ABSTRACT
Background
HIV/AIDS and potentially traumatic events (PTEs) or stressful life events (SLEs) and/or PTSD are independently associated with neurocognitive impairment (NCI). Literature suggests that HIV and PTE/SLE exposure independently and consistently affect various domains of cognition including language ability, working memory and psychomotor speed. There are limited data on the interaction between HIV infection and PTEs and their combined effect on NCI.
Objective
In this systematic review, we synthesise evidence for the combined effect of HIV infection and PTEs and SLEs and/or post-traumatic stress disorder (PTSD) on NCI of people living with HIV/AIDS (PLWHA) from high-, middle- and low- income countries.
Method
Our inclusion criteria were observational epidemiological studies (case-control, cohort and cross-sectional designs) that investigated the interaction of HIV infection, PTEs and SLEs and/or PTSD and specifically their combined effect on NCI in adults. We searched a number of electronic databases including Pubmed/Medline, PsycINFO, Scopus and Global Health using the search terms: cognition, HIV/AIDS, observational studies, trauma and permutations thereof.
Results
Fifteen studies were included in the review, of which the majority were conducted in high-income countries. Ten of the fifteen studies were conducted in the United States of America (USA) and five in South Africa. Seven of these focused on early life stress/childhood trauma. The remaining studies assessed adult-onset PTEs and SLEs only. Eight studies included women only. Overall, the studies suggest that PTE and SLE exposure and/or PTSD are a significant risk factor for NCI in adults living with HIV, with impairments in memory and executive functions being the most likely consequence of PTE and SLE exposure.
Conclusion
These findings highlight the need for trauma screening and for the integration of trauma-focused interventions in HIV care to improve outcomes.
Antecedentes: El VIH/SIDA y los eventos potencialmente traumáticos (PTEs) o los eventos estresantes de la vida (SLEs) y/o TEPT se asocian independientemente con el deterioro neurocognitivo (NCI). La literatura sugiere que la exposición al VIH, PTE y SLE afecta de manera independiente y consistente varios dominios de la cognición, incluida la capacidad del lenguaje, la memoria de trabajo y la velocidad psicomotora. Hay datos limitados sobre la interacción entre la infección por VIH y los PTE, y su efecto combinado sobre el NCI.
Objetivo: En esta revisión sistemática sintetizamos evidencia del efecto combinado de la infección por VIH, PTEs y SLEs, y/o TEPT en el NCI de personas que viven con VIH/SIDA (PLWHA) en países de ingresos altos, medios y bajos.
Método: Nuestros criterios de inclusión fueron estudios epidemiológicos observacionales (diseño de caso-control, cohortes y diseños transversales) que investigaron la interacción de la infección por VIH, PTEs y SLEs y/o TEPT, y específicamente su efecto combinado sobre el NCI en adultos. Se realizaron búsquedas en varias bases de datos electrónicas, que incluyeron a Pubmed/Medline, PsycINFO, Scopus y Global Health, utilizando los términos de búsqueda: cognición, VIH/SIDA, estudios de observación, trauma y permutaciones de los mismos.
Resultados: Quince estudios se incluyeron en la revisión, de los cuales la mayoría se realizaron en países de altos ingresos. Diez de los quince estudios fueron realizados en los Estados Unidos de América (EE.UU.) y cinco en Sudáfrica. Siete de éstos se centraron en el estrés de la vida temprana/trauma infantil. Los estudios restantes evaluaron PTEs y SLEs cuya aparición fue en la vida adulta solamente. Ocho estudios incluyeron sólo mujeres. En general, los estudios sugieren que la exposición a PTE y SLE y/o TEPT es un factor de riesgo significativo para NCI en adultos que viven con VIH, con el deterioro en la memoria y las funciones ejecutivas como la consecuencia más probable de la exposición a PTE y SLE.
Conclusión: Estos hallazgos resaltan la necesidad de la detección de traumas y la integración de intervenciones centradas en el trauma en la atención del VIH para mejorar sus resultados.
背景: HIV/AIDS和潜在创伤事件 (PTE) 或应激性生活事件 (SLE) 和/或PTSD与神经认知障碍 (NCI) 独立相关。文献表明, HIV, PTE和SLE暴露独立且持续地影响认知的各个领域, 包括语言能力, 工作记忆和精神运动速度。关于HIV感染与PTE之间的相互作用及其对NCI的综合影响的数据有限。
目的: 在本系统综述中, 我们综合了HIV感染, PTE和SLE和/或创伤后应激障碍 (PTSD) 对高, 中, 低收入国家 HIV/AIDS携带者(PLWHA)NCI的综合效应的证据。
方法: 我们的纳入标准是观察流行病学研究 (病例对照, 队列研究和横断面设计), 且考查了HIV感染, PTE和SLE和/或PTSD的交互作用, 特别是它们对成人NCI的综合效应。我们在包括Pubmed/Medline, PsycINFO, Scopus和Global Health的众多电子数据库中, 使用以下搜索词进行搜索:‘认知’, ‘HIV/AIDS’, ‘观察性研究’, ‘创伤’及其排列。
结果: 15项研究被纳入综述, 其中大多数在高收入国家进行。 15项研究中, 10项在美国 (USA) 进行, 5项在南非进行。其中有7项关注生早期生活应激/童年创伤。其余研究仅评估成年后发作型PTE和SLE。8项研究仅包括女性。总体而言, 研究表明PTE和SLE暴露和/或PTSD是HIV成年携带者NCI的显著风险因素, 记忆和执行功能损伤最有可能由PTE和SLE暴露导致。
结论: 这些发现强调了创伤筛查以及在HIV护理中整合聚焦创伤干预措施的必要性, 以改善干预结果。
1. Background
Advances in the treatment of HIV have dramatically improved survival rates. HIV has transformed from an acute terminal disease to a chronic pharmacologically-managed condition through increased access to combination antiretroviral therapy (cART) around the globe. Nevertheless, HIV infection is associated with a range of sequelae including neurocognitive impairment (NCI). The neuropathogenesis of NCI is complex and is characterized by events such as oxidative stress, neuroinflammation, synaptic pruning and neuronal death (Kumar et al., Citation2009; Valcour et al., Citation2012; Williams, Zulu, Stein, Joska, & Naude, Citation2020). HIV crosses the blood brain barrier (BBB) early in the course of infection. There is considerable evidence that NCI related to HIV has a variable clinical trajectory (Alford & Vera, Citation2018; Habib et al., Citation2013; Heaton et al., Citation2015; Rubin & Maki, Citation2019a). Symptoms of NCI in people living with HIV/AIDS (PLWHA) are generally on a spectrum ranging from asymptomatic neurocognitive impairment to mild cognitive impairment to HIV associated dementia (Antinori et al., Citation2007). The range of dysfunctions include mental slowing, memory loss, and difficulties in complex tasks, motor disorders, and behavioural abnormalities (Simioni et al., Citation2010). Studies suggest that between 30 and 50% of PLWHA experience mild forms of NCI. Data from the United States of America (USA) suggest that approximately 2% of PLWHA experience a more serious form of NCI, HIV associated dementia (Heaton et al., Citation2010). Today, while the incidence of the most severe phenotype, HIV-associated dementia has declined, subtle forms of the disease such as asymptomatic neurocognitive impairment persist despite patients being on cART (Ambrosius, Gold, Chan, & Faissner, Citation2019). This may, in part, be attributed to the limited penetration of the BBB by certain antiretrovirals (e.g. HIV protease inhibitors, nucleoside analogues). Evidence of the effectiveness of BBB penetration of antiretrovirals on cognition is equivocal, with higher and lower CNS (central nervous system) penetration effectiveness (CPE) of antiretrovirals associated with cognitive improvements, as well a few studies finding no association between CPE and cognitive benefits (Yuan & Kaul, Citation2019).
