ABSTRACT
Complicated grief (CG) is a debilitating syndrome characterized by persisting and intense distress and impairment after the death of a loved one. The biological mechanisms associated with this syndrome remain unclear but may involve neurobiological pathways implicated in the stress response and attachment systems. The neuropeptide oxytocin has been implicated in attachment and social behaviour, and loss of social bonds has been associated with disruptions in oxytocin signalling. Furthermore, prior research has reported associations between circulating oxytocin and other mental illnesses, including depression. The present pilot study aimed to examine plasma levels of oxytocin in bereaved adults with primary CG (n = 47) compared to age- and sex-matched bereaved individuals with primary Major Depressive Disorder (MDD) (n = 46), and bereaved individuals without any mental disorder (n = 46). In unadjusted analyses comparing groups according to primary diagnosis, oxytocin levels were significantly higher for primary CG compared to primary MDD (p = 0.013), but not compared to bereaved controls (p = 0.069). In adjusted regression models, having a primary or probable (Inventory of Complicated Grief ≥ 30) diagnosis of CG was associated with significantly higher oxytocin levels (p = 0.001). While additional research is needed, findings from our pilot study provide preliminary support for recent conceptualizations of CG implicating a role for oxytocin and the attachment system. Importantly, these findings contribute to the limited current knowledge about possible biological correlates of CG.
HIGHLIGHTS
• Compared to age- and sex- matched bereaved individuals with primary Major Depressive Disorders, individuals with primary Complicated Grief exhibited significantly higher circulating oxytocin levels. • Having a primary diagnosis of Complicated Grief or scoring above the threshold for probable Complicated Grief may be associated with elevated oxytocin levels. • Oxytocin and the attachment system may be implicated in the pathophysiology of Complicated Grief.
El duelo complicado (DC) es un síndrome debilitante caracterizado por intenso y persistente malestar y discapacidad, luego de la muerte de un ser querido. Los mecanismos biológicos asociados con este síndrome no están claros, pero pueden involucrar vías neurobiológicas implicadas en la respuesta al estrés y sistemas de apego. El neuropéptido oxitocina ha sido implicado en el apego y el comportamiento social, y la pérdida de vínculos sociales ha sido asociada a disrupciones en la señal de oxitocina. Más aún, la investigación previa ha reportado asociaciones entre la oxitocina circulante y otras enfermedades mentales, incluyendo depresión. El presente estudio piloto apuntó a examinar los niveles plasmáticos de oxitocina en adultos en duelo con DC primario (n = 47), comparados con personas con Trastorno Depresivo Mayor (TDM) primario emparejados por edad y sexo (n = 46), y con personas en duelo sin ningún trastorno mental (n = 46). En los análisis sin ajustar que compararon grupos de acuerdo al diagnóstico primario, los niveles de oxitocina fueron significativamente mayores para el DG primario, en comparación a TDM primario (p = 0.013), pero no en la comparación con controles en duelo (p = 0.069). En los modelos de regresión ajustados, el tener un diagnóstico primario o probable de DC (Inventario de Duelo Complicado ≥ 30) fue asociado a niveles significativamente mayores de oxitocina (p = 0.001). A pesar de que se requiere mayor investigación, los hallazgos de nuestro estudio piloto proveen soporte preliminar a las recientes conceptualizaciones del DC que implican un rol de la oxitocina y el sistema de apego. Importantemente, estos hallazgos contribuyen al limitado conocimiento actual acerca de los posibles correlatos biológicos del DC.
复杂性哀伤(CG)是一种令人衰弱的综合征,其特征是在亲人去世后有持久且强烈的痛苦和损伤。有关该综合征的生物学机制仍不清楚,但可能涉及与应激反应和依恋系统有关的神经生物学通路。神经肽催产素与依恋和社会行为有关,社会联系的丧失与催产素信号传递的中断有关。此外,先前的研究报道了循环催产素与包括抑郁症在内的其他精神疾患之间的关联。本初步研究旨在考察并比较47名患有原发性CG的丧亲成人,46名年龄、性别匹配的患有原发性抑郁症(MDD)的丧亲者以及46名未患任何精神障碍的丧亲者的血浆催产素水平。对根据初步诊断进行区分的分组进行比较时,未校正的分析显示,原发性CG组的催产素水平显著高于原发性MDD组(p = 0.013),但与对照组中的丧亲者差异不显著(p = 0.069)。在校正后的回归模型中,具有初步的或可能的CG诊断(依据《复杂性哀伤量表》)与更高的催产素水平有显著关联(p = 0.001)。虽然需要更多的研究,但我们的初步研究结果为近期提出的CG可能与催产素和依恋系统有关的理论提供了初步支持。重要的是,这些发现增加了目前有限的有关CG可能的生物学关联的了解。
1. Introduction
Complicated grief (CG) occurs in approximately 10% of bereaved adults (Lundorff, Holmgren, Zachariae, Farver-Vestergaard, & O’Connor, Citation2017) and is characterized by symptoms of both separation distress such as yearning for the deceased and proximity seeking, and traumatic distress such as intrusive thoughts and avoidance of reminders of the loss (Killikelly & Maercker, Citation2017; Shear et al., Citation2011). Theoretical conceptualizations of CG have highlighted a central role of the attachment system in its pathophysiology (Maccallum & Bryant, Citation2013; Shear et al., Citation2007), with some supporting evidence (Huh, Kim, Lee, & Chae, Citation2017; Mancini & Bonanno, Citation2012). Individuals with CG have difficulty fully processing the loss, with a mismatch between an explicit understanding that the death occurred and implicit internal representations of the deceased and their relationship with them in memory that drive persistent activation of the attachment system and failed, painful attempts at reunion (Maccallum & Bryant, Citation2013; Shear & Shair, Citation2005). Separation from an attachment figure is a threat to homeostasis (Bowlby, Citation1980) that results in emotion dysregulation and distress, and thus CG may also be conceptualized as a persistent stress response to the loss of an important attachment figure (Simon, Citation2012).
