ABSTRACT
Background: Internationally deployed humanitarian aid (HA) workers are routinely confronted with potentially traumatic stressors. However, it remains unknown whether HA deployment and related traumatic stress are associated with long-term changes in hypothalamic-pituitary-adrenal (HPA) axis function. Therefore, we investigated whether cortisol awakening response (CAR) decreased upon deployment and whether this was moderated by previous and recent trauma exposure and parallel changes in symptom severity and perceived social support.
Methods: In this prospective study, n = 86 HA workers (68% females) completed questionnaires regarding trauma exposure, posttraumatic stress disorder (PTSD), anxiety and depressive symptoms and perceived social support, as well as salivary cortisol assessments at awakening and 30 minutes post-awakening at before, early and 3–6 months post-deployment.
Results: Linear mixed models showed significantly decreased CAR (b(SE) = −.036(.011), p = .002) and awakening cortisol over time (b(SE) = −.007(.003), p = .014). The extent of awakening cortisol change was significantly moderated by interactions between previous and recent trauma exposure. Also, a steeper awakening cortisol decrease was significantly associated with higher mean anxiety and PTSD symptoms across assessments. No significant effects were found for social support.
Conclusions: We observed attenuated CAR and awakening cortisol upon HA deployment, with a dose-response effect between trauma exposure before and during the recent deployment on awakening cortisol. Awakening cortisol change was associated with PTSD and anxiety symptom levels across assessments. Our findings support the need for organizational awareness that work-related exposures may have long-lasting biological effects. Further research assessing symptoms and biological measures in parallel is needed to translate current findings into guidelines on the individual level.
HIGHLIGHTS
• Humanitarian aid work can have long-lasting biological effects.• Cortisol awakening response and awakening cortisol (AC) decreased upon deployment.• AC change was associated with prior and recent trauma combined.• AC change was associated with PTSD and anxiety symptoms.
Antecedentes: Los trabajadores de la ayuda humanitaria desplegados internacionalmente (HA) se enfrentan rutinariamente a estresores potencialmente traumáticos. Sin embargo, aún se desconoce si el despliegue de la HA y el estrés traumático relacionado están asociados con cambios a largo plazo en la función del eje hipotalámico-pituitaria-suprarrenal (HPA). Por lo tanto, investigamos si la respuesta del cortisol al despertar (CAR, en sus siglas en inglés) disminuyó en el momento del despliegue y si esto fue moderado por una anterior o reciente exposición a un trauma y los cambios paralelos en la gravedad de los síntomas y el apoyo social percibido.
Métodos: En este estudio prospectivo, x = 86 trabajadores de la HA (68% mujeres) completaron cuestionarios sobre la exposición al trauma, el trastorno de estrés postraumático (TEPT), la ansiedad y los síntomas depresivos y el apoyo social percibido, así como evaluaciones del cortisol salival al despertar y 30 minutos después del despertar, antes, durante y 3-6 meses después del despliegue.
Resultados: Los modelos lineales mixtos mostraron una disminución significativa de la CAR (b(SE) = −.036(.011), p = .002) y del cortisol al despertar, en el transcurso del tiempo (b(SE) = −.007(.003), p = .014). El grado de cambio en el cortisol al despertar fue significativamente moderado por las interacciones entre la exposición anterior y reciente al trauma. Además, una disminución más pronunciada del cortisol al despertar se asoció significativamente con una mayor media de ansiedad y síntomas de TEPT en todas las evaluaciones. No se encontraron efectos significativos en cuanto al apoyo social.
Conclusiones: Observamos CAR atenuado y cortisol al despertar en el despliegue de HA, con un efecto dosis-respuesta en el cortisol al despertar, entre la exposición al trauma antes y durante el reciente despliegue. El cambio de cortisol al despertar se asoció con el TEPT y los niveles de síntomas de ansiedad en todas las evaluaciones. Nuestros hallazgos apoyan la necesidad de la conciencia organizacional de que las exposiciones relacionadas con el trabajo pueden tener efectos biológicos duraderos. Se necesitan más investigaciones que evalúen los síntomas y las medidas biológicas en paralelo para traducir los hallazgos actuales en directrices a nivel individual.
背景:国际应征的人道主义援助 (HA) 工作者通常会面临潜在的创伤性压力源。但是, 我们仍不清楚HA应征和相关的创伤应激是否与下丘脑-垂体-肾上腺 (HPA) 轴功能的长期变化有关联。
目的:我们旨在研究皮质醇唤醒反应 (CAR) 在部署后是否下降, 以及过去和最近的创伤暴露, 症状严重程度和感知社会支持的平行变化是否对其起到调节作用。
方法: 在这项前瞻性研究中, 86名HA工作人员 (68%为女性) 在部署前期, 后期和3-6个月后填写了有关创伤暴露, 创伤后应激障碍 (PTSD), 焦虑和抑郁症状以及感知社会支持的问卷, 并在刚睡醒和30分钟后进行唾液皮质醇测量。
结果: 线性混合模型显示, CAR (b(SE) = −.036 (.011), p = .002) 和觉醒皮质醇 (b(SE) = -.007 (.003), p = . 014) 随时间显著降低。过去和最近的创伤暴露的相互作用显著调节了觉醒皮质醇变化的程度。此外, 觉醒皮质醇较快下降与焦虑和PTSD较高的跨时间点平均症状水平显著相关。没有发现对社会支持的明显效应。
结论: 我们观察到HA应征后CAR和觉醒皮质醇水平的下降。而且, 在近期部署之前和期间创伤暴露对唤醒皮质醇有剂量反应效应 (dose-response effect) 。觉醒皮质醇变化与跨时间点时的PTSD和焦虑症状水平相关。我们的发现指出, 机构组织有必要认识到与工作有关的暴露可能具有长期持续的生物学影响。但还需要进一步研究对症状和生物学指标进行同时评估, 以期将当前发现转化为个人层面的指导手册。
1. Introduction
Internationally deployed humanitarian aid (HA) workers are routinely confronted with potentially traumatic stressors, such as terrorism, violent attacks and distress from extreme environmental hardship (Eriksson et al., Citation2015; Lopes Cardozo et al., Citation2012; Strohmeier, Scholte, & Ager, Citation2018). HA deployment and its related traumatic stressors have been linked to subsequent mental health problems, including posttraumatic stress disorder (PTSD), anxiety and depression (Strohmeier et al., Citation2018). In the prospective cohort investigated in the current study, it was previously observed that the absolute prevalence rates of probable anxiety increased by 8% over the course of deployment and the absolute prevalence rates of probable depression increased by 9% (Lopes Cardozo et al., Citation2012). However, little is known about the potential long-term impact of HA deployment on biological functioning.
The hypothalamic-pituitary-adrenal (HPA) axis is a major endocrine circuit of the biological stress system. Upon activation during stress, the release of its end product cortisol results in subsequent HPA axis inhibition through negative feedback via glucocorticoid receptors (GRs) in the hypothalamus and anterior pituitary (Michaud, Matheson, Kelly, & Anisman, Citation2008). This enables termination of the acute stress response and thereby stress recovery. Cortisol release upon acute stress is superimposed on the cortisol circadian rhythm, which constitutes a sharp rise in the first 30–45 minutes immediately after awakening in the morning (i.e., cortisol awakening response, CAR; Stalder et al., Citation2016), followed by a gradual decline over the day into the first half of the night, after which levels slowly increase again (Clow, Hucklebridge, Stalder, Evans, & Thorn, Citation2010; Stalder et al., Citation2016).
Long-term changes in HPA axis functioning and circulating cortisol levels have been reported after exposure to severe and traumatic stress during childhood and adulthood (Morris, Compas, & Garber, Citation2012; Stalder et al., Citation2017). However, previous longitudinal investigations on the long-term course of cortisol upon experiencing traumatic events reported mixed results as to the magnitude and direction of the change (Aardal-Eriksson, Eriksson, & Thorell, Citation2001; Bonne et al., Citation2003; Ironson et al., Citation2014; Shalev et al., Citation2008; Söndergaard & Theorell, Citation2003; Stoppelbein & Greening, Citation2015). This can be partially attributed to between-study variability in time since trauma exposure during assessments (Morris et al., Citation2012). Other proposed explanations include between-study variability in sample characteristics (e.g., sex, age, developmental timing), cortisol specimen type and time of the day during sampling (Morris et al., Citation2012; Steudte-Schmiedgen, Kirschbaum, Alexander, & Stalder, Citation2016).
