ABSTRACT
Background: Post Traumatic Stress Disorder (PTSD) has been described as an independent risk factor for cognitive decline and dementia. At the same time, cognitive deterioration and increased loss experiences in dementia may increase liability for the reactivation of traumatic memories and thereby PTSD symptoms.
Objective: In order to investigate co-occurrence of PTSD in dementia this systematic literature review summarizes all the available evidence on reported comorbidity rates of PTSD in patients with dementia.
Method: PubMed, Embase, PsycINFO and CINAHL were searched for potential publications investigating the co-occurrence of PTSD in dementia until 25 November 2019. Cohort and cross-sectional studies were included. To assure current comorbidity of PTSD in dementia, only publications with a recent PTSD diagnosis (<2 years before onset of dementia) were selected.
Results: Of the 860 identified abstracts, three studies (0.35%) met the eligibility criteria and were included. These three studies concerned only military veteran populations, and they comprised two cross-sectional cohort studies and one prospective cohort study. The estimated comorbidity rate of PTSD in veterans with dementia varied between 4.7% and 7.8%.
Conclusions: The limited research available shows comorbidity rates only in military veterans, which were possibly dependent on investigated population with respect to dementia severity and possibly associated behavioural and psychiatric symptoms of dementia (BPSD). In dementia patients the comorbidity with PTSD may be high and we suggest that worldwide the impact of PTSD in dementia is high and probably underestimated. Research and care on this topic should improve urgently with the current expanding prevalence of dementia. A first step to improve quality of dementia research and care would be to develop a structured tool to diagnose PTSD in these patients.
HIGHLIGHTS
Dementia patients may have a high comorbidity rate of post-traumatic stress disorder.
Antecedentes: El trastorno de estrés postraumático (TEPT) ha sido descrito como un factor de riesgo independiente para el deterioro cognitivo y la demencia. De manera concurrente, el deterioro cognitivo y el incremento en la pérdida de experiencias en la demencia pueden incrementar la vulnerabilidad para la reactivación de recuerdos traumáticos y, por tanto, de síntomas del TEPT.
Objetivo: Para investigar la comorbilidad del TEPT en la demencia, esta revisión sistemática resume toda la evidencia disponible de los índices de comorbilidad del TEPT en pacientes con demencia.
Métodos: Se realizó una búsqueda de potenciales publicaciones investigando la comorbilidad del TEPT en demencia en PubMed, Embase, PsycINFO y CINAHL hasta el 25 de noviembre del 2019. Se incluyeron estudios de cohorte y estudios transversales. Para asegurar que la comorbilidad del TEPT en demencia sea actual, solo se seleccionaron publicaciones que incluyeran un diagnóstico reciente del TEPT (de menos de dos años desde el inicio de la demencia).
Resultados: De los 860 resúmenes identificados, tres estudios (0,35%) cumplieron los criterios de inclusión y fueron seleccionados. Estos tres estudios incluyeron únicamente a poblaciones de militares veteranos. Dos de estos estudios eran de cohortes transversales y uno era un estudio de cohorte prospectiva. La comorbilidad estimada del TEPT en veteranos con demencia osciló entre un 4,7% y un 7,8%.
Conclusiones: La escasa investigación disponible muestra índices de comorbilidad solo en militares veteranos, los cuales fueron posiblemente dependientes de la población investigada en relación con la severidad de la demencia y posiblemente asociados con síntomas conductuales y psiquiátricos de la demencia (BPSD por sus siglas en ingles). En los pacientes con demencia, la comorbilidad con el TEPT puede ser alta y sugerimos que, universalmente, el impacto del TEPT sobre la demencia es alto y probablemente subestimado. La investigación y la atención a este tema deberían mejorar urgentemente dado el incremento en la prevalencia de la demencia. Un primer paso para mejorar la calidad de la investigación y la atención del tema sería el desarrollar un instrumento estructurado para diagnosticar el TEPT en estos pacientes.
