating that sleepiness levels did not account for any performance differences between groups.
References
- AASM. (2012). The AASM manual for the scoring of sleep and associated events. Rules, Terminology and Technical Specifications, Darien, Illinois, American Academy of Sleep Medicine.
- Ackermann, S., Cordi, M., La Marca, R., Seifritz, E., & Rasch, B. (2019). Psychosocial stress before a nap increases sleep latency and decreases early slow-wave activity. Frontiers in Psychology, 10, 20.
- Åkerstedt, T., Gillberg, M., & Folkard, S. (1992). Slow wave activity and prior sleep/wakefulness on an irregular schedule. Journal of Sleep Research, 1(2), 118–18.
- Amir, N., Stafford, J., Freshman, M. S., & Foa, E. B. (1998). Relationship between trauma narratives and trauma pathology. Journal of Traumatic Stress, 11(2), 385–392.
- Atienza, M., & Cantero, J. L. (2008). Modulatory effects of emotion and sleep on recollection and familiarity. Journal of Sleep Research, 17(3), 285–294.
- Babson, K. A., & Feldner, M. T. (2010). Temporal relations between sleep problems and both traumatic event exposure and PTSD: A critical review of the empirical literature. Journal of Anxiety Disorders, 24(1), 1–15.
- Belleville, G., Dubé-Frenette, M., & Rousseau, A. (2019). Sleep disturbances and nightmares in victims of sexual abuse with post-traumatic stress disorder: An analysis of abuse-related characteristics. European Journal of Psychotraumatology, 10(1), 1581019.
- Bisby, J. A., & Burgess, N. (2017). Differential effects of negative emotion on memory for items and associations, and their relationship to intrusive imagery. Current Opinion in Behavioral Sciences, 17, 124–132.
- Bisby, J. A., King, J. A., Brewin, C. R., Burgess, N., & Curran, H. V. (2010). Acute effects of alcohol on intrusive memory development and viewpoint dependence in spatial memory support a dual representation model. Biological Psychiatry, 68(3), 280–286.
- Brewin, C. R. (2014). Episodic memory, perceptual memory, and their interaction: Foundations for a theory of posttraumatic stress disorder. Psychological Bulletin, 140(1), 69–97.
- Brewin, C. R., Gregory, J. D., Lipton, M., & Burgess, N. (2010). Intrusive images in psychological disorders: Characteristics, neural mechanisms, and treatment implications. Psychological Review, 117(1), 210.
- Brown, T. S. H., Akeeb, A., & Mellman, T. A. (2015). The role of trauma type in the risk for insomnia. Journal of Clinical Sleep Medicine, 11(07), 735–739.
- Buysse, D. J., Reynolds, I. I. I., Monk, C. F., Hoch, T. H., Yeager, C. C., & Kupfer, D. J. (1991). Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep, 14(4), 331–338.
- Casey, S. J., Solomons, L. C., Steier, J., Kabra, N., Burnside, A., Pengo, M. F., … Kopelman, M. D. (2016). Slow wave and REM sleep deprivation effects on explicit and implicit memory during sleep. Neuropsychology, 30(8), 931.
- Clohessy, S., & Ehlers, A. (1999). PTSD symptoms, response to intrusive memories and coping in ambulance service workers. British Journal of Clinical Psychology, 38(3), 251–265.
- Colvonen, P. J., Straus, L. D., Stepnowsky, C., McCarthy, M. J., Goldstein, L. A., & Norman, S. B. (2018). Recent advancements in treating sleep disorders in co-occurring PTSD. Current Psychiatry Reports, 20(7), 48.
- Crespo, M., & Fernández-Lansac, V. (2016). Memory and narrative of traumatic events: A literature review. Psychological Trauma: Theory, Research, Practice, and Policy, 8(2), 149.
- Daurat, A., Terrier, P., Foret, J., & Tiberge, M. (2007). Slow wave sleep and recollection in recognition memory. Consciousness and Cognition, 16(2), 445–455.
- de Vanssay-Maigne, A., Noulhiane, M., Devauchelle, A. D., Rodrigo, S., Baudoin-Chial, S., Meder, J. F., … Chassoux, F. (2011). Modulation of encoding and retrieval by recollection and familiarity: Mapping the medial temporal lobe networks. Neuroimage, 58(4), 1131–1138.