Importantly, most longitudinal studies started measuring cortisol shortly after trauma exposure. Therefore, it remains largely unclear how findings of long-term changes of cortisol within these studies should be interpreted, i.e., whether cortisol during the initial assessment reflects the pre-trauma situation; or, more likely, captures acute cortisol changes in the immediate post-trauma period. In the latter case, observed subsequent cortisol changes may actually (partially) reflect recovery of these short-term alterations. Thus, prospective longitudinal studies that start measuring cortisol before traumatic stress onset provide the means to better understand the temporal course of cortisol change following trauma exposure (Steudte-Schmiedgen et al., Citation2016). Yet, such studies remain rare given the fact that trauma exposure is generally unpredictable and heterogeneous, except in certain professional populations at increased risk for exposure during work (e.g. military or medical personnel, HA workers). Steudte-Schmiedgen et al. (Citation2015) found an increase in hair cortisol concentrations among male military personnel followed from pre-deployment until 12 months post-deployment. In contrast, no changes were observed in morning plasma cortisol in male military personnel from pre- to 6 months post-deployment (van Zuiden et al., Citation2009) and in awakening and diurnal salivary cortisol in male probationary firefighters over the first two years of their active duty (Heinrichs et al., Citation2005). To the best of our knowledge, except for Heinrichs et al. (Citation2005), no other prospective studies started assessing cortisol prior to the confrontation with potential traumatic events in non-military cohorts.
A recent model on HPA axis functioning and traumatic stress proposes that overall cortisol output changes follow a time-dependent pattern after trauma exposure, with initial elevation shortly after termination of the trauma which subsides in a later phase and eventually reverts to attenuated output, as a result of enhanced negative feedback inhibition on the HPA axis (Steudte-Schmiedgen et al., Citation2016). The model additionally proposed that repeated trauma exposure leads to a dose-dependent ‘building block’ cortisol attenuation. However, as the model is mainly based on findings in hair cortisol, which reflect average cortisol output over a longer period, it remains unknown whether the time- and dose-dependent effects also extend to cortisol’s circadian rhythm, including the CAR and awakening cortisol.
Exposure to traumatic events may lead to subsequent onset of (sub)clinical PTSD, depressive and anxiety symptoms, which in itself are also associated with long-term alterations in cortisol output (Morris et al., Citation2012; Pan, Wang, Wu, Wen, & Liu, Citation2018; Staufenbiel, Penninx, Spijker, Elzinga, & van Rossum, Citation2013). Increasing evidence on biological correlates of stress-related psychological symptoms shows that the presence, direction and magnitude of these associations depend on the exact stage of symptom development or progression during their assessment (McFarlane, Lawrence-Wood, Van Hooff, Malhi, & Yehuda, Citation2017). Most previous studies, and therefore the integrative model mentioned above, did not consider whether and how changes in concurrent symptom severity in parallel to the assessed cortisol changes moderate cortisol’s long-term course upon trauma exposure.
Similarly, concurrent perceived social support may also moderate this course as it has been frequently recognized as a key protective factor against the adverse impact of trauma exposure on mental health (Sippel, Pietrzak, Charney, Mayes, & Southwick, Citation2015; Sippel, Watkins, Pietrzak, Hoff, & Harpaz-Rotem, Citation2019). In the cohort investigated in the current study, it was previously observed that higher perceived social support was associated with lower depressive and PTSD symptoms prior to deployment (Eriksson et al., Citation2013) and lower depressive symptoms and psychological distress over the course of the deployment until at least six months after return (Lopes Cardozo et al., Citation2012). Yet, the exact mechanisms underlying this protective effect have not been fully elucidated. Perceived social support was previously found not to be associated with the CAR in a small number of cross-sectional studies among healthy populations (Chida & Steptoe, Citation2009; Heaney, Phillips, & Carroll, Citation2010). Yet as perceived social support has been repeatedly found to impact acute cortisol stress reactivity (Eisenberger, Taylor, Gable, Hilmert, & Lieberman, Citation2007), it would be of interest to investigate whether it impacts the course of CAR and awakening cortisol upon HA deployment.
Thus, in the current study, we aimed to prospectively investigate long-term changes in cortisol output in response to HA deployment; and its association with prior and current trauma exposure and with changes in PTSD, anxiety and depressive symptom severity, as well as perceived social support. We focused on the CAR as a discrete component of the diurnal rhythm thought to reflect HPA axis reactivity to awakening and preparation for the day ahead (Clow et al., Citation2010). We also investigated awakening cortisol as a distinct yet closely related parameter to CAR, reflecting the endpoint of the pre-awakening cortisol increase (Stalder et al., Citation2016). Given the previous literature (Heinrichs et al., Citation2005; Steudte-Schmiedgen et al., Citation2016, Citation2015; van Zuiden et al., Citation2009), we expected decreased CAR and awakening cortisol from pre-deployment to our final assessment at 3–6 months post-deployment. We also expected the extent of this decrease to be associated with the number of traumatic stressors encountered prior to and during the recent deployment; and with changes in concurrent PTSD, anxiety and depressive symptoms severity and in perceived social support.
2. Material and methods
2.1. Participants and procedure
The current study is part of a larger prospective cohort study conducted among n = 214 HA workers from 19 non-governmental organizations (NGOs) based in Europe or North America. All were expatriate, i.e., deployed to work in countries other than their own country of citizenship. HA workers were recruited for participation during the pre-deployment phase of their planned assignment.
International NGOs were targeted for recruitment excluding UN agencies, local aid agencies, or other governmental humanitarian efforts. Inclusion criteria for NGOs were: (1) in existence for more than five years; (2) an established record of international funding; (3) operating with a humanitarian imperative of emergency aid and development (rather than religious or political agenda); (4) a record of operations in at-risk countries; (5) deploying at least 20 expatriate staff to the field per year. In total, 88 agencies from the initial list compiled from the Relief Web archive (http://www.reliefweb.int) were contacted, among which 19 met inclusion criteria and also agreed to participate. More details on sample size justification and recruitment were described in previous publications (Eriksson et al., Citation2015, Citation2013; Lopes Cardozo et al., Citation2012).
A focal person within each agency was selected and trained to recruit eligible participants (Eriksson et al., Citation2013). Packets containing questionnaires and cortisol sampling material were distributed at pre-deployment (T1) by the focal persons and subsequently via mail at the immediate end of the deployment (post-deployment, T2) and 3–6 months after the end of the deployment (follow-up, T3). Each participant received an incentive of 50 USD for the completion of the pre-deployment questionnaires, 150 USD for the post-deployment questionnaires and another 100 USD for the follow-up questionnaires, regardless of their cortisol sample collection.
Participants were considered eligible if their planned deployment duration was of 3 to 12 months and their English reading proficiency was sufficient to complete the questionnaire materials, regardless of their previous experience in the HA field. Data was collected between December 2005 and December 2007. Of n = 214 included participants, n = 212 completed pre-deployment questionnaires, n = 170 (80%) completed post-deployment questionnaires and n = 154 (73%) completed follow-up questionnaires. For the current study, we initially included n = 107 participants who returned cortisol samples at all three assessments. After data pre-processing, our final sample consisted of n = 86 aid workers (see Section 2.3 for details).
We investigated potential differences in demographic and deployment characteristics, trauma exposure and PTSD, depressive and anxiety symptom severity at pre-deployment between the included participants (n = 86), participants with excluded cortisol data (n = 21) and participants who did not submit sufficient cortisol samples (n = 107). The included sample had significantly less depressive symptoms (p = .008) at pre-deployment than those who did not submit sufficient cortisol samples (see Supplementary Table 1). No other significant difference in study variables were found among the three groups (all p-values ≥ .140).
Written informed consent was obtained from all participants. The study protocol was reviewed and approved by the institutional review board of the Centres for Disease Control and Prevention (CDC) in Atlanta, Georgia, USA and thereafter by the partnering institutes.