NCI in PLWHA has a complex aetiology and it is likely that a number of risk factors may interact with the HIV infection to predispose its onset (Heaton et al., Citation2015). A number of studies from both high and low to middle-income countries using neuroimaging and epidemiological techniques have examined additional factors potentially associated with NCI in PLWHA. These include potentially traumatic events (PTEs), and post-traumatic stress disorder [PTSD] (a potential consequence of PTEs) (Kessler et al., Citation2014; McLaughlin et al., Citation2017; Scott et al., Citation2018), which have also been associated with NCI in HIV-negative samples (Jelinek et al., Citation2006; Lagarde, Doyon, & Brunet, Citation2010). PTEs during childhood, particularly neglect, are associated with decline in memory, executive function, and processing speed (Malan-Muller et al., Citation2013; Spies, Ahmed-Leitao, Fennema-Notestine, Cherner, & Seedat, Citation2016). PTEs encompass a broad spectrum of exposures including sexual, physical, and emotional abuse during early life, adolescence, or adulthood. PTEs have been found to be highly prevalent in PLWHA (Brief et al., Citation2004; Decker et al., Citation2016; Machtinger, Wilson, Haberer, & Weiss, Citation2012). These PTEs have also been associated with a range of additional psychopathologies, most frequently PTSD and depression (Spies et al., Citation2012). A recent systematic review by Rubin and Maki examined the relationship between depression and NCI in PLWHA and found that depression contributed to impairments particularly in the domains of executive function, processing speed, learning, and motor function (Rubin & Maki, Citation2019b). A broad body of evidence links PTSD to impairments in multiple cognitive systems, including processing speed, learning, memory, and executive function (Aupperle, Melrose, Stein, & Paulus, Citation2012; Qureshi et al., Citation2011; Schuitevoerder et al., Citation2013; Scott et al., Citation2015; Sumner et al., Citation2017). In a meta-analysis based on data from 60 studies totalling 4,108 participants, including 1,779 with PTSD, 1,446 trauma-exposed comparison participants, and 895 healthy comparison participants without trauma exposure, significant neurocognitive effects were associated with PTSD (Scott et al., Citation2015).
While literature suggests that HIV infection, PTEs, and/or PTSD independently affect various domains of cognition including language ability, working memory, and psychomotor speed (Grant, Citation2008; Heaton et al., Citation2010; Tomoda et al., Citation2011), few studies have examined the combined effect of PTEs, PTSD, and/HIV infection on NCI (i.e. HIV+PTEs and/or PTSD on NCI). Spies and colleagues who work in South Africa, against the backdrop of a substantial HIV epidemic, examined the relationship between HIV, trauma and NCI in women, both as independent and combined exposures. They found that PTEs were significantly associated with memory impairment in women living with HIV (Spies, Fennema-Notestine, Archibald, Cherner, & Seedat, Citation2012). Findings from the Women’s Inter-Agency HIV Study (WIHS) indicated an interaction between psychological risk factors, including perceived stress, anxiety, post-traumatic stress, and depressive symptoms, and NCI, such that perceived stress and anxiety were more strongly associated with deficits in learning and memory among women living with HIV compared to their uninfected counterparts (Maki et al., Citation2015). Specifically in this study, depressive symptoms were associated with a lower level of cognitive performance (Maki et al., Citation2015). The findings suggest that the neurobiological effects of psychological risk factors, such as stress on cognition, may be different in HIV-positive and HIV-negative women, with putative mechanistic links to stress responsivity and immune function (Rubin et al., Citation2017). Stress, PTSD, and depression are immunomodulatory and influence the immune response in women infected with HIV. However, stress may have a stronger influence than depression given that stress is more strongly associated with regulatory mechanisms necessary to maintain immune cell homoeostasis (Rehm & Konkle-Parker, Citation2017), in turn impacting brain homoeostasis (de Groot & Burgas, Citation2015). Despite the gradual emergence of a small body of research examining the combined effect of PTEs and SLEs and NCI, to our knowledge no review has synthesised observational epidemiological studies of PTEs or SLEs and/or PTSD and NCI in PLWHA. For this review, we sought to synthesise findings from derived from high-, middle-, and low- income countries to contribute to the growing body of research exploring the relationship between PTEs and SLEs, and/or PTSD, HIV infection and NCI.
2. Methods
The process as outlined in PRISMA was adhered to (Moher, Liberati, Tetzlaff, & Altman, Citation2009).
2.1. Inclusion and exclusion criteria
Eligible studies had to be in English and include adults (18 years and older) only. There was no limit on the date of publication. We included observational epidemiological studies (i.e. cross-sectional, cohort, or case-control studies) that examine the relationship between PTEs and/or PTSD and NCI in PLWHA. Intervention studies were also excluded. In this instance, trauma was broadly defined as traumatic or stressful events (PTEs and SLEs) during childhood and/or adulthood. PTEs included both early life and adult-onset traumatic events that met Criterion A for a traumatic event for PTSD as well as other stressful life events (SLEs, e.g. economic hardship, food insecurity) and traumatic brain injury. For a study to be included, NCI had to be measured by a full neuropsychological battery. Studies using self-reported measures of NCI were excluded unless they included z-scores based on published normative data. Moreover, studies using screening instruments only for cognitive impairment (e.g. the (International) HIV Dementia Scale and the Montreal Cognitive Assessment) were excluded. Inclusion and exclusion criteria are presented in .