背景: 创伤后应激障碍 (PTSD) 已被描述为认知能力下降和痴呆的一个独立风险因素。同时, 痴呆的认知能力下降和丧失体验增加可能增加对创伤性记忆的再激活, 从而增加PTSD症状。
目的: 为了考查痴呆患者中PTSD的并发情况, 本系统文献综述总结了痴呆患者中PTSD共病率的所有现有证据。
方法: 检索了PubMed, Embase, PsycINFO和CINAHL, 以搜索截至2019年11月25日前关于痴呆中PTSD并发的潜在出版物。纳入了队列研究和横断面研究。为确保是痴呆中当前的PTSD共病, 仅选择是近期PTSD诊断 (痴呆发作前2年以内) 的出版物。
结果: 在识别出的860篇摘要中, 有3项研究 (0.35%) 符合资格标准被纳入。这三项研究仅涉及退伍军人人群, 包括两项横断面队列研究和一项前瞻性队列研究。患有痴呆的退伍军人中PTSD共病率估计值在4.7%和7.8%之间不等。
结论: 现有有限研究表明, 共病率仅在退伍军人中较高, 这可能在痴呆严重程度以及可能的痴呆相关行为和精神症状 (BPSD) 方面取决于所考查人群。在痴呆患者中, PTSD的共病率可能很高, 我们表明在全世界范围内PTSD对痴呆症的影响很高并且可能被低估了。随着当前痴呆患病率的不断提高, 对此问题的研究和护理应亟待提高。提高痴呆研究和护理质量的第一步是开发一种结构化工具来诊断这些患者的PTSD。
1. Introduction
Posttraumatic Stress Disorder (PTSD) is a debilitating disorder with lifetime prevalence rates of 7–8% in the general population (de Vries & Olff, Citation2009; Kessler et al., Citation2017, Citation2005) and is associated with cognitive impairments (Scott et al., Citation2015). It has also been described as an independent risk factor for cognitive decline and dementia (Doyle, Dunt, & Morris, Citation2014; Qureshi et al., Citation2010). Hazard ratios for dementia range from 1.21 to 1.77 (Kang, Xu, & McConnell, Citation2019). For example, the 7-year cumulative incident dementia rate was 10.6% in an older (>55 years) veteran population with comorbid PTSD, compared to 6.6% in older veterans without PTSD (Yaffe et al., Citation2010).
The precise mechanisms of the relationship between PTSD and dementia are difficult to unravel as both illnesses share common comorbidities such as depression, alcohol abuse, hypertension and cardiovascular diseases (Clemens et al., Citation2018; Chopra et al., Citation2014; Pietrzak, Goldstein, Southwick, & Grant, Citation2012). Over the last few years, PTSD has been re-conceptualized as a full systemic disorder (Lohr et al., Citation2015) in which (cumulative life) stress is thought to develop into molecular damage, e.g. oxidative stress accompanied by epigenetic alterations, telomere shortening and long-term molecular consequences (Greenberg, Tanev, Marin, & Pitman, Citation2014; Mohlenhoff, O’Donovan, Weiner, & Neylan, Citation2017; Roberts et al., Citation2017; Rutten et al., Citation2018). Effectively, these molecular changes of the stress response system, i.e. the allostatic load concept (McEwen, Citation2013), impact among others on metabolism, sleep, the immune system, the cardiovascular system, brain structures (Avetyan, Zakharyan, Petrek, & Arakelyan, Citation2019; Lohr et al., Citation2015) and may accelerate ageing and increase the risk of eliciting or strengthening neurodegenerative biological cascades (Miller, Lin, Wolf, & Miller, Citation2017; Roberts et al., Citation2017; Wang et al., Citation2016). For example, altered functioning of brain intrinsic connectivity networks and its association with specific clinical PTSD symptoms (i.e. cognition, arousal and sense of self) have been described by Lanius, Frewen, Tursich, Jetly, and McKinnon (Citation2015). Of this, the hippocampal brain network integrates episodic memory with multimodal sensory information and anxiety-related networks involving the amygdala and medial prefrontal cortex (Çalışkan & Oliver Stork, Citation2019; Furini, Myskiw, & Izquierdo, Citation2014; Lopresto, Schipper, & Homberg, Citation2016; Yassa & Stark, Citation2011). At the cellular level, it has been observed that specifically this hippocampal network is vulnerable to age-related and dementia-related neurodegeneration (West, Citation1993) but also to traumatic stress and PTSD-related alterations. Smaller hippocampi have been described in subjects with current PTSD and hippocampal atrophy is also regarded as a main feature of Alzheimer’s disease (Jack et al., Citation1998; Josephs et al., Citation2017). Animal studies show that the CA3 region of the hippocampus, which is seen as a key region involved in the phenomenon of ‘pattern completion’, gets relatively more active during age-related neurocognitive decline, possibly due to loss of inhibitory interneuron modulation and performant path input (Dieguez & Barea-Rodriguez, Citation2004). It is tempting to speculate that both these changes shift the balance in favour of pattern completion and hence contribute to clinical anxiety associated with PTSD (Lange et al., Citation2017; Wilson, Gallagher, Eichenbaum, & Tanila, Citation2006). Stress may then impair the fear extinction process in the hippocampus, prefrontal cortex and amygdala, increasing the chance to elicit PTSD symptoms (Maren & Holmes, Citation2015).