2.2. Measures
2.2.1. Self-report questionnaires
Before deployment, participants reported their demographics, number of prior humanitarian field assignments, characteristics of the planned deployment (hardship, job function and the nature of the assignment) and prior trauma exposure during childhood, adulthood and previous deployment (if applicable). At the immediate end of the deployment, participants reported trauma exposure during the recent deployment. At each assessment, participants filled out self-report questionnaires on PTSD, anxiety and depressive symptom severity and perceived social support.
2.2.1.1. Prior childhood trauma
Participants answered two items on childhood relational trauma (injuries resulting from parents’ discipline; parents hitting or threatening to hit each other), one item on childhood sexual abuse (forced exposure to nudity, physical contact and fondling, or sexual penetration) and five items on family risk factors (parents’ divorce; removal from home; overcrowding in home; mental illness in family; and death of parent or sibling) (adapted from the Assessing Environment III, Knutson, Citation1988; the Conflict Tactics Scale, Straus & Smith, Citation2017; Resnick, Citation1996). The overall score consisted of the number of endorsed items (range 0–8).
2.2.1.2. Prior adult trauma
Participants answered two items regarding adult exposure to intimate partner violence (the Conflict Tactics Scale; Straus & Smith, Citation2017) and unwanted sexual contact after age 18 (Resnick, Citation1996) and seven items related to potential adult traumatic stressors (accidents; natural disaster; life-threatening illness; crime victimization; serious injury or threatened death; traumatic death of a family member; or witnessing threat or serious injury; Widom, Dutton, Czaja, & DuMont, Citation2005). The overall score consisted of the number of endorsed items (range 0–9).
2.2.1.3. Previous deployment trauma
For the seven abovementioned items related to potential adult traumatic stressors, participants also indicated whether they occured during a previous HA deployment. The overall score consisted of the number of endorsed items (range 0–7). For those without previous HA deployments, scores were coded as 0.
2.2.1.4. Recent deployment trauma
Participants completed 34 items on potentially traumatic events experienced during their deployment (e.g., life-threatening illness and/or limited access to necessary medical care, shootings; Cardozo & Salama, Citation2002; Eriksson, Vande, Gorsuch, Hoke, & Foy, Citation2001). A score for direct trauma exposure was calculated by summing the number of items endorsed as personally experienced (range 0–34). A score of indirect trauma exposure was calculated by adding the number of items endorsed as witnessed or heard about, including the 34 items referred to above and 9 items detailing other indirect exposure to traumatic events in HA settings (e.g., seeing mass graves, seeing children or adults die from disease or malnutrition; range 0–81). A total score was calculated by summing up the direct and indirect exposure scores (range 0–115).
2.2.1.5. PTSD
The Los Angeles Symptoms Checklist (LASC) was used at each assessment to assess the severity of DSM-IV PTSD symptoms, including re-experiencing, avoidance and hyperarousal clusters (King, King, Leskin, & Foy, Citation1995). Participants rated the 17 items on a 5-point scale ranging from 0 (not a problem) to 4 (an extreme problem). The sum score of the items reflects the overall measure of PTSD severity (range 0–68).
2.2.1.6. Anxiety and depression
The Hopkins Symptom Checklist-25 (HSCL; Derogatis, Lipman, Rickels, Uhlenhuth, & Covi, Citation1974) was used at each assessment to assess the severity of depression and anxiety. The depression subscale score consisted of the sum score of 15 items and the anxiety subscale score consisted of the sum score of 10 items on a 4-point scale ranging from 1 (not at all) to 4 (extremely) (respective ranges: 15–60; 10–40).
2.2.1.7. Perceived social support
The Social Provisions Scale (Cutrona, Citation1989) was used at each assessment to assess perceived social support in everyday life regarding shared interests, respect, guidance and advice. Participants rated the 12 items on a 5-point scale ranging from 1 (strongly disagree) to 5 (strongly agree). Five negatively phrased items were reverse-scored. The sum score of items reflects overall perceived social support (range 12–60).
2.2.1.8. Alcohol and tobacco use
Current alcohol and tobacco use were queried at each assessment as part of a questionnaire related to health habits and coded as non-user versus user.
2.2.2. Salivary cortisol sampling
We assessed CAR as our main variable of interest. As recommended by expert consensus guidelines (Stalder et al., Citation2016), we also assessed and controlled for awakening cortisol in our analyses for CAR.
Cortisol samples were collected using cotton salivettes (Sarstedt, USA). Participants received three salivettes per assessment and were instructed to collect saliva samples immediately after awakening (Cor0), 30 minutes (Cor30) and 4 hours thereafter on the same day as they filled out the questionnaires. Unfortunately, most of the third samples were found to be collected late in the evening, probably due to the busy daytime routine of HA work. Thus, we did not include the third samples in our analyses.
Participants were instructed to strictly adhere to the collection times and to keep the cotton swabs in their mouth for approximately two minutes allowing for its saturation. Time and means of awakening were not restricted because previous studies have shown that CAR is not affected by these variables (Stalder et al., Citation2016). Participants were also instructed to avoid eating, drinking, smoking and brushing teeth during the collection period of the two samples. Participants noted their behaviour and sampling times in a log form, including: waking-up time, sampling time for each tube, type of beverage and food, time of drinking and eating, time of brushing teeth. Participants also noted their body weight and height at each assessment.
Salivettes were stored in domestic refrigerators until sent back to the Antares Foundation, the Netherlands, where they were stored at −20°C until analysis. Cortisol assays were conducted at the National Institute for Public Health and the Environment (RIVM), the Netherlands.
Saliva samples were centrifuged 3000 U/min for 5 minutes. Cortisol was measured in duplicate using enzyme immunoassay kits (Salimetrics, State College, Pennsylvania). According to the manufacturer the mean intra- and inter-assay coefficients of variation were ≤7.12% and ≤6.88%, respectively. The lower limit of sensitivity is ≤0.19 nmol/L. All analyses were performed between October 2007 and August 2009. Cortisol samples of the same participant were always included in the same batch.
Of the first two samples submitted by n = 107 participants across the three assessments, 616 (95.95%) samples were detected as valid (i.e., not empty or above detection limit).
2.3. Statistical analyses
2.3.1. Data pre-processing
First, to ensure relatively homogeneity in time intervals between assessments and deployment among participants, we calculated the months between the T1 and the indicated deployment starting date and between the T3 and the indicated end date of the deployment. Samples were excluded if the respective time interval could not be calculated or exceeded median ± 2SD (n T1 = 6 and n T3 = 5). Second, 95 (15.99%) cortisol samples were excluded due to reported non-compliance (>5 minutes deviation from the required sampling time indicated on the log form) or non-valid values, as recommended by expert guidelines (Stalder et al., Citation2016).
All continuous variables were tested for normality and square root or log transformation was performed when necessary, before the investigation of the presence of outliers. For Cor0 and Cor30, n = 3 outliers were excluded for exceeding the range of mean±3SD (Stalder et al., Citation2016). Subsequently, we calculated CAR as the increase of the cortisol output with respect to the first awakening sample (Cor30-Cor0). One outlier deviating ±3SD from the mean was excluded from CAR. Log-transformed Cor0 and square root transformed CAR (hereafter all presented as transformed values if not specified otherwise) served as the outcome variables of the current study. In the end, n = 86 participants with at least one valid cortisol sample across three assessments were included in the analyses.
2.3.2. Data analyses
Linear mixed model (LMM) analyses were conducted using SPSS 24.0. Missing data in the outcome variables were handled by using restricted maximum likelihood estimation (REML) (West, Citation2009). Cor0 and CAR were used as outcome variables in separate models. Cor0 was included as a time-varying covariate in the CAR models, as recommended by Stalder et al. (Citation2016).
2.3.2.1. Basic models: Cor0 and CAR changes over time
We modelled the change of Cor0 and CAR over time from T1 to T3. For the time variables, T1 was coded as 0 while T2 and T3 were encoded as the number of months since T1 for each participant. For n = 3 (3.49%) at T2 and n = 7 (8.14%) at T3 the exact number of months since T1 was unknown, therefore these missing values were imputed by the respective medians (T2 = 8.67 months, T3 = 12.67 months).