- Diekelmann, S., & Born, J. (2010). The memory function of sleep. Nature Reviews Neuroscience, 11(2), 114.
- Drosopoulos, S., Wagner, U., & Born, J. (2005). Sleep enhances explicit recollection in recognition memory. Learning & Memory, 12(1), 44–51.
- Drummond, S. P., Anderson, D. E., Straus, L. D., Vogel, E. K., & Perez, V. B. (2012). The effects of two types of sleep deprivation on visual working memory capacity and filtering efficiency. PLoS ONE, 7(4), e35653.
- Ehlers, A., & Clark, D. M. (2000). A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy, 38(4), 319–345.
- Ehlers, A., Hackmann, A., & Michael, T. (2004). Intrusive re‐experiencing in post‐traumatic stress disorder: Phenomenology, theory, and therapy. Memory, 12(4), 403–415.
- Ehlers, A., Mauchnik, J., & Handley, R. (2012). Reducing unwanted trauma memories by imaginal exposure or autobiographical memory elaboration: An analogue study of memory processes. Journal of Behavior Therapy and Experimental Psychiatry, 43, S67–S75.
- Ehlers, A., Michael, T., Chen, Y. P., Payne, E., & Shan, S. (2006). Enhanced perceptual priming for neutral stimuli in a traumatic context: A pathway to intrusive memories? Memory, 14(3), 316–328.
- Ehring, T., & Ehlers, A. (2011). Enhanced priming for trauma-related words predicts posttraumatic stress disorder. Journal of Abnormal Psychology, 120(1), 234.
- Ehring, T., Kleim, B., & Ehlers, A. (2011). Combining clinical studies and analogue experiments to investigate cognitive mechanisms in posttraumatic stress disorder. International Journal of Cognitive Therapy, 4(2), 165–177.
- Eichenbaum, H., Yonelinas, A. P., & Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 30, 123–152.
- Ekstrand, B. R., Barrett, T. R., West, J. N., & Maier, W. G. (1977). The effect of sleep on human long-term memory. Neurobiology of Sleep and Memory (pp. 419–438).
- Eldridge, L. L., Knowlton, B. J., Furmanski, C. S., Bookheimer, S. Y., & Engel, S. A. (2000). Remembering episodes: A selective role for the hippocampus during retrieval. Nature Neuroscience, 3(11), 1149.
- Elzinga, B. M., & Bremner, J. D. (2002). Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? Journal of Affective Disorders, 70(1), 1–17.
- Filkukova, P., Jensen, T. K., Sofie Hafstad, G., Torvund Minde, H., & Dyb, G. (2016). The relationship between posttraumatic stress symptoms and narrative structure among adolescent terrorist-attack survivors. European Journal of Psychotraumatology, 7(1), 29551.
- Gais, S., Albouy, G., Boly, M., Dang-Vu, T. T., Darsaud, A., Desseilles, M., … Vandewalle, G. (2007). Sleep transforms the cerebral trace of declarative memories. Proceedings of the National Academy of Sciences, 104(47), 18778–18783.
- Giganti, F., Arzilli, C., Conte, F., Toselli, M., Viggiano, M. P., & Ficca, G. (2014). The effect of a daytime nap on priming and recognition tasks in preschool children. Sleep, 37(6), 1087–1093.
- Groch, S., Wilhelm, I., Diekelmann, S., & Born, J. (2013). The role of REM sleep in the processing of emotional memories: Evidence from behavior and event-related potentials. Neurobiology of Learning and Memory, 99, 1–9.
- Grossman, E. S., Hoffman, Y. S., Shrira, A., Kedar, M., Ben-Ezra, M., Dinnayi, M., & Zivotofsky, A. Z. (2019). Preliminary evidence linking complex-PTSD to insomnia in a sample of Yazidi genocide survivors. Psychiatry Research, 271, 161–166.
- Hackmann, A., Ehlers, A., Speckens, A., & Clark, D. M. (2004). Characteristics and content of intrusive memories in PTSD and their changes with treatment. Journal of Traumatic Stress, 17(3), 231–240.