Next, to the neurobiological link there are psychological associations. For example, unfamiliar circumstances can cause confusion and reveal feelings of anxiety and powerlessness in cognitively compromised patients, such as mobility restrictions used in psycho-geriatric care settings (e.g. closing doors). In sum, the combination of neurodegeneration, decreased cognitive reserve, increased loss experiences with ageing may increase liability for the reactivation of traumatic memories and thereby PTSD symptoms (Anderson, Fields, & Dobb, Citation2011; Khouzam, Citation2008).
Psychiatric comorbidities in dementia are referred to as ‘neuropsychiatric symptoms’ which are frequently associated with behavioural disturbances (BPSD: Behavioural and Psychological Symptoms of Dementia). A major challenge is to differentiate PTSD symptoms from other neuropsychiatric symptoms and BPSD (Martinez-Clavera, James, Bowditch, & Kuruvilla, Citation2017). This may be difficult as PTSD symptoms in dementia may differ from the classical symptoms in adults, e.g. hallucinations, vivid nightly re-experiences, screaming, wandering and aggressive incidents have been reported (Dallam, Mellman, Bhatnagar, Nguyen, & Kurukumbi, Citation2011; Johnston, Citation2000; Martinez-Clavera et al., Citation2017; van Achterberg, Rohrbaugh, & Southwick, Citation2001).
In sum, the combination of PTSD as a risk factor for dementia and increased liability for the reactivation of PTSD symptoms in dementia patient due to biological, psychological and social factors hint at a possible high prevalence of PTSD in this population. Therefore, the aim of the present study is to summarize the available evidence on reported rates of comorbid PTSD in patients with dementia in a systematic literature review.
2. Methods
2.1. Data sources and searches
The literature search was conducted in PubMed, Embase, PsycINFO and CINAHL. The broad search strategy consisted of terms related to PTSD and terms related to dementia’, as well as some specific limitations (e.g. only studies in ‘human subjects’ and ‘English language’). The complete search strategy is provided in Appendix A.
2.2. Study selection
The selection process followed the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines. Publications until 25 November 2019 were searched and found titles and abstract were screened (based on the below mentioned criteria).
Publications were included if they fulfilled the following inclusion criteria: 1) design: prospective, observational, cross-sectional studies; 2) there was a recently established PTSD diagnosis (diagnosed <2 years before study enrolment); 3) a current diagnosis of dementia; and 4) rates of PTSD in dementia were described or could be calculated on information presented in the publication. To be included in the review, the paper was appraised at the outcome level for ‘comorbidity rate’ (inclusion criterion 4). Next, reference list of publications and secondary literature (e.g. review articles, book chapters, editorials) were hand-searched for possible missing articles.
2.3. Data extraction and quality assessment
The search and operationalization of the selection criteria were established by two authors (S.S., K.D.). The first author (S.S.) screened titles and abstracts for broad suitability based on the abovementioned eligibility criteria and scrutinized the full-text versions of potentially relevant citations. A standardized data collection form was used to extract information like sample size, setting, age range, follow-up period, outcome (dementia diagnosis), measurement of PTSD, measurement of (neuro)psychiatric symptoms (or BPSD) and comorbidity rates. Data extraction was checked by the last author (K.D.) and possible discrepancies were discussed in the study team.
The Newcastle-Ottawa Scale (NOS) (Wells et al., Citation2000) was used to assess study quality. For cross-sectional studies, an adapted version of the NOS was applied (see Appendix B).