First, we determined the optimal error covariance structure of the repeated measurements among three common error covariance structures in models with fixed effects (i.e., first-order autoregressive (AR1), unstructured (UN), or diagonal (DIAG)). Model fit was investigated by conducting χ 2 tests on the changes of −2 restricted log -likelihood (−2 restricted LL) (West, Citation2009). We subsequently added (1) random intercept effects and then (2) random slope effects. Models with the lowest Akaike Information Criteria (AIC) and Bayesian Information Criteria (BIC) were selected and retained as the basic models for the later investigation of moderators (West, Citation2009).
2.3.2.2. Associations of trauma exposure, psychological symptoms and social support with Cor0 and CAR changes over time
Subsequently, we investigated effects of potential moderators on changes in the two cortisol parameters over the course of the deployment. Grand mean centering was conducted for continuous variables to decrease the risk of multicollinearity and to facilitate interpretation of interaction terms (Hox, Moerbeek, & van de Schoot, Citation2017). First, the effects of demographic and assay characteristics (age, sex and batch) and potential confounding effects of health-related characteristics impacting cortisol levels (time-varying BMI, alcohol and tobacco use) were investigated in separate models to assess whether they should be controlled for in the subsequent analyses. Second, we tested main and interaction effects of prior trauma exposure (i.e., childhood trauma, adult trauma or previous deployment trauma) and recent deployment trauma exposure in separate models.
Finally, we investigated associations of Cor0 and CAR changes with time-varying PTSD, anxiety and depressive symptom severity and perceived social support in separate models. Between-subject and within-subject effects of all time-varying covariates were disaggregated using a subject-mean approach (Curran & Bauer, Citation2011). Thus, between-subject effects contained the differences between participants in their mean scores across three assessments (i.e., subject-mean), while the within-subject effects consisted of the differences between the score at each assessment from their respective subject-mean. Between-subject and within-subject effects were included together in the models as potential moderators of cortisol change.
P-values below 0.05 were considered significant in all analyses. Due to the exploratory nature of analyses, corrections for multiple testing were not applied (Bender & Lange, Citation2001).
3. Results
3.1. Sample characteristics
The participant characteristics including severity of PTSD, depressive and anxiety symptoms and perceived social support are shown in . The included sample consisted of n = 86 expatriate HA workers with 24 different nationalities (23.3% Belgian, 14.0% French, 10.5% British, 10.5% American or dual). Overall, the sample was mostly female (n = 58, 67.4%), single (n = 55, 64.0%) and with education at college level or above (n = 76, 88.4%). The mean age prior to deployment was 32.92 years (SD = 7.67).
Table 1. Participants characteristics for the current sample (n = 86) at three assessments
Modes of the wake-up time in the morning were 7:30 at pre-deployment (range 4:30–11:25), 8:30 (range 4:00–12:30) at post-deployment, and 8:00 (range 4:30–11:40) at follow-up. Participants on average reported collecting their first cortisol sample 2.20 ± 1.86 minutes (range 0–5 min) after waking up at pre-deployment, 2.69 ± 1.78 minutes (range 0–5 min) at post-deployment, and 2.29 ± 1.73 minutes (range 0–5 min) at follow-up.
3.2. Basic model: Cor0 and CAR changes over time
We observed significant decreases from T1 to T3 for Cor0 (time: b = −0.007, SE = 0.003, p = .014) and CAR (time: b = −0.036, SE = 0.011, p = .002) (see Supplementary Tables 2 and 3). No significant effects were found for demographic variables and other potential confounders of cortisol levels (all p-values > .174; see Supplementary Tables 4 and 5). Therefore, none of these variables were included as covariates in the subsequent analyses.
3.3. Effects of prior and recent trauma exposure on Cor0 and CAR changes over time
Prior childhood and adult trauma exposure and recent deployment trauma were not significantly associated with Cor0 or CAR change over time. However, the number of recent deployment traumatic events significantly interacted with both childhood trauma (b = 0.006, SE = 0.003, p = .034; see , ) and adult trauma (b = 0.006, SE = 0.002, p = .014; see Supplementary Table 6) on the change of Cor0 over time. No significant interaction effects were observed for CAR change as the outcome, nor for previous deployment trauma as the moderator (see and Supplementary Tables 6 and 7).
Table 2. Linear mixed model results for the interaction effects of childhood trauma and recent deployment trauma on cortisol parameters
Figure 1. Awakening cortisol (Cor0) change over time from pre-deployment to follow-up. Participants with high levels of childhood trauma exposure and low levels of recent deployment trauma (▲) showed the sharpest decrease of Cor0 over time, compared to the other groups. CT: childhood trauma; DT: recent deployment trauma; Cor0: the first cortisol sample at awakening, values log transformed; Pre: pre-deployment, Post: immediate post-deployment, Follow-up: 3–6 months post-deployment. For visualization purposes, we obtained the model-estimated means of exposure variables at mean-SD (low level group) and mean + SD (high level group). Estimated means are presented, thus no SDs are reported
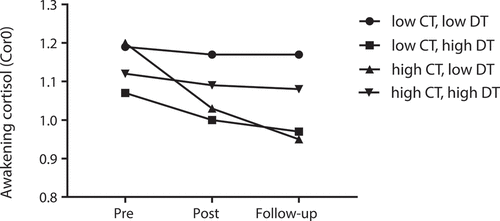
illustrates the significant interaction effect between childhood trauma exposure and recent deployment trauma exposure on the change of Cor0 over time. Participants exposed to high levels of childhood trauma and low levels of recent deployment trauma showed the steepest decrease in Cor0 over time compared to the other groups.
3.4. Associations of concurrent symptoms with Cor0 and CAR changes over time
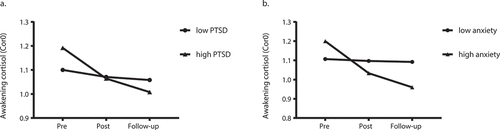
Here we investigated whether time-varying PTSD, depressive and anxiety symptom severity were associated with changes in Cor0 and CAR over time (see for PTSD and anxiety symptom severity and Supplementary Table 8 for depressive symptom severity). Between-subject differences in PTSD (b = −0.022, SE = 0.011, p = .049) and anxiety (b = −0.087, SE = 0.030, p = .004) symptoms were significantly associated with changes over time in Cor0. Specifically, Cor0 decreased more sharply over time in case of relatively high mean anxiety and PTSD symptoms across assessments (see ). This effect was not observed for CAR.
Table 3. Linear mixed model results for the effect of time-varying PTSD symptoms on cortisol parameters
Table 4. Linear mixed model results for the effect of time-varying anxiety symptoms on cortisol parameters
Figure 2. Awakening cortisol (Cor0) change over time from pre-deployment to follow-up of participants with overall low (●) and high (▲) levels of PTSD (a) or anxiety (b) symptoms. The within-subject variation of PTSD and anxiety symptoms were fixed to 0 to generate the plots. Thus high and low levels refer to the group with overall high or low symptom levels across three assessments. Participants with overall high level (▲) of PTSD or anxiety symptoms showed the sharpest decrease of Cor0 over time, compared to the low level (●) groups. PTSD: posttraumatic stress disorder. Cor0: the first cortisol sample at awakening, values log transformed. Pre: pre-deployment, Post: immediate post-deployment, Follow-up: 3–6 months post-deployment. For visualization purposes, we fixed the model-estimated values of the covariates/predictors at mean-SD (low level group) and mean+SD (high level group). Estimated means are presented, thus no SDs are reported
3.5. Associations of perceived social support with Cor0 and CAR changes over time
Here we investigated whether time-varying perceived social support was associated with Cor0 and CAR over time. Between-subject and within-subject levels of perceived social support were not significantly associated with Cor0 and CAR changes over time (all p-values ≥ .085, see Supplementary Table 9).
4. Discussion
We prospectively examined whether CAR and awakening cortisol changed during HA deployment, a period of routine confrontation with potentially traumatic stress. As hypothesized, we observed decreased CAR and awakening cortisol from pre-deployment to 3–6 months post-deployment. The extent of prior trauma exposure combined with recent deployment trauma exposure moderated the observed attenuation in awakening cortisol. Furthermore, mean PTSD and anxiety symptom severity across assessments was significantly associated with awakening cortisol decrease.