- Halligan, S. L., Michael, T., Clark, D. M., & Ehlers, A. (2003). Posttraumatic stress disorder following assault: The role of cognitive processing, trauma memory, and appraisals. Journal of Consulting and Clinical Psychology, 71(3), 419.
- Hayes, J. P., LaBar, K. S., McCarthy, G., Selgrade, E., Nasser, J., Dolcos, F., & Morey, R. A. (2011). Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. Journal of Psychiatric Research, 45(5), 660–669.
- Hayes, J. P., VanElzakker, M. B., & Shin, L. M. (2012). Emotion and cognition interactions in PTSD: A review of neurocognitive and neuroimaging studies. Frontiers in Integrative Neuroscience, 6, 89.
- Herscovitch, J., & Broughton, R. (1981). Sensitivity of the Stanford sleepiness scale to the effects of cumulative partial sleep deprivation and recovery oversleeping. Sleep, 4(1), 83–92.
- Hoffman, Y., Shrira, A., Bodner, E., & Ben-Ezra, M. (2017). Prime and prejudice: The effect of priming context and prejudicial attitudes on post-traumatic stress disorder symptoms following immigrant violence. Psychiatry Research, 254, 224–231.
- Holz, E., Lass-Hennemann, J., Streb, M., Pfaltz, M., & Michael, T. (2014). Effects of acute cortisol administration on perceptual priming of trauma-related material. PLoS ONE, 9(9), e104864.
- Hutchison, I. C., & Rathore, S. (2015). The role of REM sleep theta activity in emotional memory. Frontiers in Psychology, 6, 1439.
- Iyadurai, L., Blackwell, S. E., Meiser-Stedman, R., Watson, P. C., Bonsall, M. B., Geddes, J. R., … Holmes, E. A. (2018). Preventing intrusive memories after trauma via a brief intervention involving Tetris computer game play in the emergency department: A proof-of-concept randomized controlled trial. Molecular Psychiatry, 23(3), 674.
- Jelinek, L., Randjbar, S., Seifert, D., Kellner, M., & Moritz, S. (2009). The organization of autobiographical and nonautobiographical memory in posttraumatic stress disorder (PTSD). Journal of Abnormal Psychology, 118(2), 288.
- Jones, C., Harvey, A. G., & Brewin, C. R. (2007). The organisation and content of trauma memories in survivors of road traffic accidents. Behaviour Research and Therapy, 45(1), 151–162.
- Kleim, B., & Ehlers, A. (2008). Reduced autobiographical memory specificity predicts depression and posttraumatic stress disorder after recent trauma. Journal of Consulting and Clinical Psychology, 76(2), 231.
- Kleim, B., Ehring, T., & Ehlers, A. (2012). Perceptual processing advantages for trauma-related visual cues in post-traumatic stress disorder. Psychological Medicine, 42(1), 173–181.
- Kleim, B., Graham, B., Bryant, R. A., & Ehlers, A. (2013). Capturing intrusive re-experiencing in trauma survivors’ daily lives using ecological momentary assessment. Journal of Abnormal Psychology, 122(4), 998.
- Kleim, B., Wallott, F., & Ehlers, A. (2008). Are trauma memories disjointed from other autobiographical memories in posttraumatic stress disorder? An experimental investigation. Behavioural and Cognitive Psychotherapy, 36(2), 221–234.
- Kleim, B., Wysokowsky, J., Schmid, N., Seifritz, E., & Rasch, B. (2016). Effects of sleep after experimental trauma on intrusive emotional memories. Sleep, 39(12), 2125–2132.
- Krans, J., Näring, G., Holmes, E. A., & Becker, E. S. (2009). Tell me more: Can a memory test reduce analogue traumatic intrusions? Behaviour Research and Therapy, 47(5), 426–430.
- Kroenke, K., Spitzer, R. L., & Williams, J. B. (2001). The PHQ‐9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613.
- Lakens, D., Scheel, A., & Isager, P. (2018). Equivalence testing for psychological research: A tutorial. Advances in Methods and Practices in Psychological Science, 1(2), 259–269.
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1997). International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention (pp. 39–58).
- Lissek, S., & van Meurs, B. (2015). Learning models of PTSD: Theoretical accounts and psychobiological evidence. International Journal of Psychophysiology, 98(3), 594–605.