Table 1. Study inclusion and exclusion criteria.
2.2. Search strategies
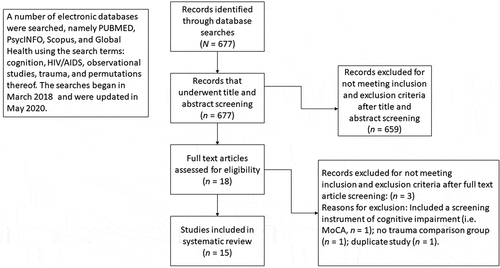
Prior to conducting the searches, we collaboratively decided on the search terms. A number of electronic databases were searched, namely PUBMED, PsycINFO, Scopus, and Global Health using the search terms: cognition, HIV/AIDS, observational studies, trauma, and permutations thereof. The searches began in March 2018. A total of 677 abstracts were extracted to a Rayyan database, a web and mobile application for systematic reviews (Ouzzani, Hammady, Fedorowicz, & Elmagarmid, Citation2016). The searches were updated in May 2020 using the following electronic databases: PUBMED, Psych Info, and Google Scholar. Reference lists of eligible studies were also scanned. The abstracts were then reviewed independently by three reviewers to see if they met inclusion criteria. A face-to-face meeting was subsequently held whereby reviewers discussed discrepancies and reached consensus about which studies to include. Each reviewer flagged duplicate entries on Rayyan, with removal of the duplicates from the database via consensus. See Prisma flow diagram ().
2.3. Data analysis
Data were extracted by two independent reviewers using a standard data extraction form. The form included the following fields: author and date, country, study design, sample, trauma, and PTSD measurement and summary of NCI measures. Quality appraisal of included studies was conducted by GS and SM. However, given that the seminal studies were conducted by two independent reviewers, a third objective reviewer was brought in to assist with the quality appraisal of these studies. The quality appraisal was guided by the Systematic Appraisal of Quality in Observational Research (SAQOR) tool that comprises six domains (each containing two to five questions): sample, control/comparison group, exposure/outcome measurements, follow-up, confounders, and reporting of data (Ross et al., Citation2011). presents the quality appraisal.
Table 2. Quality assessment of papers included in the systematic review (SAQOR tool).
3. Results
3.1. Quality assessment of included studies
The majority of studies (n = 12; 80%) included were deemed to be of high quality. Two studies were of moderate quality and only one study was deemed to be of low quality due to inadequate description of distorting influences/confounders and the sample and comparison groups. See for more detail on quality appraisal of included studies.
3.2. Study characteristics
and provide characteristics of the fifteen selected studies meeting inclusion criteria. No studies including a mixture of eligible and non-eligible participants (e.g. both adults and children) were found; therefore, data disaggregation was not necessary. Collectively the fifteen studies included 5140 participants (3866 PLWHA), with the average age of participants in most studies in the 30s and 40s. The average age for fourteen of fifteen studies, (one study reported the age range only) was 40 years. Eight were all-female studies (Malan-Muller et al., Citation2013; Rubin et al., Citation2017, Citation2015, Citation2016; Spies et al., Citation2016, Citation2012; Spies, Fennema-Notestine, Cherner, & Seedat, Citation2017; Womersley, Spies, Seedat, & Hemmings, Citation2019) and two were all-male studies (Deiss et al., Citation2019; Pukay-Martin, Cristiani, Saveanu, & Bornstein, Citation2003), with the remainder (five studies) including both men and women (Clark, Arce Renteria, Hegde, & Morgello, Citation2018; Clark et al., Citation2012; Kapetanovic et al., Citation2020; Lin et al., Citation2011; Watson et al., Citation2019).
Table 3. Studies included in the comprehensive review of the literature on the association between potentially traumatic or stressful events and cognitive function among PLWHA.
Table 4. HIV disease parameters of included studies.
3.2.1. Study design
There were ten case-control studies (Clark et al., Citation2012; Kapetanovic et al., Citation2020; Malan-Muller et al., Citation2013; Pukay-Martin et al., Citation2003; Rubin et al., Citation2017, Citation2015; Spies et al., Citation2016, Citation2012, Citation2017; Watson et al., Citation2019) and five cross-sectional studies (Clark et al., Citation2018; Deiss et al., Citation2019; Lin et al., Citation2011; Rubin et al., Citation2016; Womersley et al., Citation2019). Eight studies were longitudinal in design (Malan-Muller et al., Citation2013; Rubin et al., Citation2017, Citation2015, Citation2016; Spies et al., Citation2016, Citation2012, Citation2017; Womersley et al., Citation2019) but only one of these presented longitudinal data examining the relationship between childhood trauma and HIV and change in cognition over time (Spies et al., Citation2017) and one examined the relationship between perceived stress and PTSD and NCI over time (Rubin et al., Citation2017).
3.2.2. Study location
All fifteen studies were limited to two countries; ten studies (67%) were from the USA (high-income country) and five (33%) from South Africa (middle-income country). No studies from low-income countries/regions met entry criteria.
3.2.3. Trauma measurement
The majority of studies (n = 12) measured trauma using self-report questionnaires, with the exception of one study which included self-reported medical histories of Traumatic Brain Injury (TBI) to examine if PLWHA with a clear history of TBI had greater risk of NCI compared to those without TBI (Lin et al., Citation2011), one study which included the Composite International Diagnostic Interview (CIDI) to assess for a DSM-IV diagnosis of PTSD and other mental health conditions (Deiss et al., Citation2019), and another study which included the Client Diagnostic Questionnaire (CDQ) to assess for interpersonal trauma (IPT history) and PTSD. Overall, current or past PTSD was assessed for in four studies (Deiss et al., Citation2019; Kapetanovic et al., Citation2020; Rubin et al., Citation2017, Citation2016).
3.2.4. Trauma exposure
Of the fifteen studies, seven studies (Clark et al., Citation2018, Citation2012; Malan-Muller et al., Citation2013; Spies et al., Citation2016, Citation2012, Citation2017; Womersley et al., Citation2019) examined early life trauma only (experienced before the age of 18) and seven studies (Deiss et al., Citation2019; Lin et al., Citation2011; Pukay-Martin et al., Citation2003; Rubin et al., Citation2017, Citation2015, Citation2016; Watson et al., Citation2019) examined adult-onset trauma only (experienced after age 18). Only one study considered both early life and adult-onset trauma (Kapetanovic et al., Citation2020) but no studies sought to separate out the effects of early-onset versus adult-onset trauma. One study from the USA assessed the combined effects of PTEs (through a perceived stress measure) and PTSD (PTEs + PTSD) on NCI (Rubin et al., Citation2017). Across the studies, sample sizes ranged from 38 to 1003 PLWHA. In ten of the fifteen studies (67%) a HIV-negative control group was included, with HIV-negative control groups ranging in size from 45 to 496. The remaining five studies did not include a HIV-negative control group but included a comparison group comprised of PLWHA.