Our main finding supports a long-term attenuation in CAR and awakening cortisol, which was sustained until at least 3–6 months after return from HA deployment. Our findings thus indicate an attenuated cortisol release during the distinct morning component of cortisol’s diurnal rhythm and thereby complement the model of Steudte-Schmiedgen et al. (Citation2016) which integrated mainly hair cortisol studies, reflecting cortisol output across aggregated periods. Our findings indicate that changes in awakening cortisol and CAR follow a time-dependent decreasing pattern as postulated by the model. However, as we measured cortisol only at three assessments and not immediately upon trauma exposure, we were unable to capture the potential quadratic course and acute post-trauma increase preceding the decrease in cortisol output postulated by the model.
In the only prospective study investigating changes in morning cortisol – although not specifically the CAR – upon trauma exposure thus far, Heinrichs et al. (Citation2005) did not find significant change within the first-hour post-awakening among male probationary firefighters during their first 24 months of service. Their study repeatedly measured cortisol during ongoing potential exposure to work-related traumatic stressors and did not include follow-up assessments during a period without exposure, which may explain the difference in findings, in addition to the differences in cortisol parametrization between studies.
In the current study, the amount of recent deployment trauma exposure did not affect the observed cortisol changes during deployment per se. Instead, we observed interactions of prior adult and childhood trauma with recent deployment trauma on awakening cortisol. Interestingly, prior deployment trauma did not moderate the effect of recent deployment trauma on cortisol changes, indicating that only prior exposure in personal lives moderated the neuroendocrine effects during the recent deployment.
Several of our findings on awakening cortisol fit with the dose-dependent effects described by Steudte-Schmiedgen et al. (Citation2016). First, attenuated awakening cortisol were observed for participants with high levels of recent trauma in the context of low levels of prior trauma. Also, no or minimal awakening cortisol changes were observed for participants reporting low levels of prior and recent trauma. Expanding on the model, we found that participants with high prior trauma and low recent trauma levels showed the steepest awakening cortisol decrease over time. One possible explanation for this initially counterintuitive findings stems from the perspective of the developmental match/mismatch model (Daskalakis, Bagot, Parker, Vinkers, & de Kloet, Citation2013) posing that a mismatch between the early-life environment and the later-life environment (i.e., the current deployment) may negatively impact the ability to cope with the demands from the later-life environment, which is linked to heightened susceptibility to subsequent development of stress-related symptoms and presumably underlying biological correlates. Following this model, potentially the high level of prior exposure during childhood resulted in ongoing distress (i.e., sustained chronic stress) as a result of continued anticipated exposure over the course of the deployment in spite of low levels of actual exposure, resulting in long-term attenuated cortisol output (Miller, Chen, & Zhou, Citation2007; Stalder et al., Citation2017).
Interestingly, we found that participants with both high levels of prior exposure and recent deployment exposure (a ‘double hit’) showed no or minimal cortisol attenuation, inconsistent with the dose-response curve of increasing trauma load (Steudte-Schmiedgen et al., Citation2016). From the perspective of the match/mismatch model introduced above (Daskalakis et al., Citation2013) we may interpret this null finding in participants with a ‘double hit’ as an adaptive or resilient response to the exposure during the recent deployment due to ‘stress inoculation’ by prior experiences which were moderately stressful. On the other hand, from a cumulative stress exposure perspective, GR sensitivity in these participants might have become blunted as consequences of repeated exposure, thus no subsequent cortisol attenuation due to negative feedback inhibition within the HPA axis was induced. Unfortunately, in the current study we cannot infer whether any of these interpretations is correct. Nevertheless, our results indicate that the effects of prior and recent trauma exposure on awakening cortisol output are not necessarily cumulative. Also, effects differ depending on cortisol parameters, as no significant effects of the amount of trauma exposure on the CAR were observed. Thus our findings may concur with previous interpretations that the CAR is specifically sensitive to effects of chronic stress and anticipation of upcoming challenges, but not to trauma exposure per se (Clow et al., Citation2010; Stalder et al., Citation2016), although in that instance we may have expected to observe changes in CAR in participants with high prior trauma and low recent deployment trauma, who may have potentially experienced sustained anticipatory distress throughout the deployment as a result of their prior experiences.
We did not observe significant effects of social support on any cortisol output parameter. This concurs with previous meta-analytic evidence (Stalder et al., Citation2017) in which no consistent associations between social support and hair cortisol were observed. However, psychological symptom severity did impact cortisol changes over time. The extent of concurrent change in symptom severity was not significantly associated with changes of any cortisol parameter, but participants with relatively high mean levels of PTSD and anxiety symptoms across assessments showed the sharpest parallel decrease in awakening cortisol over time. The relatively high mean symptom severity could result from continuously high symptom levels from baseline onwards or increased high symptoms immediately post-deployment which had not recovered at follow-up. Thus, we may conclude that the observed decrease in awakening cortisol for these participants continued after symptom onset. Notably, these effects were observed in the absence of a high prevalence of above clinical-threshold symptoms, indicating effects across the whole spectrum of symptom severity. The absence of similar effects for depressive symptoms may indicate disorder-specificity but may also be related to our observation that participants with higher pre-deployment depressive symptoms were less likely to complete all cortisol assessments.
Pre-deployment cortisol levels were not significantly associated with overall symptom levels nor with symptom changes over time. Previous prospective studies investigating the predictive effects of pre-trauma cortisol levels on subsequent PTSD symptoms in high-risk professionals, reported null findings (Heinrichs et al., Citation2005; van Zuiden et al., Citation2011, Citation2012), with the exception of Steudte-Schmiedgen et al. (Citation2015) who reported lower hair cortisol levels and lower cortisol stress reactivity predicting higher PTSD symptoms after military deployment upon accounting for the amount of the deployment trauma. However, prospective studies found that the onset of PTSD and depressive symptoms in response to military deployment could be predicted by high and low GR function and sensitivity in immune cells at pre-deployment respectively, irrespective of the amount of the deployment trauma. Immune GR function and sensitivity did not change from pre- to six months post-military deployment, neither in military personnel with high nor low levels of psychological symptoms at the final assessment (van Zuiden et al., Citation2012, Citation2012). Thus we may infer our observed attenuated awakening cortisol output in those with relatively high PTSD and anxiety symptom severity may have resulted from compensatory mechanisms to ongoing high GR signalling and sensitivity.
In line with this, Morris et al. (Citation2012) found in a meta-regression that afternoon/evening and daily cortisol output in PTSD patients decreased with increasing time since trauma and symptom onset, while negative feedback of GRs in the HPA axis increased over time. This effect was not observed for morning/8 a.m. cortisol, but they did not refer to the awakening period which may explain the difference with our findings. The studies in the meta-regression had a wide range of within-study average time since trauma, with an overall mean of 17 years. Our findings indicate that the decrease in cortisol output is already present relatively early after trauma and presumably related symptom onset. Our findings thus support that biological correlates of trauma exposure and related psychological symptoms are influenced by the time since exposure (Steudte-Schmiedgen et al., Citation2016) and the exact stage of symptom progression (McFarlane et al., Citation2017).
Thus, to better understand the biological consequences of trauma exposure and psychological symptoms, it is pivotal to assess psychological symptom severity and biological measures repeatedly in parallel and to take the time since trauma into account.