To date, little is known about the pathophysiology of CG, limiting development of targeted pharmacotherapy as well as diagnostic biomarkers. Oxytocin, a neuropeptide produced in the hypothalamus, has been consistently implicated in attachment and social behaviours (e.g. Theodoridou, Rowe, Penton-Voak, & Rogers, Citation2009). Animal studies most notably in prairie voles have demonstrated that the loss of a mating partner is associated with perturbations in oxytocin signalling, including decreased oxytocin release in the striatum linked to corticotropin-releasing factor (CRF) activation (for review see: Bosch & Young, Citation2017). Human research examining maternal-infant bonding has also identified a potential role for oxytocin, including links to dopamine in the medial amygdala network (nucleus accumbens, amygdala and medial prefrontal cortex) hypothesized to contribute to affiliative bonding (Atzil et al., Citation2017).
Given the central role of attachment and the disruption of social bonds associated with CG, a potential role for oxytocin in this condition can be hypothesized but has been minimally studied. Building on earlier work supporting a role for oxytocin in social distress (Taylor et al., Citation2000; Taylor, Saphire-Bernstein, & Seeman, Citation2010), Hurlemann and Scheele (Citation2016) recently proposed that the loss of important positive attachments might lead to elevations in oxytocin which then drive efforts towards restoring social contact or affiliation; they note that bonding and affiliative behaviour promoted by oxytocin may interact with brain reward systems, and when this occurs in the absence of social support, can increase stress and related conditions. Such a model would suggest that those with CG could have alterations in oxytocin function consistent with failed attempts to reunite with the deceased. To our knowledge, however, only one investigation of CG symptoms and oxytocin systems has been conducted in humans. Although this study did not directly examine the association between oxytocin function and CG, it provides some support for a potential role of oxytocin signaling in the pathophysiology of CG by providing evidence for an interactive effect of behavioral inhibition with a genetic variant of the oxytocin receptor (single nucleotide polymorphism rs2254298) on greater CG symptom severity (Schiele et al., Citation2018).
Prior data suggest that plasma oxytocin might be elevated in MDD reflecting emotional distress and impaired social relationships in this condition (Parker et al., Citation2010). Because oxytocin effects vary by sex (Bredewold & Veenema, Citation2018) and CG occurs more commonly in women (Kersting, Brahler, Glaesmer, & Wagner, Citation2011), investigations of circulating levels of oxytocin should also account for sex as a potential moderator. In line with this, a recent study found that plasma oxytocin was lower in adults with Major Depressive Disorder (MDD) compared to Controls, but there was a significant moderating effect of sex (Yuen et al., Citation2014).
Although there is debate about whether peripheral levels of oxytocin reflect levels in the central nervous system, this association has been shown to be higher in situations of stress (Valstad et al., Citation2017), as might be the case for individuals with CG. The present pilot study aims to examine plasma levels of oxytocin in bereaved individuals with a primary diagnosis of CG in comparison to two age- and sex-matched control groups: bereaved individuals with a primary diagnosis of MDD, and bereaved controls without any mental disorder. We hypothesized that plasma oxytocin would be higher in individuals with CG compared to the other groups, potentially reflecting stress-induced activation of the attachment system in response to the death of a loved one. We further tested whether there was an association of CG symptom severity and plasma oxytocin levels among those with a CG diagnosis. Lastly, due to the high association between CG and MDD reported in prior research (Heeke, Kampisiou, Niemeyer, & Knaevelsrud, Citation2019), and a considerable comorbidity of CG and MDD diagnoses in both the primary CG and primary MDD groups, we explore an alternative modelling approach to examine the simple main effects of meeting symptom severity criteria for CG regardless of primary diagnosis, as well as interactions between CG, MDD, and gender.