3. Results
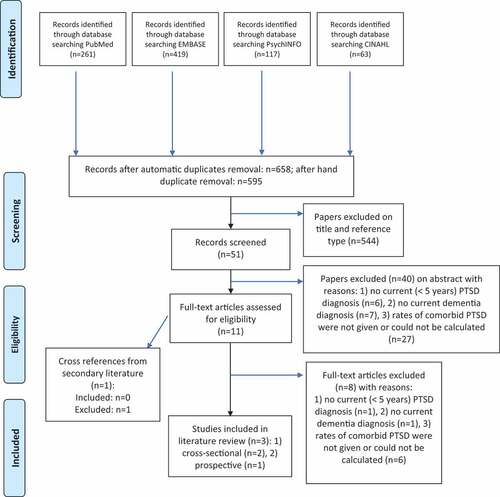
The literature search resulted in 860 abstracts of which 11 (1.28%) were included for full-text screening. Of these, three studies (0.35%) met the eligibility criteria () (Ball et al., Citation2009; Pinciotti et al., Citation2017; Verma et al., Citation2001). Eight papers did not meet the inclusion criteria. 1) There was no current PTSD diagnosis (<2 years) (n = 1) in the study of Sperling, Kreil, and Biermann (Citation2011). This paper was a cross-sectional study of medical documents of Holocaust survivors (Sperling et al., Citation2011) and association was assessed between diagnoses of lifetime PTSD and dementia, both according to the medical documents. 2) In one paper cognition was only described as cognitive functioning (without diagnosing dementia) (Burri, Maercker, Krammer, & Simmen-Janevska, Citation2013). 3) Rates of PTSD in dementia were not described or could not be calculated on information in the paper (n = 6) (Delrue & Plagnol, Citation2017; Flatt, Gilsanz, Quesenberry, Albers, & Whitmer, Citation2018; Mawanda, Wallace, McCoy, & Abrams, Citation2017; Qureshi et al., Citation2010; van Achterberg, et al., Citation2001; Yaffe et al., Citation2010). These were retrospective cohort studies (n = 2) (Mawanda et al., Citation2017; Yaffe et al., Citation2010) or prospective cohort studies (n = 3) (Flatt et al., Citation2018; Qureshi et al., Citation2010). In the retrospective cohort studies, associations of a life history of PTSD and a current dementia diagnosis were assessed. In the prospective cohort studies, patients with current or history of PTSD were followed and risk of dementia was investigated. Delrue and Plagnol (Citation2017) included solely patients with dementia and PTSD in an observational design. No information about comorbidity rate was given. The same applies to van Achterberg et al. (Citation2001) who described serial case-reports.
Three papers were incorporated into this review of which two studies used a cross-sectional design (Pinciotti et al., Citation2017; Verma et al., Citation2001) and one was prospective cohort study using a 2 year follow-up of patients with new onset dementia (Ball et al., Citation2009). No additional studies were found from cross-references. shows the characteristics, quality and results of the included studies. Quality assessment of the three included studies was sufficient (mean NOS score: 6.33; SD: 0.58).
Table 1. Studies (n = 3) in which comorbidity rates of PTSD in dementia patients have been described or can be calculated based on reported findings. Variables to describe the population with respect to setting, age, cognition, PTSD and other psychiatric symptoms or behavioural and psychological symptoms of dementia (BPSD) are shown
3.1. Comorbidity rates of PTSD in dementia
The three included studies described comorbidity rates of PTSD in veterans with dementia (Ball et al., Citation2009; Pinciotti et al., Citation2017; Verma et al., Citation2001). In the study of Verma et al., patients with a diagnosis of dementia and BPSD at a clinical geropsychiatric inpatient unit were screened for PTSD. Diagnoses of dementia and PTSD were determined by consensus using a standardized battery of psychological rating scales (see ) in combination with clinical diagnoses by two independent geriatric psychiatrists, a gero-psychologist or other members of the research team. Mean Mini Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, Citation1975) was 18.07 (SD = 5.26, range 5–25). Of the 252 consecutive admissions with dementia, 16 were diagnosed with PTSD according to the criteria of Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV). Hence the comorbidity rate in this study may be determined as 6.3% (16/252 * 100%). Mean age and gender of dementia patients with PTSD were not specified in the paper. Of the patients with PTSD 25% (4/16 * 100%) were combat veterans who were former Prisoners Of War (POW). POW veterans showed a quite different psychiatric presentation compared to veterans without a history of POW, i.e. more paranoia and less verbal agitation as measured by items on Brief Psychiatric Rating Scale (BPRS) (Overall & Gorham, Citation1962) and Positive and Negative Syndrome Scale for Schizophrenia (PANSS) (Kay, Opler, & Fiszbein, Citation1986) but not on the Cohen-Mansfield Aggression Inventory (CMAI) (Cohen-Mansfield, Marx, & Rosenthal, Citation1989). It was not described whether these psychiatric symptoms were due to differences in kind and severity of trauma. Relevant reported limitations of this study were the relatively few subjects, who were solely male and all recruited from an inpatient setting. PTSD was not measured on a standardized scale. Reporter bias may be present as the assessment tools were all completed by the attending (blinded) psychiatrist. In the community sample of Pinciotti et al. (Citation2017), 486 veterans with dementia were included. Record information of the last 2 years, including diagnostic data International Classification of Diseases-9 (ICD-9), were extracted from the Veterans Administration (VA) database. There were no descriptions of how the diagnoses were established. Of them, 38 patients of 486 patients had a comorbid PTSD, leading to a comorbidity rate in this population of 7.8% (38/486 * 100%). Mean age of these patients with PTSD was 77.3 years, gender was not specified. A hetero-anamnestic cognitive 7-item questionnaire was used to assess cognitive impairments and a hetero-anamnestic 4-item questionnaire was used to assess behaviour (neuropsychiatric) problems. Both questionnaires used a Likert-scale ranging from 1 (not applicable) to 4 (most applicable). Compared to the ‘dementia only’ group, cognitive impairments (severity not presented) did not differ in the ‘PTSD-dementia’ group, but there were significantly more behaviour problems (mean 4.0 compared to 2.7 on the Likert scales). Reported relevant limitations were the small sample of patients with both PTSD and dementia, the cross-sectional design. Next the data on PTSD were limited, e.g. no information was available on when PTSD was diagnosed, the severity of current or past PTSD symptoms. Ball et al. (Citation2009) included 215 veterans who were identified with recently diagnosed dementia (<1 year) at a Veterans Affairs medical centre. Dementia severity was categorized as severe, moderate and mild based on the Dementia Rating Scale-2 (DemRS2) (Jurica, Leitten, & Mattis, Citation2001). The administrative databases (i.e. patient treatment files and outpatient clinic files) were searched for PTSD diagnosis 2 years pre- and post-study enrolment (ICD-9). There was no description of the diagnosis of PTSD. Of these 215 patients, 10 patients had a diagnosis of PTSD (mean age: 75.8 years, gender not specified). The comorbidity rate in this cohort may therefore be determined at 4.7% (10/215 * 100%). Sixty-percent (6/10 * 100%) of the patients with PTSD had combat exposure and 40% did not (4/10 *100%). Kind of trauma was not further specified. Authors did not distinguish PTSD comorbidity rates in the three categories of dementia severity. However, they found that aggression (as measure of BPSD) indicated by Cohen-Mansfield Agitation Inventory (CMAI) (Cohen-Mansfield, Citation1986) was more prevalent in the more cognitively impaired group, but no association with PTSD was found. Limitations of relevance, the authors reported were: the limited sample size, possible selection bias (recruited only at a single veteran medical centre, and only patients with newly diagnosed dementia), lack of information on PTSD diagnosis (not using a structured tool), severity of PTSD and dementia type.
4. Discussion
In this systemic literature review we examined comorbidity rates of PTSD in patients with a diagnosis of dementia. Comorbidity rates were described in only three studies, using three different veteran patient populations. Our main findings show that these estimated comorbidity rates ranged between 4.7% and 7.8% possibly depending on the investigated population, i.e. severity of dementia. Current clinical PTSD in a clinical veteran population with moderate dementia (mean MMSE-score: 18.07) and BPSD was 6.3% (Verma et al., Citation2001). When PTSD diagnoses were extracted from patient records (last 2 years) comorbidity rates were respectively 7.8% in a veteran population with dementia (duration and severity not specified) (Pinciotti et al., Citation2017) and 4.7% in a veteran male population with new onset dementia (<1-year, severity not specified) of which 60% were traumatized by combat exposure (Ball et al., Citation2009).
These preliminary findings suggest a comorbidity rate between 4.7% and 7.8% of PTSD in veteran patients with dementia. The comorbid PTSD rates in dementia may be considered relatively high compared to one-month PTSD prevalence of 1.3%, 12-months PTSD prevalence of 3.3% in the general population (de Vries & Olff, Citation2009) or 12-months PTSD prevalence of 3% in older adults (age 65+) (Reynolds, Pietrzak, Mackenzie, Chou, & Sareen, Citation2016). However in US veterans (Richardson, Frueh, & Acierno, Citation2010), prevalence rates of combat-related PTSD in veterans are relatively higher and may range from 2% to 37% depending on period of military service and sociohistorical context of the war and applied diagnostic criteria (Baker et al., Citation2009; Hoge, Riviere, Wilk, Herrell, & Weathers, Citation2014; Lee, Citation2019). As veterans are known to underuse mental health difficulties, this may also be an underestimation (Mellotte, Murphy, Rafferty, & Greenberg, Citation2017).