Several limitations of the current study should be considered. First, without a non-recently deployed control group, it remains difficult to disentangle whether the observed cortisol attenuation resulted from the deployment and subsequent symptom development, or from pre-existing symptoms or other confounding factors. Also, we cannot exclude potential confounders as all participants were internationally deployed to a (post-)emergency or development context with probable exposure to new pathogens and potential health problems (e.g., injuries and infections) which could result in immune activation. Additionally, the deployment may have had acute and more long-term effects on participants’ sleep quality and quantity as well as their circadian rhythm, because of e.g., being deployed to a different time zone and potential shift work. Information regarding these factors and participants’ chronotype (i.e., endogenous circadian rhythms) was unfortunately not collected, while known to influence cortisol’s diurnal rhythm including the CAR (Dayan, Rauchs, & Guillery-Girard, Citation2016; Germain, McKeon, & Campbell, Citation2017; Koch, Leinweber, Drengberg, Blaum, & Oster, Citation2017; Landgraf, McCarthy, & Welsh, Citation2014). Moreover, it would have been interesting to assess whether these factors moderated the longitudinal associations between cortisol output and symptom development, as there is increasing evidence linking inter-individual differences in sleep quality, sleep quantity and circadian rhythm to differential susceptibility for developing mental health problems (Acheson et al., Citation2019; Dayan et al., Citation2016; Germain et al., Citation2017; Koch et al., Citation2017; Landgraf et al., Citation2014; Lewis et al., Citation2020; Teicher et al., Citation2017). Nevertheless, as we observed that the amount of deployment-related traumatic exposure (combined with prior trauma) significantly impacted the cortisol decrease and as pre-deployment symptom levels were generally low, we remain confident that we captured the actual effects of deployment-related trauma exposure and associated changes in symptom severity.
Second, with retrospective self-report questionnaires, we cannot rule out recall bias, especially regarding the assessment of prior trauma exposure. A recent meta-analysis reported discrepancies between prospective and retrospective measures of childhood trauma (Baldwin, Reuben, Newbury, & Danese, Citation2019). In addition, our questionnaires on prior and recent trauma exposure did not measure frequency and severity, nor subjective interpretation or impact of exposure to the various trauma types. Curvilinear effects of adversity on subsequent mental health and biological correlates have been reported previously, with more beneficial effects of moderate amounts of adversity (Daskalakis et al., Citation2013). Also, our childhood trauma measure queried participants’ overall childhood, without differentiating exposure during developmentally sensitive periods from exposure outside of these windows. Furthermore, in the current analysis we specifically focused on deployment-related traumatic stressors, without taking the potential effects of chronic, non-traumatic stressors during the deployment into account. Additionally, to contain the number of analyses performed, we did not investigate potential differential effects according to trauma type during the respective exposure periods under investigation. Peritraumatic or acute psychological responses to trauma (e.g., peritraumatic distress) are among the strongest predictors of PTSD currently identified (Brunet et al., Citation2001; Ozer, Best, Lipsey, & Weiss, Citation2003) and therefore such responses to traumatic exposure during the deployment may have been of relevance in the current investigation, in addition to measuring objective exposure to traumatic events. However, as our data collection preceded the rapid technological developments facilitating personalized timing of acute assessment after the occurrence of a potentially traumatic event (Lorenz et al., Citation2019; van der Meer, Bakker, Schrieken, Hoofwijk, & Olff, Citation2017), we would only have been able to measure these responses retrospectively, well after the return from deployment. Thus, more in-depth assessments of characteristics of prior and recent (traumatic) stress exposure as well as its subjective impact may have provided additional nuance to our results.
Third, for feasibility reasons cortisol levels were only determined for participants who returned their saliva samples at all three assessments. This constitutes 50% of the original cohort. We cannot exclude this may have influenced our findings. Yet the differences in demographics, prior and recent trauma exposure and baseline symptoms between included and excluded participants were quite limited, aside from lower baseline depressive symptom severity.
Fourth, to maximize the feasibility of and compliance with the saliva collection protocol, we limited cortisol assessments to a single day assessment of two samples in the first 30 minutes post-awakening, while collecting 4–5 samples until 60 minutes post-awakening during multiple days is recommended to capture temporal CAR dynamics (Stalder et al., Citation2016). Nevertheless, two awakening samples provide a general approximation of the CAR and our rigorous screening of sampling compliance (±5 minutes allowed) increased our results’ reliability. Yet it should be emphasized that due to our single day assessments, we could not control for intra-individual variability and the effects of situational factors on awakening cortisol and the CAR. Finally, although we did not observe sex differences in cortisol parameter changes, we were unable to control for reproductive factors such as menstrual cycle phase and hormonal contraception use which were found to influence HPA axis function including CAR (Fries, Dettenborn, & Kirschbaum, Citation2009) and PTSD symptom course post-trauma (Engel et al., Citation2019).
In conclusion, in this prospective cohort study we observed attenuated CAR and awakening cortisol during HA deployment, with a non-cumulative dose-response interaction effect between the amount of prior and recent trauma on the extent of attenuation in awakening cortisol. HA workers who entered deployment with high levels of non-work-related trauma exposure seem to be the most vulnerable to long-term consequences of the deployment on their neuroendocrine functioning, in terms of awakening cortisol. Additionally, the attenuation in awakening cortisol was the strongest in those HA workers who developed or maintained relatively high levels of PTSD or anxiety symptoms over the course of the deployment. While the CAR also decreased during HA deployment, the extent of trauma exposure or PTSD, depressive and anxiety symptom severity did not moderate this course and mechanisms underlying the observed decrease still need further investigation.
The HPA axis and GR are pivotal modulators of physical health, including metabolic and immune function. Therefore, its altered functioning may be involved in the increased risk for subsequent physical disorders and mortality in individuals with trauma exposure (Dedert, Calhoun, Watkins, Sherwood, & Beckham, Citation2010) and psychological symptoms (Adam et al., Citation2017). Therefore, our findings are relevant for the HA field and other occupational fields with routine confrontations with potentially traumatic stressors. Yet, further prospective research including more rigorous cortisol sampling, more detailed assessments of stress exposure and psychological symptoms over longer follow-up periods is needed before the current findings could be translated into guidelines and recommendations for targeted primary or secondary prevention of adverse (mental) health outcome. Nevertheless, our findings support the importance of organizational awareness that non-work-related trauma exposure and (sub)clinical levels of psychological symptoms impact the biological effects of work-related exposure to (potential) traumatic stress. This emphasizes the importance of offering resilience-building resources and low-threshold psychological support and treatment for HA workers during and after the deployment cycle.
Supplemental Material
Download MS Word (37.3 KB)Acknowledgments
We thank the Antares Foundation for their pivotal contribution to this study by coordinating the data collection. Additionally, we thank all the humanitarian aid workers for participating in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, Miranda Olff. The data are not publicly available due to their containment of information that could compromise the privacy of research participants.
Additional information
Funding
References
- Aardal-Eriksson, E. , Eriksson, T. E. , & Thorell, L.-H. (2001). Salivary cortisol, posttraumatic stress symptoms, and general health in the acute phase and during 9-month follow-up. Biological Psychiatry , 50(12), 986–15. https://doi.org/10.1016/S0006-3223(01)01253-7
- Acheson, D. T. , Kwan, B. , Maihofer, A. X. , Risbrough, V. B. , Nievergelt, C. M. , Clark, J. W. , … Baker, D. G. (2019). Sleep disturbance at pre-deployment is a significant predictor of post-deployment re-experiencing symptoms. European Journal of Psychotraumatology , 10, 1. https://doi.org/10.1080/20008198.2019.1679964
- Adam, E. K. , Quinn, M. E. , Tavernier, R. , McQuillan, M. T. , Dahlke, K. A. , & Gilbert, K. E. (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology , 83(May), 25–41.https://doi.org/10.1016/j.psyneuen.2017.05.018
- Baldwin,J.R. , Reuben, A. , Newbury, J.B. , & Danese, A. (2019).Agreement between prospective and retrospective measures of childhood maltreatment. JAMA Psychiatry , 76(6), 584. https://doi.org/10.1001/jamapsychiatry.2019.0097
- Bender, R. , & Lange, S. (2001). Adjusting for multiple testing—when and how? Journal of Clinical Epidemiology , 54(4), 343–349. https://doi.org/10.1016/S0895-4356(00)00314-0
- Bonne, O. , Brandes, D. , Segman, R. , Pitman, R. K. , Yehuda, R. , & Shalev, A. Y. (2003). Prospective evaluation of plasma cortisol in recent trauma survivors with posttraumatic stress disorder. Psychiatry Research , 119(1–2), 171–175. https://doi.org/10.1016/S0165-1781(03)00098-2
- Brunet, A. , Weiss, D. S. , Metzler, T. J. , Best, S. R. , Neylan, T. C. , Rogers, C. , … Marmar, C. R. (2001). The Peritraumatic Distress Inventory: A proposed measure of PTSD criterion A2. American Journal of Psychiatry , 158(9), 1480–1485. https://doi.org/10.1176/appi.ajp.158.9.1480
- Cardozo, B. L. , & Salama, P. (2002). Mental health of humanitarian aid workers in complex emergencies. In Y. Danieli (Ed.), Sharing the front line and the back hills: International protectors and providers: Peacekeepers, humanitarian aid workers and the media in the midst of crisis (pp. 242–255). New York: Baywood Publishing Co.