2. Material and methods
Clinical measures and peripheral blood samples were collected as part of an ancillary study of characteristics of adults with stress- or anxiety-related disorders and healthy controls who screened to participate in parent-affiliated studies, including an NIH-funded randomized controlled CG treatment study (NCT01179568; Shear et al., Citation2016). For individuals with MDD and controls, primary data and samples were collected as part of a study of stress, depression and cellular ageing (Simon et al., Citation2015). All study procedures were approved by the Institutional Review Board of Partners Healthcare.
2.1. Subjects
The sample consisted of n = 47 adults with a primary diagnosis of CG recruited January 2009 to August 2013 and age- (± 5 years) and sex-matched bereaved adults with MDD (n = 46, recruited October 2007 to September 2011), and age- and sex-matched psychiatrically healthy bereaved controls (n = 46, recruited November 2008 to July 2013). All participants were 21 to 70 years old, willing and able to provide informed consent, and had experienced the death of a loved one at least 6 months prior. Participants with primary CG were treatment-seeking individuals identifying grief as their primary concern, with an Inventory of Complicated Grief (ICG) ≥ 30, and a CG diagnosis confirmed using the Structured Clinical Interview for Complicated Grief by a trained clinician (SCI-CG; Bui et al., Citation2015). Among participants with CG, 69% (n = 31) met criteria for secondary MDD, and among participants with primary MDD, 22% (n = 10) met the symptom threshold (ICG ≥ 30) for CG. Controls were individuals with no current DSM-IV Axis I psychiatric disorders. Both parent studies excluded pregnancy or lactation, severe or unstable medical illness, lifetime diagnosis of a psychotic disorder, bipolar disorder, or mental disorder due to a medical condition or substance, current antidepressant medication, current alcohol or substance abuse or dependence within the past 6 months, or a positive toxicology screen for drugs of abuse at baseline, and serious acute suicidal risk were exclusionary. Minor differences in exclusion criteria of the parent studies included allowed stable benzodiazepines for the CG group only and excluded daily anti-inflammatory medication use in the MDD and control sample (NCT01179568; Shear et al., Citation2016; Simon et al., Citation2015).
2.2. Measures
Participants were assessed for the presence or absence of psychiatric diagnoses using the Structured Clinical Interview for DSM-IV (SCID; First et al. Citation1994). The 19-item self-report Inventory of Complicated Grief (ICG; Prigerson et al., Citation1995) (total range 0–76) was used to assess CG symptom severity, and the SCI-CG to confirm primary CG diagnosis (Bui et al., Citation2015). Women were also assessed for their menopausal status, and a ‘premenopausal status’ variable was coded ‘1’ for premenopausal women, and ‘0’ for other women and men.
2.3. Blood assays and OXT measurement
At the time of collection, blood was collected in EDTA tubes and centrifuged at 3,500 rpm for 20 min at 4°C. Plasma (citrated) was then transferred into 2 cc plastic tubes, capped and frozen at – 80°C until processing. Oxytocin immunoreactivity levels were quantified using a commercial oxytocin ELISA kit and thawed samples on ice were assayed according to manufacturer’s instructions (Enzo Life Sciences, NY, USA). The oxytocin assay was performed without extraction or dilution, and had a sensitivity of 15.6 pg/ml, with inter- and intra-assay coefficient of variations below 16% in the present study. Although the validity of oxytocin immunoassays from unextracted plasma has been previously questioned (McCullough, Churchland, & Mendez, Citation2013), oxytocin levels from unextracted plasma are strongly correlated with levels from extracted plasma (MacLean et al., Citation2018). Furthermore, mass spectrometry data suggest that the majority of oxytocin is removed during extraction as it is bound to other proteins (Brandtzaeg et al., Citation2016). This may decrease assay precision, as the quantity of oxytocin left after extraction is often close to detection limits (Sunahara et al., Citation2018). Finally, while it may be interesting to estimate absolute levels of free (unbound) oxytocin, this was not particularly advantageous for our present study that aimed to compare overall levels of oxytocin between a group of individuals with CG and two control groups.
2.4. Statistical analyses
Between-group differences in socio-demographic, blood draw, and clinical characteristics were evaluated using analyses of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. For the main outcome analysis, we used an analysis of variance with restricted maximum likelihood estimation to examine the variance in plasma oxytocin levels across diagnostic groups, testing the difference between individuals with primary CG compared to each of the two control groups in post hoc pairwise comparisons consistent with study aims. The model was not adjusted for age or sex, as the diagnostic groups were age- and sex-matched. We inspected model residuals and influence diagnostic plots visually and found no notable departures from the assumptions of normality and homogeneity of variance, nor any unduly influential data points. In a follow-up regression analysis among individuals with primary CG, we tested whether higher symptom severity (as measured by ICG total scores) predicted elevated plasma oxytocin levels, adjusting for both age and sex; this analysis excluded two participants due to missing ICG scores.