3.3. Findings on trauma and NCI
All fifteen included studies that assessed NCI in PLWHA demonstrated a significant association with trauma exposure (). Five studies controlled for additional confounding variables such as depression, substance use, and antidepressant use (Clark et al., Citation2018, Citation2012; Pukay-Martin et al., Citation2003; Rubin et al., Citation2015; Watson et al., Citation2019). Among the fifteen studies measuring both trauma and NCI in PLWHA, three studies (conducted in the USA) also estimated the prevalence of NCI (Deiss et al., Citation2019; Kapetanovic et al., Citation2020; Watson et al., Citation2019). The prevalence of NCI in these studies was 19% (Deiss et al., Citation2019), 39% (Watson et al., Citation2019), and 27% (Kapetanovic et al., Citation2020) respectively. One study that recruited participants from the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) estimated NCI using a global deficit score (Lin et al., Citation2011). The remaining studies reported associations between exposure to PTEs and specific cognitive domains ().
Table 5. Relationship between potentially traumatic or stressful life events and cognitive function among PLWHA.
3.3.1. Cognitive domains
In these studies, a cognitive domain was assessed using more than one test. Five studies demonstrated an association between PTEs and SLEs and executive function deficits (Lin et al., Citation2011; Pukay-Martin et al., Citation2003; Spies et al., Citation2016, Citation2017; Watson et al., Citation2019). Regarding verbal fluency, five studies demonstrated an association with PTEs and SLEs (Malan-Muller et al., Citation2013; Pukay-Martin et al., Citation2003; Rubin et al., Citation2017; Spies et al., Citation2016, Citation2017; Watson et al., Citation2019). For attention/working memory, seven studies demonstrated an association with trauma (Clark et al., Citation2018; Kapetanovic et al., Citation2020; Lin et al., Citation2011; Pukay-Martin et al., Citation2003; Spies et al., Citation2016; Watson et al., Citation2019; Womersley et al., Citation2019); four studies demonstrated an association with processing speed, (Clark et al., Citation2012; Pukay-Martin et al., Citation2003; Spies et al., Citation2016, Citation2012); six studies demonstrated an association with learning (Rubin et al., Citation2017, Citation2015, Citation2016; Spies et al., Citation2016, Citation2012; Watson et al., Citation2019) while three studies demonstrated an association with motor function (Pukay-Martin et al., Citation2003; Spies et al., Citation2016; Womersley et al., Citation2019). Two studies (Deiss et al., Citation2019; Pukay-Martin et al., Citation2003) reported an association between global NCI and trauma (i.e. not distinguished by cognitive domain).
3.3.2. Associations between PTEs and SLEs and NCI
Across all fifteen studies, there was consistent evidence for an association between PTEs and SLEs and NCI. In the nine studies that included a HIV-negative control group, results consistently showed that trauma was a significant risk factor for neurocognitive dysfunction among PLWHA. For example, a recent study showed that interpersonal trauma (IPT) exposure and HIV infection have a synergistic effect on daily functioning and cortical thickness in PLWHA (Kapetanovic et al., Citation2020). Participants were classified as IPT+ if they experienced any of the following: childhood physical or sexual abuse, intimate partner violence as an adult, physical, or sexual assault as an adult, direct combat, seeing people harming one another in the family as a child, or losing a child to death (Kapetanovic et al., Citation2020). Of all fifteen studies included, this was the only study that considered both early life and adult-onset trauma, although this study did not separate out the effects of early-onset versus adult-onset trauma on NCI in PLWHA. This study found that attention/working memory test performances were significantly worse in PLWHA with IPT compared with PLWHA without IPT and HIV-negative counterparts without IPT. The HIV+ IPT+ group had significantly greater compromise in activities of daily living and also had ‘impaired’ scores on a measure of subjective cognitive function compared with the remaining three groups (Kapetanovic et al., Citation2020). In another study, a case control design, stressful life events (47 events in seven different categories: relationships and love, family, residence, crime and legal matters, finances, health, and other), measured by the Psychiatric Epidemiology Research Interview (PERI) Life Events Scale, were associated with cognitive impairment only among male PLWHA (Pukay-Martin et al., Citation2003). In a cohort study, where participants were recruited across six USA based sites, a significant HIV by stress interaction was found for verbal memory among HIV-infected women only, with high stress associated with lower performance on verbal memory tests compared to women with low stress (Rubin et al., Citation2015). In another cohort study, assessing the combined effects of multiple traumatic and stressful experiences among PLWHA, a higher composite stress score (consisting of multiple adverse experiences including trauma, economic hardship, and stress) was associated with poorer executive function, learning, working memory, and greater decline in activities of daily living, even after controlling for relevant demographic, psychiatric, substance use, and HIV disease covariates. On their own, individual stress composite scores did not predict these outcomes, suggesting that the accumulation of adverse experiences may additively or synergistically harm cognitive health (Watson et al., Citation2019). In the remaining five studies, that included a comparator group of PLWHA, similar results were demonstrated. In two studies, one a USA based cross-sectional study design and the other a USA based MRI study, HIV-positive participants were categorised into low stress and high stress groups (Clark et al., Citation2018; Rubin et al., Citation2016). In one of these studies by Rubin et al., which compared HIV-infected women with low stress PSS-10 scores in the lower two tertiles) and HIV-infected women with high stress (PSS-10 scores in the top tertile), high stress and HIV seemed to be associated with worse performance on measures of verbal learning and memory and smaller brain volumes (Rubin et al., Citation2016). Similarly in the other study by Clark et al, participants with high early life stress (ELS) (defined as an endorsement of three or more adverse childhood events [ACEs]) exhibited poorer working memory compared to those with low-ELS (defined by endorsement of fewer than three ACEs) (Clark et al., Citation2018). In studies that included comparison groups with and without trauma (viz. childhood trauma) (Womersley et al., Citation2019), PTSD (Deiss et al., Citation2019), and TBI (Lin et al., Citation2011), PLWHA who were trauma exposed consistently performed more poorly than their counterparts without trauma.