Differences in reported comorbidity rates in the included studies seem to be related to differences in the investigated populations. For example, Verma et al. (Citation2001), included veterans with moderate dementia and BPSD from a geropsychiatric hospital unit. As BPSD symptom patterns can be identified in different forms of dementia (Majer et al., Citation2019), the population of Verma constitutes a population with possibly more advanced and specific subtypes of dementia than the population of Ball et al. (Citation2009). The latter included patients with a new onset diagnosis of dementia (<1 year) who were not aggressive during the previous year. This may have excluded specific forms of dementia, e.g. dementias with frontal lobe dysfunction (Senanarong et al., Citation2004), or patients with PTSD, as both are usually identified by increased impulsivity and aggression (Dallam et al., Citation2011; Bonanni et al., Citation2018). Evidence that PTSD is associated with development of specific dementia type, i.e. semantic frontotemporal dementia, was presented by a recent prospective study (Bonanni et al., Citation2018). Therefore, severity and subtypes of dementia might also be determining factors in co-occurrence of PTSD in dementia.
Another explanation for the relatively low comorbidity rate reported by Ball et al. (Citation2009), compared to the other two studies, might be the retro and prospective data generation in the cohort which differs from the cross-sectional set-up of the other two studies.
The largest variance was seen in the studies that were both in the community/outpatient setting (Ball et al., Citation2009; Pinciotti et al., Citation2017). We suggest that this variance may possibly also be due to differences in research populations. Pinciotti et al. (Citation2017) recruited veterans from a care and support service ‘Partners in Dementia Care (PDC)’. This service involves partnerships between veteran administration (VA). Veterans who use dementia care were selected. As severity of dementia and care use are positively associated it may be speculated that this population concerns a more severe dementia population (with possibly BPSD) than the population of Ball et al. who were recruited with using a newly dementia diagnosis from a Veterans Affairs Medical Centre without previous reports of aggression (Ball et al., Citation2009).
Severity of trauma may be a confounding factor in clinical presentation of PTSD in dementia. Verma et al. (Citation2001) found an association between combat trauma (POW) and a different clinical presentation of PTSD in dementia, i.e. more paranoia, less verbal agitation. Possibly, combat trauma may be regarded as severe trauma. The other reviewed studies did not take kind or severity of trauma into account. Previously, it has been described that the risk of cognitive decline and developing dementia seems to be associated with the severity of PTSD complaints (Wang et al., Citation2016). Interestingly enough, in a meta-analysis by Nelson and Tumpap (Citation2017) it appeared that reduction of volume of the left hippocampus is associated with exaggerated PTSD symptom severity, which may emphasize the neurobiological impact of trauma on the brain.
Of interest is the investigated association of PTSD and BPSD in the reviewed studies. Pinciotti et al. (Citation2017) described increased BPSD in the PTSD group, the other two reviewed papers did not (Ball et al., Citation2009; Verma et al., Citation2001). Severity of dementia and information about what the behavioural problems compromised in the study of Pinciotti et al. remain unclear as the used hetero-anamnestic questionnaire was not elaborated on. Though, it may be suggested that i) a valid rating of BPSD was incomplete in the reviewed studies by using questionnaires which are not validated in dementia (i.e. BPRS and PANNS), ii) as BPSD occurs mostly in more advanced dementia (Nunes et al., Citation2019), co-occurrence of PTSD in combination with BPSD might have been lower in the population with new-onset dementia (Ball et al., Citation2009). Accordingly, in elaborating the clinical presentation of PTSD in dementia, recurrent assessments of BPSD, with validated instruments (e.g. Neuro Psychiatric Inventory, NPI) (Cummings et al., Citation1994), are needed.
Worldwide, the number of patients with dementia is estimated at 35.6 million in 2010 and will almost double every 20 years to about 115.4 million in 2050 (Patterson, Citation2018; Prince et al., Citation2013). In light of the current findings, affected patients with comorbid PTSD will be substantial. As both impact on quality of life, disease burden and health costs, research and care for these patients must improve urgently (van Zelst, de Beurs, Beekman, van Dyck, & Deeg, Citation2006).