Due to comorbidities of primary CG with secondary MDD and of primary MDD with probable secondary CG (i.e. ICG ≥ 30), we also conducted a set of secondary regression analyses examining plasma oxytocin by any ‘probable CG diagnosis’ (primary CG as confirmed by structured clinician interview combined with any ‘probable CG’ based on ICG score), any current MDD diagnosis, sex, all two-way interactions of diagnoses and of diagnoses with sex (full model), and using age and ‘premenopausal status’ as covariates. We then dropped all non-significant interaction terms (p > 0.15) to retain the most parsimonious regression model (reduced model). The regression analyses used effects coding for diagnosis and sex effects to allow easy interpretation of beta coefficients for both main and interactions effects (UCLA: Statistical Consulting Group) and were adjusted for sex and age effects, because in the regression analyses we no longer used the age- and sex-matched primary diagnostic group variable. Five participants were excluded from the regression analyses due to missing ICG scores (2 controls, 1 MDD) or missing MDD diagnosis (2 CG). Means are presented as unadjusted raw means with standard deviations and effect sizes reported as Hedges’ g (Hedges, Citation1981), that is typically preferred for small samples (Rosnow & Rosenthal, Citation2003). Analyses were performed using SAS for Windows v9.4 and the alpha level of significance was set to 0.05 (two-tailed).
3. Results
Primary diagnosis groups were matched for age and sex and did not differ significantly in racial or ethnic distribution or education status (). Individuals with primary MDD were less likely to be married or in a partnership than participants with primary CG or bereaved controls. Participants with primary CG diagnoses were more likely (41%) to report the loss of their spouse as their most distressing loss, compared to either bereaved controls without primary MDD (9%) or with primary MDD (12%: ). As expected, CG symptom severity significantly differed across the three groups (F(2,130) = 112.5, p < 0.001) with individuals with primary CG scoring significantly higher on the ICG than bereaved controls (p < 0.001) and those with MDD (p < 0.001). Although the time of the blood draws differed slightly across the three diagnostic groups (), oxytocin levels were not significantly associated with time of draw (r = −0.094, p = 0.286), or morning draw (t(128) = 0.520, p = 0.604). Across the whole sample, oxytocin levels did not differ by relationship to the deceased (F(2, 96) = 0.40, p = 0.67), nor by current PTSD status (t(135) = 0.5731, p = 0.57).
Table 1. Sociodemographic and clinical characteristics of n = 47 adults with complicated grief and age- and sex-matched bereaved controls (n = 46), and adults with major depressive disorder (n = 46).
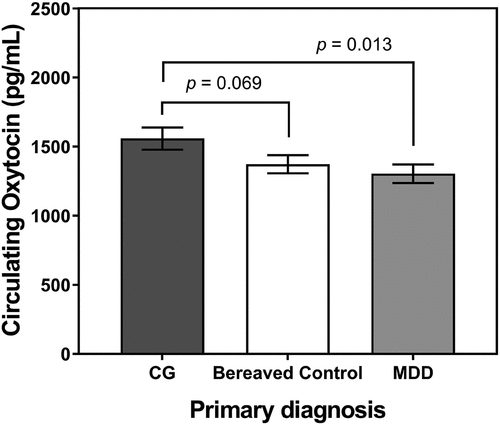
The primary outcome analysis indicated that primary diagnosis was significantly associated with plasma oxytocin levels (F(2,136) = 3.38, p = 0.037). Plasma oxytocin levels were significantly higher for primary CG compared to primary MDD (plasma oxytocin M± SD: 1,558.6 ± 551.8 pg/mL vs. 1,304.4 ± 461.1 pg/mL; t(136) = −2.51, p = 0.013, g = 0.50), though not different from bereaved controls (1,558.6 ± 551.8 pg/mL vs. 1,372.9 ± 443.6 pg/mL; t(136) = −1.83, p = 0.069, g = 0.37; ) in pair-wise comparisons. The multiple regression analysis predicting plasma oxytocin within the group of participants with primary CG indicated that age, sex and ICG severity explained only 2% of the variation in plasma oxytocin levels (R2 = 0.02, F(3,41) = 0.27, p = 0.84); neither grief symptom severity (b± SE:-0.7 ± 8.4, t(1) = −0.08, p = 0.938), nor age (b± SE:-2.2 ± 6.5, t(1) = −0.35, p = 0.731), nor sex (b± SE:-144.4 ± 183.8, t(1) = −0.079, p = 0.437) were significantly associated with plasma oxytocin levels.