- Chida, Y. , & Steptoe, A. (2009). Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology , 80(3), 265–278. https://doi.org/10.1016/j.biopsycho.2008.10.004
- Clow, A. , Hucklebridge, F. , Stalder, T. , Evans, P. , & Thorn, L. (2010). The cortisol awakening response: More than a measure of HPA axis function. Neuroscience and Biobehavioral Reviews , 35(1), 97–103. https://doi.org/10.1016/j.neubiorev.2009.12.011
- Curran, P. J. , & Bauer, D. J. (2011). The disaggregation of within-person and between-person effects in longitudinal models of change. Annual Review of Psychology , 62(1), 583–619. https://doi.org/10.1146/annurev.psych.093008.100356
- Cutrona, C. E. (1989). Ratings of social support by adolescents and adult informants: Degree of correspondence and prediction of depressive symptoms. Journal of Personality and Social Psychology , 57(4), 723–730. https://doi.org/10.1037/0022-3514.57.4.723
- Daskalakis, N. P. , Bagot, R. C. , Parker, K. J. , Vinkers, C. H. , & de Kloet, E. R. (2013). The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology , 38(9), 1858–1873. https://doi.org/10.1016/j.psyneuen.2013.06.008
- Dayan, J. , Rauchs, G. , & Guillery-Girard, B. (2016). Rhythms dysregulation: A new perspective for understanding PTSD? Journal of Physiology Paris , 110(4), 453–460. https://doi.org/10.1016/j.jphysparis.2017.01.004
- Dedert, E. A. , Calhoun, P. S. , Watkins, L. L. , Sherwood, A. , & Beckham, J. C. (2010). Posttraumatic stress disorder, cardiovascular, and metabolic disease: A review of the evidence. Annals of Behavioral Medicine , 39(1), 61–78. https://doi.org/10.1007/s12160-010-9165-9
- Dedovic, K. , & Ngiam, J. (2015). The cortisol awakening response and major depression: Examining the evidence. Neuropsychiatric Disease and Treatment , 1181. https://doi.org/10.2147/NDT.S62289
- Derogatis, L. R. , Lipman, R. S. , Rickels, K. , Uhlenhuth, E. H. , & Covi, L. (1974). The Hopkins symptom checklist (HSCL): A self-report symptom inventory. Behavioral Science , 19(1), 1–15. https://doi.org/10.1002/bs.3830190102
- Eisenberger, N. I. , Taylor, S. E. , Gable, S. L. , Hilmert, C. J. , & Lieberman, M. D. (2007). Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage , 35(4), 1601–1612. https://doi.org/10.1016/j.neuroimage.2007.01.038
- Engel, S. , van Zuiden, M. , Frijling, J. L. , Koch, S. B. J. , Nawijn, L. , Schumacher, S. , … Olff, M. (2019). Patterns of recovery from early posttraumatic stress symptoms after a preventive intervention with oxytocin: Hormonal contraception use is a prognostic factor. Biological Psychiatry , 85(12), e71–e73. https://doi.org/10.1016/j.biopsych.2019.01.014
- Eriksson, C. B. , Holland, J. M. , Currier, J. M. , Snider, L. M. , Ager, A. K. , Kaiser, R. E. R. , & Simon, W. S. (2015). Trajectories of spiritual change among expatriate humanitarian aid workers: A prospective longitudinal study. Psychology of Religion and Spirituality , 7(1), 13–23. https://doi.org/10.1037/a0037703
- Eriksson, C. B. , Lopes Cardozo, B. , Foy, D. W. , Sabin, M. , Ager, A. , Snider, L. , … Simon, W. (2013). Predeployment mental health and trauma exposure of expatriate humanitarian aid workers: Risk and resilience factors. Traumatology , 19(1), 41–48. https://doi.org/10.1177/1534765612441978
- Eriksson, C. B. , Vande, Gorsuch, K. H. , Hoke, R. , & Foy, D. W. (2001). Trauma exposure and PTSD symptoms in international relief and development personnel. Journal of Traumatic Stress , 14(1), 205–212. https://doi.org/10.1023/A:1007804119319
- Fries, E. , Dettenborn, L. , & Kirschbaum, C. (2009). The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology , 72(1), 67–73. https://doi.org/10.1016/j.ijpsycho.2008.03.014
- Germain, A. , McKeon, A. B. , & Campbell, R. L. (2017). Sleep in PTSD: Conceptual model and novel directions in brain-based research and interventions. Current Opinion in Psychology , 14, 84–89. https://doi.org/10.1016/j.copsyc.2016.12.004
- Heaney, J. L. J. , Phillips, A. C. , & Carroll, D. (2010). Ageing, depression, anxiety, social support and the diurnal rhythm and awakening response of salivary cortisol. International Journal of Psychophysiology , 78(3), 201–208. https://doi.org/10.1016/j.ijpsycho.2010.07.009
- Heinrichs, M. , Wagner, D. , Schoch, W. , Soravia, L. M. , Hellhammer, D. H. , & Ehlert, U. (2005). Predicting posttraumatic stress symptoms from pretraumatic risk factors: A 2-year prospective follow-up study in firefighters. American Journal of Psychiatry , 162(12), 2276–2286. https://doi.org/10.1176/appi.ajp.162.12.2276
- Hox, J. J. , Moerbeek, M. , & van de Schoot, R. (2017). Multilevel analysis: Techniques and applications (3rd ed.). New York: Routledge. https://doi.org/10.4324/9781315650982
- Ironson, G. , Kumar, M. , Greenwood, D. , Schneiderman, N. , Cruess, D. , Kelsch, C. B. , … Baum, A. (2014). Posttraumatic stress symptoms, intrusive thoughts, and disruption are longitudinally related to elevated cortisol and catecholamines following a major hurricane. Journal of Applied Biobehavioral Research , 19(1), 24–52. https://doi.org/10.1111/jabr.12014
- King, L. A. , King, D. W. , Leskin, G. , & Foy, D. W. (1995). The Los Angeles symptom checklist: A self report measure of posttraumatic stress disorder. Assessment , 2(1), 1–17. https://doi.org/10.1177/1073191195002001001
- Knutson, J. F. (1988). Physical and sexual abuse in children. In D. K. Routh (Ed.), Handbook of pediatric psychology (pp. 32–70). New York: Guilford.