4.1. Methodological considerations
The scientific evidence of this paper is mainly restricted by the low number of included papers. Use of different research settings (clinical or community based), study designs, research questions and possibly research populations (veterans in different stages of dementia) hampered a direct comparison. Investigated patients were all veterans (possibly mainly men with combat trauma) and findings can therefore not automatically be generalized to the entire population.
Information bias might have occurred on several levels. First, the original aims of the included articles were not to measure comorbidity rates of PTSD and dementia. Second, no structured diagnostic tool for PTSD in dementia patients was used in these studies. Consequently, it is plausible that the comorbidity rates in the included cohort studies are underestimated. By lack of a valid assessment method, the comprehensive multidisciplinary clinical evaluation to diagnose PTSD applied by Verma et al. (Citation2001), seems an applicable alternative. Information on the diagnostic processes of dementia, its severity and subgroups were also incomplete. In case of severe dementia, anamnestic information about traumatic events and PTSD symptoms is more difficult to obtain, and PTSD symptoms could therefore be more easily missed. The complex interaction between PTSD and dementia can only be unscrambled when both diagnostic processes are qualitative, complete and clear.
Secondly, the underreporting of diagnostic information on PTSD may also impact on selection bias. Two studies did not describe how PTSD was determined as the most recent PTSD diagnoses (<2 years) were retrieved from a database (Ball et al., Citation2009; Pinciotti et al., Citation2017). Possibly this information was not representative of the current state at moment of assessment.
4.2. Conclusion and recommendations
We found comorbidity rates of PTSD in veterans with dementia between 4.7% and 7.8% possibly depending on the investigated population, i.e. severity of dementia. Overall, studies on comorbidity rates of PTSD in dementia are sparse and quality is limited by not using a structured method to diagnose current PTSD, and not specifying the dementia population with respect to kind of dementia and its severity. Taken together, we suggest that these comorbidity rates are underestimated due to lack of a valid diagnostic tool to diagnose current PTSD in dementia patients. Many questions arise: Which symptoms of PTSD are present in dementia? How to obtain valid and reliable assessment in this population? What is the influence of kind and severity of dementia on symptoms of PTSD? Would it be relevant to screen for PTSD in older adults prior to cognitive decline?
It is likely that the worldwide impact of PTSD in dementia is high and underestimated with respect to number of patients, impact of quality of life and health care burden.
This review emphasizes the need for a structured method to diagnose PTSD and its severity in dementia patients. A diagnostic tool should not only improve personal care and serve the individual patient, but will also improve quality of research by, i.e., reducing diagnostic bias in epidemiological studies. In future research, not only PTSD must be validly diagnosed, but also diagnostic information on duration, severity and subtypes of dementia should be taken into account. Only then the complex association between PTSD and cognitive deterioration and its impact on disease burden in dementia can be further elucidated.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statements
The authors confirm that there are no data used in this study.
Additional information
Funding
References
- Anderson, K. A., Fields, N. L., & Dobb, L. A. (2011). Understanding the impact of early-life trauma in nursing home residents. Journal of Gerontological Social Work, 54(8), 755–11. doi:10.1080/01634372.2011.596917.
- Avetyan, D., Zakharyan, R., Petrek, M., & Arakelyan, A. (2019). Telomere shortening in blood leukocytes of patients with posttraumatic stress disorder. Journal of Psychiatric Research, 111, 83–88. doi:10.1016/j.jpsychires.2019.01.018.
- Baker, D. G., Heppner, P., Afari, N., Nunnink, S., Kilmer, M., Simmons, A., … Bosse, B. (2009). Trauma exposure, branch of service, and physical injury in rela- tion to mental health among U.S. veterans returning from Iraq and Afghanistan. Military Medicine, 8, 773–778.
- Ball, V. L., Hudson, S., Davila, J., Morgan, R., Walder, A., Graham, D. P., … Kunik, M. E. (2009). Post-traumatic stress disorder and prediction of aggression in persons with dementia. International Journal of Geriatric Psychiatry, 24(11), 1285–1290. doi:10.1002/gps.2258.
- Bonanni, L., Franciotti, R., Martinotti, G., Vellante, F., Flacco, M. E., Di Giannantonio, M., … Onofrj, M. (2018). Post traumatic stress disorder heralding the onset of semantic frontotemporal dementia. Journal of Alzheimer’s Disease, 63(1), 203–215. doi:10.3233/JAD-171134.