3.3.3. PTSD findings
Overall, current or past PTSD was assessed for in four studies (Deiss et al., Citation2019; Kapetanovic et al., Citation2020; Rubin et al., Citation2017, Citation2016). PTSD was not dealt with as a mediator of trauma exposure and NCI but rather as an independent predictor of NCI in one study (Deiss et al., Citation2019). Although PLWHA were assessed for PTSD, the independent effect of PTSD on NCI was not reported in two of these studies (Kapetanovic et al., Citation2020; Rubin et al., Citation2016). Instead, the effects of high vs. low stress (viz. perceived stress) (Rubin et al., Citation2016) and interpersonal trauma (IPT) (Kapetanovic et al., Citation2020) on NCI were reported. In the study including PTSD as an independent predictor of NCI, lifetime history of PTSD was independently associated with NCI in a cohort of 189 U.S. military men living with HIV (Deiss et al., Citation2019). Individuals with a lifetime history of PTSD were six times more likely to be diagnosed with NCI than individuals without PTSD (Deiss et al., Citation2019).
3.3.4. PTE exposure and PTSD findings
The fourth study reporting on current or past PTSD sought to assess both the separate and interactive effects of HIV and stress or PTSD on NCI (Rubin et al., Citation2017). This longitudinal study from the USA assessed the combined effects of PTEs (through a perceived stress measure) and PTSD on NCI (Grant, Citation2008), finding that higher levels of stress (viz. perceived stress) and PTSD symptoms, more so than depressive symptoms, may contribute to different patterns of detrimental neurocognitive performance among PLWHA, compared to lower levels of stress and PTSD (Rubin et al., Citation2017). Across time points, PTSD was associated with lower performance on both learning and memory among women living with HIV but not HIV-negative counterparts. This longitudinal study provides evidence that elevated perceived stress and PTSD symptoms in the context of HIV are linked to alterations in verbal abilities (learning, memory, fluency) over time (Rubin et al., Citation2017).
4. Discussion
4.1. Associations between PTEs and SLEs and NCI
In this paper we present, to our knowledge, the most comprehensive review of evidence of the relationship between broad concepts of PTEs and NCI in PLWHA. Despite the small evidence base (especially from low and middle-income countries [LMIC]) and the methodological limitations of the available studies, it is notable that in all the studies included there was an association between trauma and NCI in PLWHA. In order to provide a robust body of evidence, the studies in the present review include measures of different kinds of adversity including exposure to PTEs (both early life and adult-onset trauma), economic hardship (food insecurity and low SES), perceived stress, and traumatic brain injury. These findings of the relationship between NCI and PTE have emerged in several previous studies (Pavlovic, Pekic, Stojanovic, & Popovic, Citation2019). Perceived stress may include stressful life events considered as potentially traumatic events. Stressful life events are thought to increase risk for disease when one perceives that the demands these events impose tax or exceed a person’s adaptive capacity (Cohen, Janicki-Deverts, & Miller, Citation2007). Exposures to chronic stress are considered the most toxic because they are most likely to result in long-term or permanent changes in the emotional, physiological, and behavioural responses that influence susceptibility to and course of disease (McEwen, Citation2006). A high level of perceived stress has been shown to be accompanied by symptoms of depression and/or anxiety (Wiegner, Hange, Björkelund, & Ahlborg, Citation2015). Irrespective of the type of exposure, the results show compelling evidence for the contributory role of trauma in NCI in PLWHA. All 15 included studies that assessed NCI in PLWHA demonstrated a significant association with PTEs and SLE and/or PTSD as broadly defined in our study. Three studies reported prevalences of 19%, 39%, and 27% of NCI (Deiss et al., Citation2019; Kapetanovic et al., Citation2020; Watson et al., Citation2019). All 15 included studies demonstrated an association of PTEs and SLEs and/or PTSD and various cognitive domains, including executive function (Lin et al., Citation2011; Pukay-Martin et al., Citation2003; Spies et al., Citation2016, Citation2017; Watson et al., Citation2019), attention/working memory (Clark et al., Citation2018; Kapetanovic et al., Citation2020; Lin et al., Citation2011; Pukay-Martin et al., Citation2003; Spies et al., Citation2016; Watson et al., Citation2019; Womersley et al., Citation2019), processing speed (Clark et al., Citation2012; Pukay-Martin et al., Citation2003; Spies et al., Citation2016, Citation2012), verbal fluency (Malan-Muller et al., Citation2013; Pukay-Martin et al., Citation2003; Rubin et al., Citation2017; Spies et al., Citation2016, Citation2017), learning/memory (Rubin et al., Citation2017, Citation2015, Citation2016; Spies et al., Citation2016, Citation2012; Watson et al., Citation2019), and motor function (Pukay-Martin et al., Citation2003; Spies et al., Citation2016; Womersley et al., Citation2019), with some studies showing an association with global cognitive function among PLWHA (Deiss et al., Citation2019; Pukay-Martin et al., Citation2003).
4.2. Methodological considerations of included studies
Potential confounders were adequately controlled for in the majority of studies including (Clark et al., Citation2018; Deiss et al., Citation2019; Lin et al., Citation2011; Malan-Muller et al., Citation2013; Pukay-Martin et al., Citation2003; Rubin et al., Citation2017, Citation2015, Citation2016; Spies et al., Citation2016, Citation2012; Watson et al., Citation2019). A recent systematic review by Rubin and Maki found that depression contributed to impairments particularly in the domains of executive function, processing speed, learning, and motor function (Rubin & Maki, Citation2019b), highlighting the importance for studies to adequately consider the effects of depression on NCI or control for these effects. In some studies included in this review, depression was controlled for or included in analyses to determine the independent effects thereof on NCI (Clark et al., Citation2018, Citation2012; Deiss et al., Citation2019; Lin et al., Citation2011; Pukay-Martin et al., Citation2003; Rubin et al., Citation2017, Citation2015). The majority of studies were cross-sectional and as such, a causal relationship between trauma and NCI cannot be inferred. However, in the longitudinal study by Spies et al., the effects of HIV and childhood trauma on NCI were sustained at 12-month follow-up despite better ART uptake and improved HIV disease status (Spies et al., Citation2017). In the era of effective ART and increased virologic suppression, the experience of trauma over the life course, the occurrence of stressors, or PTSD among individuals living with HIV infection may hold more relevance in the development of NCI than was previously recognised. Future longitudinal studies are needed. Across studies there were discrepancies in the way trauma, stressors and adversity were defined and measured. Few HIV-trauma-NCI studies included mental health measures (e.g. PTSD, depression etc.) and those that did mostly included self-report screeners of PTSD and depression. Only one study included a structured diagnostic interview to assess PTSD and depression (Deiss et al., Citation2019). The majority of studies either assessed early-onset or adult-onset trauma. Only one study considered both (Kapetanovic et al., Citation2020) but no studies sought to separate out the effects of early-onset versus adult-onset trauma. Across studies there were also discrepancies in the measurement of the severity of exposures and in the timing of trauma in relation to NCI. There was a lack of consistency in instrumentation used, both for the assessment of trauma exposure and NCI. Moreover, only three studies estimated the prevalence of NCI based on available norms (Deiss et al., Citation2019; Kapetanovic et al., Citation2020; Watson et al., Citation2019), overwhelmingly studies reported associations between trauma and NCI in the absence of neuropsychological norms (Malan-Muller et al., Citation2013; Spies et al., Citation2016, Citation2012, Citation2017; Womersley et al., Citation2019). Across studies, there were inconsistencies in how HIV-related clinical characteristics such as HIV RNA, use of ART, illness stage etc. were dealt with. Moreover, the characterisation of participants across studies in terms of parameters such as gender, age, and HIV co-morbidities was varied. To our knowledge, there are no studies from Europe, South America, or Asia, highlighting the lack of geographical diversity. Finally, more studies are needed to parse out the effects of HIV and PTEs and SLEs on NCI from the effects of HIV and mental health disorders (e.g. PTSD) on NCI.