- Macmillan, N. A., & Kaplan, H. L. (1985). Detection theory analysis of group data: estimating sensitivity from average hit and false-alarm rates. Psychological Bulletin, 98(1), 185.
- Maher, M. J., Rego, S. A., & Asnis, G. M. (2006). Sleep disturbances in patients with post-traumatic stress disorder: Epidemiology, impact and approaches to management. CNS Drugs, 20(7), 567–590.
- Marcks, B. A., Weisberg, R. B., Edelen, M. O., & Keller, M. B. (2010). The relationship between sleep disturbance and the course of anxiety disorders in primary care patients. Psychiatry Research, 178(3), 487–492.
- Marshall, L., & Born, J. (2007). The contribution of sleep to hippocampus-dependent memory consolidation. Trends in Cognitive Sciences, 11(10), 442–450.
- Mayes, A. R., Montaldi, D., Roper, A., Migo, E. M., Gholipour, T., & Kafkas, A. (2019). Amount, not strength of recollection, drives hippocampal activity: A problem for apparent word familiarity‐related hippocampal activation. Hippocampus, 29(1), 46–59.
- Merkow, M. B., Burke, J. F., & Kahana, M. J. (2015). The human hippocampus contributes to both the recollection and familiarity components of recognition memory. Proceedings of the National Academy of Sciences, 112(46), 14378–14383.
- Meyer, T., Krans, J., van Ast, V., & Smeets, T. (2017). Visuospatial context learning and configuration learning is associated with analogue traumatic intrusions. Journal of Behavior Therapy and Experimental Psychiatry, 54, 120–127.
- Michael, T., & Ehlers, A. (2007). Enhanced perceptual priming for neutral stimuli occurring in a traumatic context: Two experimental investigations. Behaviour Research and Therapy, 45(2), 341–358.
- Michael, T., Ehlers, A., Halligan, S., & Clark, D. (2005a). Unwanted memories of assault: What intrusion characteristics are associated with PTSD? Behaviour Research and Therapy, 43(5), 613–628.
- Michael, T., Ehlers, A., & Halligan, S. L. (2005b). Enhanced priming for trauma-related material in posttraumatic stress disorder. Emotion, 5(1), 103–112.
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113.
- Pace-Schott, E. F., Germain, A., & Milad, M. R. (2015). Sleep and REM sleep disturbance in the pathophysiology of PTSD: The role of extinction memory. Biology of Mood & Anxiety Disorders, 5, 3.
- Pace-Schott, E. F., Shepherd, E., Spencer, R. M., Marcello, M., Tucker, M., Propper, R. E., & Stickgold, R. (2011). Napping promotes inter-session habituation to emotional stimuli. Neurobiology of Learning and Memory, 95(1), 24–36.
- Parsons, R. G., & Ressler, K. J. (2013). Implications of memory modulation for post-traumatic stress and fear disorders. Nature Neuroscience, 16, 146.
- Pfaltz, M. C., Michael, T., Grossman, P., Margraf, J., & Wilhelm, F. H. (2010). Instability of physical anxiety symptoms in daily life of patients with panic disorder and patients with posttraumatic stress disorder. Journal of Anxiety Disorders, 24(7), 792–798.
- Philip, P., Sagaspe, P., Prague, M., Tassi, P., Capelli, A., Bioulac, B., … Taillard, J. (2012). Acute versus chronic partial sleep deprivation in middle-aged people: Differential effect on performance and sleepiness. Sleep, 35(7), 997–1002.
- Plihal, W., & Born, J. (1999). Effects of early and late nocturnal sleep on priming and spatial memory. Psychophysiology, 36(5), 571–582.
- Porcheret, K., Holmes, E. A., Goodwin, G. M., Foster, R. G., & Wulff, K. (2015). Psychological effect of an analogue traumatic event reduced by sleep deprivation. Sleep, 38(7), 1017–1025.
- Rajaram, S. (1993). Remembering and knowing: Two means of access to the personal past. Memory & Cognition, 21(1), 89–102.
- Rajaram, S., & Roediger, H. L. (1993). Direct comparison of four implicit memory tests. Journal of Experimental Psychology: Learning, Memory, and Cognition, 19(4), 765.
- Randler, C. (2013). German version of the reduced Morningness–Eveningness Questionnaire (rMEQ). Biological Rhythm Research, 44(5), 730–736.