Figure 1. Plasma circulating oxytocin levels (pg/mL) in bereaved people with a primary diagnosis of complicated grief (CG; n = 47), a primary diagnosis of major depressive disorder (MDD, n = 46), and without psychiatric disorder (bereaved controls; n = 46). Note that the p-values of the pairwise comparisons shown were not adjusted for age, sex, comorbid diagnosis, or menopausal status, as reported elsewhere in the paper.
In the follow-up regression models, in which we coded the presence or absence of any primary and secondary MDD, and primary plus probable secondary CG diagnoses, the final reduced model explained approximately 10% of the observed variation in circulating oxytocin levels (R2 = 0.15, adj. R2 = 0.10, F(7, 126) = 3.23, p = 0.004). A primary or probable CG diagnosis was associated with higher plasma oxytocin levels (plasma oxytocin M± SD: 1,526.8 ± 543.8 with (n = 56) vs. 1,327.8 ± 442.0 without primary or probable CG (n = 78), g = 0.41), while primary or secondary MDD diagnoses only had a sex-specific association with plasma oxytocin (). We were unable to detect any interactive effects between CG and MDD diagnoses (Probable CG × Current MDD effect) or between CG and sex (Probable CG × Sex effect), or a main effect of MDD diagnoses (Current MDD effect; full model, ). The MDD by sex interaction indicated that the presence of MDD was associated with lower oxytocin levels in women (plasma oxytocin M± SD: 1,358.8 ± 491.5 pg/mL with (n = 53) vs. 1,510.9 ± 540.2 pg/mL without MDD (n = 39), g = 0.30), but higher oxytocin levels in men (1,475.9 ± 519.5 pg/mL with (n = 23) vs. 1,272.8 ± 334.8 pg/mL without MDD (n = 19), g = 0.46), an effect that persisted even when adjusting for overall MDD diagnosis, sex, age, and premenopausal status effects (full and reduced models, ).
Table 2. Follow-up regression models predicting plasma (circulating) oxytocin levels with probable complicated grief (CG) and current major depressive disorder (MDD) diagnoses, sex, and their interactions, adjusting for age and premenopausal status.
4. Discussion
To our knowledge, our pilot study is the first investigation of circulating oxytocin in individuals with CG. Our preliminary results indicate that circulating plasma oxytocin may be moderately elevated in individuals with a primary diagnosis of CG compared to those with MDD, suggesting that the oxytocin pathway may be uniquely involved in the pathophysiology of CG and a potential biomarker for this condition. Although oxytocin was not significantly higher in the CG group compared to the age- and sex-matched bereaved controls, the effect size was small to moderate. Further, when examined together, primary and probable CG were associated with higher oxytocin levels across the full sample. It is possible that this result was due to greater power and precision with the combination of the probable CG group (comorbid with MDD) and primary CG, and may indicate that an elevation in oxytocin levels is present even for threshold CG symptoms co-occurring with other conditions such as MDD.
Administration of oxytocin has been previously shown to elicit approach behaviours in animal and human studies (Gordon, Zagoory-Sharon, Leckman, & Feldman, Citation2010; Robinson, Twiss, Hazon, Moss, & Pomeroy, Citation2017). While our data are limited by their cross-sectional nature, it is possible that increased levels of circulating oxytocin might be related to core CG symptoms such as persistent yearning and proximity seeking (e.g. spending time with ashes or belongings of the loved one) in an ineffective attempt at reunion, as hypothesized. Consistent with others who reported that depression was associated with decreased oxytocin levels (Gordon et al., Citation2008; Yuen et al., Citation2014), we found lower oxytocin levels in individuals with MDD compared to those with CG. This difference provides support for a distinct pathophysiology that may identify CG as distinct from depression, as previously suggested by clinical data (Jacobsen, Zhang, Block, Maciejewski, & Prigerson, Citation2010). In particular, given the role of oxytocin in social sensitivity and social needs (Bartz, Zaki, Bolger, & Ochsner, Citation2011; Taylor et al., Citation2000, Citation2010), this elevation of oxytocin levels in individuals CG might reflect the social attachment loss-related distress that is specific to CG (vs. depression) (Shear et al., Citation2011). Prior data also suggest that salivary oxytocin levels might be lower in male individuals with PTSD compared to trauma-exposed controls (Frijling et al., Citation2015). Here, our results of elevated plasma oxytocin in CG provide support for a potentially distinct pathophysiology between CG and PTSD, despite both conditions involving exposure to a major stressor and some overlapping stress response-related phenomenology (Maercker & Znoj, Citation2010). More research is needed to fully understand the interactions of oxytocin with sex in adults with trauma, loss and depression-related conditions.