- Koch, C. E. , Leinweber, B. , Drengberg, B. C. , Blaum, C. , & Oster, H. (2017). Interaction between circadian rhythms and stress. Neurobiology of Stress , 6, 57–67. https://doi.org/10.1016/j.ynstr.2016.09.001
- Landgraf, D. , McCarthy, M. J. , & Welsh, D. K. (2014). Circadian Clock and stress interactions in the molecular biology of psychiatric disorders. Current Psychiatry Reports , 16, 10. https://doi.org/10.1007/s11920-014-0483-7
- Lewis, C. , Lewis, K. , Kitchiner, N. , Isaac, S. , Jones, I. , & Bisson, J. I. (2020). Sleep disturbance in post-traumatic stress disorder (PTSD): A systematic review and meta-analysis of actigraphy studies. European Journal of Psychotraumatology , 11, 1. https://doi.org/10.1080/20008198.2020.1767349
- Lopes Cardozo, B. , Gotway Crawford, C. , Eriksson, C. , Zhu, J. , Sabin, M. , Ager, A. , … Simon, W. (2012). Psychological distress, depression, anxiety, and burnout among international humanitarian aid workers: A longitudinal study. PLoS ONE , 7(9), e44948. https://doi.org/10.1371/journal.pone.0044948
- Lorenz, P. , Schindler, L. , Steudte-schmiedgen, S. , Kirschbaum, C. , Schellong, J. , Lorenz, P. , … Schellong, J. (2019). Ecological momentary assessment in posttraumatic stress disorder and coping . An eHealth study protocol. European Journal of Psychotraumatology , 00, 00. https://doi.org/10.1080/20008198.2019.1654064
- McFarlane, A. C. , Lawrence-Wood, E. , Van Hooff, M. , Malhi, G. S. , & Yehuda, R. (2017). The need to take a staging approach to the biological mechanisms of PTSD and its treatment. Current Psychiatry Reports , 19, 2. https://doi.org/10.1007/s11920-017-0761-2
- Michaud, K. , Matheson, K. , Kelly, O. , & Anisman, H. (2008). Impact of stressors in a natural context on release of cortisol in healthy adult humans: A meta-analysis. Stress , 11(3), 177–197. https://doi.org/10.1080/10253890701727874
- Miller, G. E. , Chen, E. , & Zhou, E. S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin , 133(1), 25–45. https://doi.org/10.1037/0033-2909.133.1.25
- Morris, M. C. , Compas, B. E. , & Garber, J. (2012). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clinical Psychology Review , 32(4), 301–315. https://doi.org/10.1016/j.cpr.2012.02.002
- Ozer, E. J. , Best, S. R. , Lipsey, T. L. , & Weiss, D. S. (2003). Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychological Bulletin , 129(1), 52–73. https://doi.org/10.1037/0033-2909.129.1.52
- Pan, X. , Wang, Z. , Wu, X. , Wen, S. W. , & Liu, A. (2018). Salivary cortisol in post-traumatic stress disorder: A systematic review and meta-analysis. BMC Psychiatry , 18(1), 324. https://doi.org/10.1186/s12888-018-1910-9
- Resnick,H.S. (1996). Psychometric review of trauma assessment for adults (TAA). In B. H. Stamm (Ed.), Measurement of stress, trauma, and adaptation (pp. 362–365). Lutherville: Sidran Press.
- Shalev, A. Y. , Videlock, E. J. , Peleg, T. , Segman, R. , Pitman, R. K. , & Yehuda, R. (2008). Stress hormones and post-traumatic stress disorder in civilian trauma victims: A longitudinal study. Part I: HPA axis responses. International Journal of Neuropsychopharmacology , 11(3), 365–372. https://doi.org/10.1017/S1461145707008127
- Sippel, L. M. , Pietrzak, R. H. , Charney, D. S. , Mayes, L. C. , & Southwick, S. M. (2015). How does social support enhance resilience in the trauma-exposed individual? Ecology and Society , 20(4), art10. https://doi.org/10.5751/ES-07832-200410
- Sippel, L. M. , Watkins, L. E. , Pietrzak, R. H. , Hoff, R. , & Harpaz-Rotem, I. (2019). Heterogeneity of posttraumatic stress symptomatology and social connectedness in treatment-seeking military veterans: A longitudinal examination. European Journal of Psychotraumatology , 10(1), 1646091. https://doi.org/10.1080/20008198.2019.1646091
- Söndergaard, H. P. , & Theorell, T. (2003). A longitudinal study of hormonal reactions accompanying life events in recently resettled refugees. Psychotherapy and Psychosomatics , 72(1), 49–58. https://doi.org/10.1159/000067185
- Stalder, T. , Kirschbaum, C. , Kudielka, B. M. , Adam, E. K. , Pruessner,J.C. , Wüst, S. , … Clow, A. (2016). Assessment of the cortisol awakening response: Expert consensusguidelines. Psychoneuroendocrinology ,63,414–432. https://doi.org/10.1016/j.psyneuen.2015.10.010
- Stalder, T. , Steudte-Schmiedgen, S. , Alexander, N. , Klucken, T. , Vater, A. , Wichmann, S. , … Miller, R. (2017). Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology , 77, 261–274. https://doi.org/10.1016/j.psyneuen.2016.12.017
- Staufenbiel, S.M. , Penninx,B.W.J. H. , Spijker, A. T. , Elzinga, B. M. , & van Rossum, E. F. C. (2013). Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology ,38(8),1220–1235.https://doi.org/10.1016/j.psyneuen.2012.11.015
- Steudte-Schmiedgen, S. , Kirschbaum, C. , Alexander, N. , & Stalder, T. (2016). An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neuroscience and Biobehavioral Reviews , 69, 124–135. https://doi.org/10.1016/j.neubiorev.2016.07.015
- Steudte-Schmiedgen, S. , Stalder, T. , Schönfeld, S. , Wittchen, H.-U.-U. , Trautmann, S. , Alexander, N. , … Kirschbaum, C. (2015). Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology , 59, 123–133. https://doi.org/10.1016/j.psyneuen.2015.05.007
- Stoppelbein, L. , & Greening, L. (2015). A longitudinal study of the role of cortisol in posttraumatic stress disorder symptom clusters. Anxiety, Stress and Coping , 28(1), 17–30. https://doi.org/10.1080/10615806.2014.923844
- Straus, M. A. , & Smith, C. (2017). Violence in hispanic families in the USA: Incidence rates and structural interpretations. In C. Smith Ed., Physical violence in American families: Risk factors and adaptations to violence in 8,145 families (Vol. 41, pp. 341–368). New York: Routledge. https://doi.org/10.4324/9781315126401
- Strohmeier, H. , Scholte, W. F. , & Ager, A. (2018). Factors associated with common mental health problems of humanitarian workers in South Sudan. PLoS ONE , 13(10), 1–19. https://doi.org/10.1371/journal.pone.0205333
- Teicher, M. H. , Ohashi, K. , Khan, A. , Garcia, L. C. H. , Klengel, T. , Anderson, C. M. , & Silveri, M. M. (2017). Does sleep disruption mediate the effects of childhood maltreatment on brain structure? European Journal of Psychotraumatology , 8, 00. https://doi.org/10.1080/20008198.2018.1450594
- van der Meer, C.A.I. , Bakker, A. , Schrieken, B. A. L. , Hoofwijk, M. C. , & Olff, M. (2017). Screening for trauma-related symptoms via a smartphone app: The validity of Smart Assessment on your Mobile in referred police officers. International Journal of Methods in Psychiatric Research , 26(3), 1–8. https://doi.org/10.1002/mpr.1579
- van Zuiden, M. , Geuze, E. , Maas, M. , Vermetten, E. , Heijnen, C. J. , & Kavelaars, A. (2009). Deployment-related severe fatigue with depressive symptoms is associated with increased glucocorticoid binding to peripheral blood mononuclear cells. Brain, Behavior, and Immunity , 23(8), 1132–1139. https://doi.org/10.1016/j.bbi.2009.07.004
- van Zuiden, M. , Geuze, E. , Willemen, H. L. D. M. , Vermetten, E. , Maas, M. , Amarouchi, K. , … Heijnen, C. J. (2012). Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: A prospective study. Biological Psychiatry , 71(4), 309–316. https://doi.org/10.1016/j.biopsych.2011.10.026
- vanZuiden, M. , Geuze,E. , Willemen, H.L.D.M. , Vermetten, E. , Maas, M. , Heijnen, C. J. , & Kavelaars, A. (2011). Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. American Journal of Psychiatry , 168(1), 89–96. https://doi.org/10.1176/appi.ajp.2010.10050706
- van Zuiden, M. , Heijnen, C. J. , Maas, M. , Amarouchi, K. , Vermetten, E. , Geuze, E. , & Kavelaars, A. (2012). Glucocorticoid sensitivity of leukocytes predicts PTSD, depressive and fatigue symptoms after military deployment: A prospective study. Psychoneuroendocrinology , 37(11), 1822–1836. https://doi.org/10.1016/j.psyneuen.2012.03.018
- West, B. T. (2009). Analyzing longitudinal data with the linear mixed models procedure in SPSS. Evaluation & the Health Professions , 32(3), 207–228. https://doi.org/10.1177/0163278709338554
- Widom, C. S. , Dutton, M. A. , Czaja, S. J. , & DuMont, K. A. (2005). Development and validation of a new instrument to assess lifetime trauma and victimization history. Journal of Traumatic Stress , 18(5), 519–531. https://doi.org/10.1002/jts.20060