4.3. Clinical implications
The experience of trauma over the life course, the occurrence of highly stressful events, and PTSD deserve more scrutiny in the clinical assessment and management of PLWHA as they may signal the presence of NCI, more than was previously recognised. The findings of the present review, therefore, highlight the need for PTE and SLE screening and detection and implementation of secondary prevention strategies in PLWHA who have PTEs and SLEs. PTEs and SLEs and PTSD symptoms are treatable targets and early intervention may serve to improve cognitive abilities in PLWHA. Trauma-focused interventions in youth and adults, that include resilience building and coping strengthening elements, may be beneficial in mitigating the negative psychological sequelae of PTEs and SLEs. The integration of trauma-focused interventions in primary HIV care aimed at improving cognitive and HIV disease outcomes, as well as emotional well-being and quality of life, among PLWHA, warrants investigation.
4.4. Implications for research
The quality analysis of included studies in this review highlights the need for future studies to report their methodology and findings in a standardised way to ultimately improve methodological rigour in observational epidemiological studies. Future research should aim to unpack the mechanisms underlying the association between trauma and NCI among PLWHA. Prospective studies in a range of LMIC and controlling for different types of trauma are needed. Measurement of the severity of exposure and the timing of trauma in relation to NCI should be considered. Due to the cross-sectional nature of these studies, it is not possible to determine the temporal pattern of these changes. Future longitudinal studies that track the course of neurocognitive changes are needed to provide more clarity. Such studies have the potential to provide greater insights into potential targets for therapeutic interventions.
5. Limitations
5.1. Limitations of reviewed studies
First, the use of self-report screeners of PTSD and other psychiatric diagnoses rather than a structured diagnostic interview in the majority of studies may overestimate the number of individuals meeting criteria for a clinical diagnosis. Second, the heterogeneity of trauma measures and variations in sample size, sampling strategy, and study design, all of which may have impacted on the results reported herein. Third, publication bias, namely a tendency for journals to publish positive findings, may overestimate the strength or consistency of the association between trauma and NCI.
5.2. Limitations of this study
In our study, trauma was broadly defined as potentially traumatic events (PTEs) and stressful live events (SLEs) during childhood and/or adulthood. Given the paucity of studies in this area, both studies of potentially TEs (PTEs) and stressful life events (SLEs) were included. For example, studies assessing high levels of stress (viz. perceived stress) in the context of everyday stressors were also included. Measuring trauma exposure without PTSD means that these events are not DSM Criterion A events that cause PTSD and therefore should be considered potentially traumatic events. In our review, studies that assessed for potentially traumatic and stressful life events were included. This should therefore be taken into account when interpreting the findings of these studies. The reliability and validity of the SAQOR tool need to be established. As with other quality assessment tools, the potential impact of differential weighting of the component domains of the SAQOR tool should also be determined. In our use of this instrument, each of the five component domains (sample, control, outcome/exposure, follow‐up, distorting influences, reporting of data) was given equal weighting in the overall quality level assigned. Variations in weighting may be appropriate depending upon the research question.
6. Conclusion
The results of the included studies underscore the importance of considering trauma, PTSD, and other common mental health outcomes when conducting research on cognition in PLWHA, as well as when making a diagnosis of HIV associated Neurocognitive Disorders (HAND). In particular, these studies highlight the need for trauma screening over the life course in PLWHA. Stress reduction and post-traumatic stress prevention and treatment programmes could help improve and maintain cognitive function in PLWHA and in turn may contribute to optimal HIV treatment adherence, viral suppression and improved quality of life.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alford, K., & Vera, J. H. (2018, June 2). Cognitive Impairment in people living with HIV in the ART era: A review. British Medical Bulletin [Internet], 127(1), 55–18.
- Ambrosius, B., Gold, R., Chan, A., & Faer, S. (2019, May 1). Antineuroinflammatory drugs in HIV-associated neurocognitive disorders as potential therapy. Neurology - Neuroimmunology Neuroinflammation [Internet], 6(3), e551. Retrieved from http://nn.neurology.org/content/6/3/e551.abstract
- Antinori, A., Arendt, G., Becker, J. T., Brew, B. J., Byrd, D. A., Cherner, M., & Wojna, V. E. (2007, October). Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 69(18), 1789–1799.
- Aupperle, R. L., Melrose, A. J., Stein, M. B., & Paulus, M. P. (2012, February). Executive function and PTSD: Disengaging from trauma. Neuropharmacology, 62(2), 686–694.
- Brief, D. J., Bollinger, A. R., Vielhauer, M. J., Berger-Greenstein, J. A., Morgan, E. E., Brady, S. M., … The Hivaids treatment adherenc, F. (2004). Understanding the interface of HIV, trauma, post-traumatic stress disorder, and substance use and its implications for health outcomes. AIDS Care, 16(Suppl 1), S97–120.
- Clark, U. S., Arce Renteria, M., Hegde, R. R., & Morgello, S. (2018). Early life stress-related elevations in reaction time variability are associated with brain volume reductions in HIV+ adults. Frontiers in Behavioral Neuroscience, 12, 6.