- Rasch, B., & Born, J. (2013). About sleeps role in memory. Physiological Reviews, 93(2), 681–766.
- Rasch, B., Papassotiropoulos, A., & de Quervain, D. F. (2010). Imaging genetics of cognitive functions: Focus on episodic memory. Neuroimage, 53(3), 870–877.
- Roach, G. D., Dawson, D., & Lamond, N. (2006). Can a shorter psychomotor vigilance task be usedas a reasonable substitute for the ten‐minute psychomotor vigilance task? Chronobiology International, 23(6), 1379–1387.
- Roediger, H. L., & Blaxton, T. A. (1987). Effects of varying modality, surface features, and retention interval on priming in word-fragment completion. Memory & Cognition, 15(5), 379–388.
- Rubin, D. C., Berntsen, D., & Bohni, M. K. (2008). A memory-based model of posttraumatic stress disorder: Evaluating basic assumptions underlying the PTSD diagnosis. Psychological Review, 115(4), 985.
- Sachschal, J., Woodward, E., Wichelmann, J., Haag, K., & Ehlers, A. (2019). Differential effects of poor recall and memory disjointedness on trauma symptoms. Clinical Psychological Science.
- Sopp, M. R., Brueckner, A. H., Schäfer, S. K., Lass-Hennemann, J., & Michael, T. (2019). REM theta activity predicts re-experiencing symptoms after exposure to a traumatic film. Sleep Medicine, 54, 142–152.
- Sopp, M. R., Michael, T., & Mecklinger, A. (2018). Effects of early morning nap sleep on associative memory for neutral and emotional stimuli. Brain Research, 1698, 29–42.
- Sopp, M. R., Michael, T., Weess, H. G., & Mecklinger, A. (2017). Remembering specific features of emotional events across time: The role of REM sleep and prefrontal theta oscillations. Cognitive, Affective, & Behavioral Neuroscience, 17(6), 1186–1209.
- Squire, L. R., Wixted, J. T., & Clark, R. E. (2007). Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience, 8(11), 872.
- Stickgold, R., & Manoach, D. S. (2017). The importance of sleep in fear conditioning and posttraumatic stress disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(2), 109–110.
- Streb, M., Conway, M. A., & Michael, T. (2017). Conditioned responses to trauma reminders: How durable are they over time and does memory integration reduce them? Journal of Behavior Therapy and Experimental Psychiatry, 57, 88–95.
- Sündermann, O., Hauschildt, M., & Ehlers, A. (2013). Perceptual processing during trauma, priming and the development of intrusive memories. Journal of Behavior Therapy and Experimental Psychiatry, 44(2), 213–220.
- Tulving, E. (1985). Memory and consciousness. Canadian Psychology, 26(1), 1.
- Wamser-Nanney, R., & Chesher, R. E. (2018). Trauma characteristics and sleep impairment among trauma-exposed children. Child Abuse & Neglect, 76, 469–479.
- Wang, W. C., Ranganath, C., & Yonelinas, A. P. (2014). Activity reductions in perirhinal cortex predict conceptual priming and familiarity-based recognition. Neuropsychologia, 52, 19–26.
- Wegerer, M., Blechert, J., Kerschbaum, H., & Wilhelm, F. H. (2013). Relationship between fear conditionability and aversive memories: Evidence from a novel conditioned-intrusion paradigm. PLoS ONE, 8(11), e79025.
- Woud, M. L., Cwik, J. C., Blackwell, S. E., Kleim, B., Holmes, E. A., Adolph, D., … Margraf, J. (2018). Does napping enhance the effects of cognitive bias modification-appraisal training? An experimental study. PLoS ONE, 13(2), e0192837.
- Yonelinas, A. P. (2002). The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language, 46(3), 441–517.
- Yonelinas, A. P., & Jacoby, L. L. (1994). Dissociations of processes in recognition memory: Effects of interference and of response speed. Canadian Journal of Experimental Psychology, 48(4), 516.
- Yonelinas, A. P., Ranganath, C., Ekstrom, A. D., & Wiltgen, B. J. (2019). A contextual binding theory of episodic memory: Systems consolidation reconsidered. Nature Reviews Neuroscience, 1, 364–375.