Studies have also uncovered complex relationships between the stress response, HPA axis, oxytocin, and dopamine (Bale, Davis, Auger, Dorsa, & McCarthy, Citation2001; Hashiguchi, Ye, Morris, & Alexander, Citation1997). Prior reports of corticotropin-releasing hormone inducing oxytocin signalling with partner loss in animals (Bosch & Young, Citation2017) suggest that elevated peripheral oxytocin may be a biomarker of a downstream stress response related to attachment loss. Conversely, it is possible that perturbed oxytocin signalling might provoke disturbances in the HPA axis and reward system, and contribute to maintaining an acute stress state and dysfunctional behaviours. This would be consistent with hypotheses that gaps in social relationships (e.g. due to loss) may enhance oxytocin signalling in support of an affiliative effort. These efforts when frustrated by the absence of social support or reunion may maintain the stress response and thus elevate risk for stress-related psychiatric disorders (Hurlemann & Scheele, Citation2016).
While quite speculative, elevated levels of oxytocin could also be the result of a positive feedback loop caused by changes in oxytocin receptors. While one study reported that a variant of a gene that codes for the oxytocin receptor (rs2254298) was associated with CG symptoms in interaction with behavioural inhibition measures, the function of this variant remains speculative (Schiele et al., Citation2018). The context dependent presence of this association only for those with behavioural inhibition, however, suggests that attending to pre-existing risk factors such as mood and anxiety disorders, prior trauma, and other factors related to the lost relationship might be important to consider in the role of oxytocin in grief-related psychopathology. This would be consistent with the social salience hypothesis of oxytocin, suggesting that oxytocin’s effects may vary depending on the context and intra-individual differences; for example, in the context of unpredictable threat, oxytocin release may heighten attention to threat and enhance distress rather than being anxiolytic (Shamay-Tsoory & Abu-Akel, Citation2016).
While our pilot study provides preliminary evidence for a potential role for oxytocin in adults with CG, more work is needed to understand the mechanism through which the oxytocin system is involved. Some limitations should also be acknowledged, including the unclear relationship between peripheral and central levels of oxytocin, and the possibility that peripheral oxytocin levels are not closely related to those found in the central nervous system; the cross-sectional nature of the study; its relatively small sample size; the absence of a non-bereaved control group; the slightly different exclusionary criteria in the parent studies; a single blood collection; and the notable overlap in MDD and CG symptoms in our age and gender matched primary diagnosis groups. Further, future studies should also investigate the potential mediating or moderating role of variables not assessed in our study including the type of death (e.g. violent or natural illness), menstrual phases, and separation anxiety. Despite these limitations, our preliminary findings combined with the rapidly growing neurobiological understanding of the roles of oxytocin in attachment and attachment loss support that additional studies to elucidate the role of the oxytocin system in CG are warranted. In addition, while psychotherapeutic interventions for CG are available (Boelen, de Keijser, van Den Hout, & van Den Bout, Citation2007; Bryant et al., Citation2014; Shear et al., Citation2016; Smid et al., Citation2015), no evidence supported pharmacotherapy is currently available for CG; the oxytocin system may be a potentially promising target for future research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Atzil, S., Touroutoglou, A., Rudy, T., Salcedo, S., Feldman, R., Hooker, J. M., … Barrett, L. F. (2017). Dopamine in the medial amygdala network mediates human bonding. Proceedings of the National Academy of Sciences of the USA, 114(9), 2361–8.
- Bale, T. L., Davis, A. M., Auger, A. P., Dorsa, D. M., & McCarthy, M. M. (2001). CNS region-specific oxytocin receptor expression: Importance in regulation of anxiety and sex behavior. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21(7), 2546–2552.
- Bartz, J. A., Zaki, J., Bolger, N., & Ochsner, K. N. (2011). Social effects of oxytocin in humans: Context and person matter. Trends in Cognitive Sciences, 15(7), 301–309.
- Boelen, P. A., de Keijser, J., van Den Hout, M. A., & van Den Bout, J. (2007). Treatment of complicated grief: A comparison between cognitive-behavioral therapy and supportive counseling. Journal of Consulting and Clinical Psychology, 75(2), 277–284.
- Bosch, O. J., & Young, L. J. (2017). Oxytocin and social relationships: from attachment to bond disruption. Current Topics in Behavioral Neurosciences, 35, 97–117. doi:10.1007/7854_2017_10
- Bowlby, J. (1980). Attachment and loss, volume 3: Loss; sadness and depression. London: Hogarth Press.
- Brandtzaeg, O. K., Johnsen, E., Roberg-Larsen, H., Seip, K. F., MacLean, E. L., Gesquiere, L. R., … Wilson, S. R. (2016). Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Scientific Reports, 6, 31693.
- Bredewold, R., & Veenema, A. H. (2018). Sex differences in the regulation of social and anxiety-related behaviors: Insights from vasopressin and oxytocin brain systems. Current Opinion in Neurobiology, 49, 132–140.