- Clark, U. S., Cohen, R. A., Sweet, L. H., Gongvatana, A., Devlin, K. N., Hana, G. N., & Tashima, K. T. (2012, July). Effects of HIV and early life stress on amygdala morphometry and neurocognitive function. Journal of the International Neuropsychological Society, 18(4), 657–668.
- Cohen, S., Janicki-Deverts, D., & Miller, G. E. (2007, October). Psychological stress and disease. JAMA, 298(14), 1685–1687.
- de Groot, N. S., & Burgas, M. T. (2015, December). Is membrane homeostasis the missing link between inflammation and neurodegenerative diseases? Cellular and Molecular Life Sciences, 72(24), 4795–4805.
- Decker, M. R., Benning, L., Weber, K. M., Sherman, S. G., Adedimeji, A., Wilson, T. E., & Golub, E. T. (2016, November). Physical and sexual violence predictors: 20 Years of the women’s interagency HIV study cohort. American Journal of Preventive Medicine, 51(5), 731–742.
- Deiss, R., Campbell, C. J., Watson, C. W.-M., Moore, R. C., Crum-Cianflone, N. F., Wang, X., … Moore, D. J. (2019). Posttraumatic stress disorder and neurocognitive impairment in a U.S. military cohort of persons living with HIV. Psychiatry, 82(3), 228–239.
- Grant, I. (2008). Neurocognitive disturbances in HIV. International Review of Psychiatry, 20(1), 33–47.
- Habib, A. G., Yakasai, A. M., Owolabi, L. F., Ibrahim, A., Habib, Z. G., Gudaji, M., … Nashabaru, I. (2013, October). Neurocognitive impairment in HIV-1-infected adults in Sub-Saharan Africa: A systematic review and meta-analysis. International Journal of Infectious Diseases, 17(10), e820–31.
- Heaton, R. K., Clifford, D. B., Franklin, D. R. F., Woods, S. P., Ake, C., Vaida, F., … Grant, I. (2010, December). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology, 75(23), 2087–2096.
- Heaton, R. K., Jr, D. R. F., Deutsch, R., Letendre, S., Ellis, R. J., Casaletto, K., … Teshome, M. (2015, February). Neurocognitive change in the era of HIV combination antiretroviral therapy: The longitudinal CHARTER study. Clinical Infectious Diseases, 60(3), 473–480.
- Jelinek, L., Jacobsen, D., Kellner, M., Larbig, F., Biesold, K. H., Barre, K., & Moritz, S. (2006, August). Verbal and nonverbal memory functioning in posttraumatic stress disorder (PTSD). Journal of Clinical and Experimental Neuropsychology, 28(6), 940–948.
- Kapetanovic, S., Norato, G., Nair, G., Julnes, P. S., Traino, K. A., Geannopoulos, K., & Nath, A. (2020, March). Effect of HIV and interpersonal trauma on cortical thickness, cognition, and daily functioning. JAIDS Journal of Acquired Immune Deficiency Syndromes. doi:10.1097/QAI.0000000000002358
- Kessler, R. C., Rose, S., Koenen, K. C., Karam, E. G., Stang, P. E., Stein, D. J., … Carmen Viana, M. (2014, October). How well can post-traumatic stress disorder be predicted from pre-trauma risk factors? An exploratory study in the WHO World Mental Health Surveys. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 13(3), 265–274.
- Kumar, A. M., Fernandez, J. B., Singer, E. J., Commins, D., Waldrop-Valverde, D., Ownby, R. L., & Kumar, M. (2009, May). Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. Journal of Neurovirology, 15(3), 257–274.
- Lagarde, G., Doyon, J., & Brunet, A. (2010, May). Memory and executive dysfunctions associated with acute posttraumatic stress disorder. Psychiatry Research, 177(1–2), 144–149.
- Lin, K., Taylor, M. J., Heaton, R., Franklin, D., Jernigan, T., Fennema-Notestine, C., … The CHARTER Group, F. (2011, March). Effects of traumatic brain injury on cognitive functioning and cerebral metabolites in HIV-infected individuals. Journal of Clinical and Experimental Neuropsychology, 33(3), 326–334.
- Machtinger, E. L., Wilson, T. C., Haberer, J. E., & Weiss, D. S. (2012, November). Psychological trauma and PTSD in HIV-positive women: A meta-analysis. AIDS and Behavior, 16(8), 2091–2100.
- Maki, P. M., Rubin, L. H., Valcour, V., Martin, E., Crystal, H., Young, M., … Anastos, K. (2015, January). Cognitive function in women with HIV: Findings from the women’s interagency HIV study. Neurology, 84(3), 231–240.
- Malan-Muller, S., Hemmings, S. M., Spies, G., Kidd, M., Fennema-Notestine, C., & Seedat, S. (2013). Shorter telomere length - A potential susceptibility factor for HIV-associated neurocognitive impairments in South African women [corrected]. PloS One, 8(3), e58351.
- McEwen, B. S. (2006). Protective and damaging effects of stress mediators: Central role of the brain. Dialogues in Clinical Neuroscience, 8(4), 367–381.
- McLaughlin, K. A., Koenen, K. C., Bromet, E. J., Karam, E. G., Liu, H., Petukhova, M., & Kessler, R. C. (2017, November). Childhood adversities and post-traumatic stress disorder: Evidence for stress sensitisation in the World Mental Health Surveys. British Journal of Psychiatry, 211(5), 280–288.
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009, July). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097.
- Ouzzani, M., Hammady, H., Fedorowicz, Z., & Elmagarmid, A. (2016). Rayyan-a web and mobile app for systematic reviews. Systematic Reviews, 5(1), 210.
- Pavlovic, D., Pekic, S., Stojanovic, M., & Popovic, V. (2019, June). Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary, 22(3), 270–282.
- Pukay-Martin, N. D., Cristiani, S. A., Saveanu, R., & Bornstein, R. A. (2003). The relationship between stressful life events and cognitive function in HIV-infected men. The Journal of Neuropsychiatry and Clinical Neurosciences, 15(4), 436–441.
- Qureshi, S. U., Long, M. E., Bradshaw, M. R., Pyne, J. M., Magruder, K. M., Kimbrell, T., & Kunik, M. E. (2011). Does PTSD impair cognition beyond the effect of trauma? Journal of Neuropsychiatry, 23(1), 16–28.
- Rehm, K. E., & Konkle-Parker, D. (2017, September). Association of CD4+ T cell subpopulations and psychological stress measures in women living with HIV. AIDS Care, 29(9), 1107–1111.