- Bryant, R. A., Kenny, L., Joscelyne, A., Rawson, N., Maccallum, F., Cahill, C., … Nickerson, A. (2014). Treating prolonged grief disorder: A randomized clinical trial. JAMA Psychiatry (Chicago, Ill.), 71(12), 1332–1339.
- Bui, E., Mauro, C., Robinaugh, D. J., Skritskaya, N. A., Wang, Y., Gribbin, C., … Shear, M. K. (2015). The Structured Clinical Interview for Complicated Grief: Reliability, Validity, and Exploratory Factor Analysis. Depression and Anxiety, 32(7), 485–492.
- First, M. S., Gibbon, R. L., & Williams, M. (1994). Structured clinical interview for Axis I DSM-IV disorders - patient version (SCID-1/P version 2.0). New York, NY: New York State Psychiatric Institute Biometrics Research Department.
- Frijling, J. L., van Zuiden, M., Nawijn, L., Koch, S. B., Neumann, I. D., Veltman, D. J., & Olff, M. (2015). Salivary Oxytocin and Vasopressin Levels in Police Officers With and Without Post-Traumatic Stress Disorder. Journal of Neuroendocrinology, 27(10), 743–751.
- Gordon, I., Zagoory-Sharon, O., Leckman, J. F., & Feldman, R. (2010). Oxytocin and the development of parenting in humans. Biological Psychiatry, 68(4), 377–382.
- Gordon, I., Zagoory-Sharon, O., Schneiderman, I., Leckman, J. F., Weller, A., & Feldman, R. (2008). Oxytocin and cortisol in romantically unattached young adults: Associations with bonding and psychological distress. Psychophysiology, 45(3), 349–352.
- Hashiguchi, H., Ye, S. H., Morris, M., & Alexander, N. (1997). Single and repeated environmental stress: Effect on plasma oxytocin, corticosterone, catecholamines, and behavior. Physiology & Behavior, 61(5), 731–736.
- Hedges, L. V. (1981). Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics, 6(2), 107–128.
- Heeke, C., Kampisiou, C., Niemeyer, H., & Knaevelsrud, C. (2019). A systematic review and meta-analysis of correlates of prolonged grief disorder in adults exposed to violent loss. European Journal of Psychotraumatology, 10(1), 1583524.
- Huh, H. J., Kim, K. H., Lee, H. K., & Chae, J. H. (2017). Attachment styles, grief responses, and the moderating role of coping strategies in parents bereaved by the Sewol ferry accident. European Journal of Psychotraumatology, 8(sup6), 1424446.
- Hurlemann, R., & Scheele, D. (2016). Dissecting the Role of Oxytocin in the Formation and Loss of Social Relationships. Biological Psychiatry, 79(3), 185–193.
- Jacobsen, J. C., Zhang, B., Block, S. D., Maciejewski, P. K., & Prigerson, H. G. (2010). Distinguishing symptoms of grief and depression in a cohort of advanced cancer patients. Death Studies, 34(3), 257–273.
- Kersting, A., Brahler, E., Glaesmer, H., & Wagner, B. (2011). Prevalence of complicated grief in a representative population-based sample. Journal of Affective Disorders, 131(1–3), 339–343. doi:10.1016/j.jad.2010.11.032
- Killikelly, C., & Maercker, A. (2017). Prolonged grief disorder for ICD-11: The primacy of clinical utility and international applicability. European Journal of Psychotraumatology, 8(Suppl 6), 1476441.
- Lundorff, M., Holmgren, H., Zachariae, R., Farver-Vestergaard, I., & O’Connor, M. (2017). Prevalence of prolonged grief disorder in adult bereavement: A systematic review and meta-analysis. Journal of Affective Disorders, 212, 138–149.
- Maccallum, F., & Bryant, R. A. (2013). A Cognitive Attachment Model of prolonged grief: Integrating attachments, memory, and identity. Clinical Psychology Review, 33(6), 713–727.
- MacLean, E. L., Gesquiere, L. R., Gee, N., Levy, K., Martin, W. L., & Carter, C. S. (2018). Validation of salivary oxytocin and vasopressin as biomarkers in domestic dogs. Journal of Neuroscience Methods, 293, 67–76.
- Maercker, A., & Znoj, H. (2010). The younger sibling of PTSD: Similarities and differences between complicated grief and posttraumatic stress disorder. European Journal of Psychotraumatology, 1.
- Mancini, A. D., & Bonanno, G. A. (2012). The persistence of attachment: Complicated grief, threat, and reaction times to the deceased’s name. Journal of Affective Disorders, 139(3), 256–263.
- McCullough, M. E., Churchland, P. S., & Mendez, A. J. (2013). Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted?. Neuroscience and Biobehavioral Reviews, 37(8), 1485–1492.
- Parker, K. J., Kenna, H. A., Zeitzer, J. M., Keller, J., Blasey, C. M., Amico, J. A., & Schatzberg, A. F. (2010). Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Research, 178(2), 359–362.
- Prigerson, H. G., Maciejewski, P. K., Reynolds, C. F., 3rd, Bierhals, A. J., Newsom, J. T., Fasiczka, A., … Miller, M. (1995). Inventory of Complicated Grief: A scale to measure maladaptive symptoms of loss. Psychiatry Research, 59(1–2), 65–79.
- Robinson, K. J., Twiss, S. D., Hazon, N., Moss, S., & Pomeroy, P. P. (2017). Positive social behaviours are induced and retained after oxytocin manipulations mimicking endogenous concentrations in a wild mammal. Proceedings. Biological sciences / The Royal Society, 284(1855), 20170554.
- Rosnow, R. L., & Rosenthal, R. (2003). Effect sizes for experimenting psychologists. Canadian Journal of Experimental Psychology/Revue Canadienne de Psychologie Expérimentale, 57(3), 221.
- Schiele, M. A., Costa, B., Abelli, M., Martini, C., Baldwin, D. S., Domschke, K., & Pini, S. (2018). Oxytocin receptor gene variation, behavioural inhibition, and adult separation anxiety: Role in complicated grief. The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry, 1–9.
- Shamay-Tsoory, S. G., & Abu-Akel, A. (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79(3), 194–202.
- Shear, K., Monk, T., Houck, P., Melhem, N., Frank, E., Reynolds, C., & Sillowash, R. (2007). An attachment-based model of complicated grief including the role of avoidance. European Archives of Psychiatry and Clinical Neuroscience, 257(8), 453–461.
- Shear, K., & Shair, H. (2005). Attachment, loss, and complicated grief. Developmental Psychobiology, 47(3), 253–267.
- Shear, M. K., Reynolds, C. F., 3rd, Simon, N. M., Zisook, S., Wang, Y., Mauro, C., … Skritskaya, N. (2016). Optimizing Treatment of Complicated Grief: A Randomized Clinical Trial. JAMA Psychiatry (Chicago, Ill.).
- Shear, N. M., Wall, S. M., Zisook, S., Neimeyer, R., Duan, N., Reynolds, C., … Keshaviah, A. (2011). Complicated grief and related bereavement issues for DSM-5. Depression and Anxiety, 28(2), 103–117.
- Simon, N. M. (2012). Is complicated grief a post-loss stress disorder? Depression and Anxiety, 29(7), 541–544.
- Simon, N. M., Walton, Z. E., Bui, E., Prescott, J., Hoge, E., Keshaviah, A., … Wong, K. K. (2015). Telomere length and telomerase in a well-characterized sample of individuals with major depressive disorder compared to controls. Psychoneuroendocrinology, 58, 9–22.
- Smid, G. E., Kleber, R. J., de la Rie, S. M., Bos, J. B., Gersons, B. P., & Boelen, P. A. (2015). Brief Eclectic Psychotherapy for Traumatic Grief (BEP-TG): Toward integrated treatment of symptoms related to traumatic loss. European Journal of Psychotraumatology, 6, 27324.
- Sunahara, C. S., Zelkowitz, P., Bolger, N., Sadikaj, G., Samuel, S., Gold, I., … Bartz, J. A. (2018). Maternal oxytocin predicts relationship survival during the perinatal transition period: Preliminary evidence. International Journal of Psychophysiology, 136, 33–38.
- Taylor, S. E., Klein, L. C., Lewis, B. P., Gruenewald, T. L., Gurung, R. A., & Updegraff, J. A. (2000). Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review, 107(3), 411–429.
- Taylor, S. E., Saphire-Bernstein, S., & Seeman, T. E. (2010). Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science, 21(1), 3–7.
- Theodoridou, A., Rowe, A. C., Penton-Voak, I. S., & Rogers, P. J. (2009). Oxytocin and social perception: Oxytocin increases perceived facial trustworthiness and attractiveness. Hormones and Behavior, 56(1), 128–132.
- UCLA: Statistical Consulting Group. FAQ: What is Effect coding?. Retrieved from https://stats.idre.ucla.edu/other/mult-pkg/faq/general/faqwhat-is-effect-coding/
- Valstad, M., Alvares, G. A., Egknud, M., Matziorinis, A. M., Andreassen, O. A., Westlye, L. T., & Quintana, D. S. (2017). The correlation between central and peripheral oxytocin concentrations: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 78, 117–124.
- Yuen, K. W., Garner, J. P., Carson, D. S., Keller, J., Lembke, A., Hyde, S. A., … Parker, K. J. (2014). Plasma oxytocin concentrations are lower in depressed vs. healthy control women and are independent of cortisol. Journal of Psychiatric Research, 51, 30–36.