- Ross, L. E., Grigoriadis, S., Mamisashvili, L., Koren, G., Steiner, M., Dennis, C.-L., & Mousmanis, P. (2011, December). Quality assessment of observational studies in psychiatry: An example from perinatal psychiatric research. International Journal of Methods in Psychiatric Research [Internet], 20(4), 224–234. Retrieved from http://www.scopus.com/inward/record.url?eid=2-s2.0-79951999327&partnerID=tZOtx3y1
- Rubin, L. H., Cook, J. A., Springer, G., Weber, K. M., Cohen, M. H., Martin, E. M., & Maki, P. M. (2017, November). Perceived and post-traumatic stress are associated with decreased learning, memory, and fluency in HIV-infected women. AIDS, 31(17), 1401–2393.
- Rubin, L. H., Cook, J. A., Weber, K. M., Cohen, M. H., Martin, E., Valcour, V., & Maki, P. M. (2015, August). The association of perceived stress and verbal memory is greater in HIV-infected versus HIV-uninfected women. Journal of NeuroVirology, 21(4), 422–432.
- Rubin, L. H., & Maki, P. M. (2019a, August). Neurocognitive complications of HIV infection in women: Insights from the WIHS cohort. Current Topics in Behavioral Neurosciences.
- Rubin, L. H., & Maki, P. M. (2019b, February). HIV, depression, and cognitive impairment in the era of effective antiretroviral therapy. In Current HIV/AIDS Reports. Berlin, Heidelberg: Springer. doi:10.1007/7854_2019_101
- Rubin, L. H., Meyer, V. J. J., Conant, R., Sundermann, E. E., Wu, M., Weber, K. M., & Maki, P. M. (2016, August). Prefrontal cortical volume loss is associated with stress-related deficits in verbal learning and memory in HIV-infected women. Neurobiology of Disease, 92(Pt B), 166–174.
- Rubin, L. H., Wu, M., Sundermann, E. E., Meyer, V. J., Smith, R., Weber, K. M., & Maki, P. M. (2016, December). Elevated stress is associated with prefrontal cortex dysfunction during a verbal memory task in women with HIV. Journal of NeuroVirology, 22(6), 840–851.
- Schuitevoerder, S., Rosen, J. W., Twamley, E. W., Ayers, C. R., Sones, H., Lohr, J. B., & Thorp, S. R. (2013, August). A meta-analysis of cognitive functioning in older adults with PTSD. Journal of Anxiety Disorders, 27(6), 550–558.
- Scott, J. C., Matt, G. E., Wrocklage, K. M., Crnich, C., Jordan, J., Southwick, S. M., & Schweinsburg, B. C. (2015, January). A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bulletin, 141(1), 105–140.
- Scott, K. M., Koenen, K. C., King, A., Petukhova, M. V., Alonso, J., Bromet, E. J., & Kessler, R. C. (2018, January). Post-traumatic stress disorder associated with sexual assault among women in the WHO World Mental Health Surveys. Psychological Medicine, 48(1), 155–167.
- Simioni, S., Cavassini, M., Annoni, J.-M., Rimbault Abraham, A., Bourquin, I., Schiffer, V., … Du Pasquier, R. A. (2010, June). Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS, 24(9), 1243–1250.
- Spies, G., Afifi, T. O., Archibald, S. L., Fennema-Notestine, C., Sareen, J., & Seedat, S. (2012). Mental health outcomes in HIV and childhood maltreatment: A systematic review. Systematic Reviews, 1, 30. doi:10.1186/2046-4053-1-30.
- Spies, G., Ahmed-Leitao, F., Fennema-Notestine, C., Cherner, M., & Seedat, S. (2016, April). Effects of HIV and childhood trauma on brain morphometry and neurocognitive function. Journal of NeuroVirology, 22(2), 149–158.
- Spies, G., Fennema-Notestine, C., Archibald, S. L., Cherner, M., & Seedat, S. (2012). Neurocognitive deficits in HIV-infected women and victims of childhood trauma. AIDS Care, 24(9), 1126–1135.
- Spies, G., Fennema-Notestine, C., Cherner, M., & Seedat, S. (2017, January). Changes in cognitive function in women with HIV infection and early life stress. AIDS Care, 29(1), 14–23.
- Sumner, J. A., Hagan, K., Grodstein, F., Roberts, A. L., Harel, B., & Koenen, K. C. (2017, April). Posttraumatic stress disorder symptoms and cognitive function in a large cohort of middle-aged women. Depression and Anxiety, 34(4), 356–366.
- Tomoda, A., Sheu, Y.-S., Rabi, K., Suzuki, H., Navalta, C. P., Polcari, A., & Teicher, M. H. (2011, January). Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage, 54, S280–6.
- Valcour, V., Chalermchai, T., Sailasuta, N., Marovich, M., Lerdlum, S., Suttichom, D., … Ananworanich, J. (2012, July). Central nervous system viral invasion and inflammation during acute HIV infection. The Journal of Infectious Diseases, 206(2), 275–282.
- Watson, C. W.-M., Sundermann, E. E., Hussain, M. A., Umlauf, A., Thames, A. D., Moore, R. C., … Moore, D. J. (2019, January). Effects of trauma, economic hardship, and stress on neurocognition and everyday function in HIV. Health Psychology, 38(1), 33–42.
- Wiegner, L., Hange, D., Björkelund, C., & Ahlborg, G. J. (2015, March). Prevalence of perceived stress and associations to symptoms of exhaustion, depression and anxiety in a working age population seeking primary care–an observational study. BMC Family Practice, 16(1), 38.
- Williams, M. E., Zulu, S. S., Stein, D. J., Joska, J. A., & Naude, P. J. W. (2020, March). Signatures of HIV-1 subtype B and C Tat proteins and their effects in the neuropathogenesis of HIV-associated neurocognitive impairments. Neurobiology of Disease, 136, 104701.
- Womersley, J. S., Spies, G., Seedat, S., & Hemmings, S. M. J. (2019, April). Childhood trauma interacts with ApoE to influence neurocognitive function in women living with HIV. Journal of NeuroVirology, 25(2), 183–193.
- Yuan, N. Y., & Kaul, M. (2019, August). Beneficial and Adverse Effects of cART Affect Neurocognitive Function in HIV-1 Infection: Balancing Viral Suppression against Neuronal Stress and Injury [published online ahead of print, 2019 Aug 6]. J Neuroimmune Pharmacol. doi:10.1007/s11481-019-09868-9