- Burri, A., Maercker, A., Krammer, S., & Simmen-Janevska, K. (2013). Childhood trauma and PTSD symptoms increase the risk of cognitive impairment in a sample of former indentured child laborers in old age. PLoS One, 8(2), e57826. doi:10.1371/journal.pone.0057826.
- Çalışkan, G., & Oliver Stork, O. (2019). Hippocampal network oscillations at the interplay between innate anxiety and learned fear. Psychopharmacology, 236, 321–338. doi:10.1007/s00213-018-5109-z.
- Chopra, M. P., Zhang, H., Pless Kaiser, A., Moye, J. A., Llorente, M. D., Oslin, D. W., & Spiro, A., 3rd. (2014). PTSD is a chronic, fluctuating disorder affecting the mental quality of life in older adults. American Journal of Geriatric Psychiatry, 22(1), 86–97. doi:10.1016/j.jagp.2013.01.064.
- Clemens, V., Huber-Lang, M., Plener, P. L., Brahler, E., Brown, R. C., & Fegert, J. M. (2018). Association of child maltreatment subtypes and long-term physical health in a German representative sample. European Journal of Psychotraumatology, 9(1), 1510278. doi:10.1080/20008198.2018.1510278.
- Cohen-Mansfield, J. (1986). Agitated behaviors in the elderly II. Preliminary results in the cognitively deteriorated. Journal of the American Geriatrics Society, 34, 722–727. doi:10.1111/j.1532-5415.1986.tb04303.x.
- Cohen-Mansfield, J., Marx, M. S., & Rosenthal, A. S. (1989). A description of agitation in a nursing home. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 44, 77–84. doi:10.1093/geronj/44.3.M77.
- Cummings, J. L., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D. A., & Gornbein, J. (1994). The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology, 44(12), 2308–2314. doi:10.1212/WNL.44.12.2308.
- Dallam, D. L., Mellman, T. A., Bhatnagar, A., Nguyen, S., & Kurukumbi, M. (2011). Trauma reenactments in aging Veterans with dementia. Journal of the American Geriatrics Society, 59(4), 766–768. doi:10.1111/j.1532-5415.2011.03344.x.
- de Vries, G. J., & Olff, M. (2009). The lifetime prevalence of traumatic events and posttraumatic stress disorder in the Netherlands. Journal of Traumatic Stress, 22(4), 259–267. doi:10.1002/jts.20429.
- Delrue, N., & Plagnol, A. (2017). Post-traumatic stress disorder in Alzheimer’s disease. Counselling Psychology Review, 32(4), 58–69.
- Dieguez, D. J., & Barea-Rodriguez, E. J. (2004). Aging impairs the late phase of long-term potentiation at the medial perforant path-CA3 synapse in awake rats. Synapse, 52, 53–61. doi:10.1002/syn.20004.
- Doyle, C., Dunt, D., & Morris, P. (2014). Stress and dementia. International Psychogeriatrics, 26(8), 1235–1236. doi:10.1017/S1041610214001033.
- Flatt, J. D., Gilsanz, P., Quesenberry, C. P., Jr., Albers, K. B., & Whitmer, R. A. (2018). Post-traumatic stress disorder and risk of dementia among members of a health care delivery system. Alzheimer’s & Dementia, 14(1), 28–34. doi:10.1016/j.jalz.2017.04.014.
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “ Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:10.1016/0022-3956(75)90026-6.
- Furini, C., Myskiw, J., & Izquierdo, I. (2014). The learning of fear extinction. Neuroscience & Biobehavioral Reviews, 47, 670–683.
- Greenberg, M. S., Tanev, K., Marin, M. F., & Pitman, R. K. (2014). Stress, PTSD, and dementia. Alzheimer’s & Dementia, 10(3 Suppl), S155–165. doi:10.1016/j.jalz.2014.04.008.
- Hoge, C. W., Riviere, L. A., Wilk, J. E., Herrell, R. K., & Weathers, F. W. (2014). The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: A head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry, 1(4), 269–277. doi:10.1016/S2215-0366(14)70235-4.
- Jack, C. R., Jr., Petersen, R. C., Xu, Y., O’Brien, P. C., Smith, G. E., Ivnik, R. J., … Kokmen, E. (1998). Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology, 51, 993–999. doi:10.1212/WNL.51.4.993.
- Johnston, D. (2000). A series of cases of dementia presenting with PTSD symptoms in World War II combat veterans. Journal of the American Geriatrics Society, 48(1), 70–72. doi:10.1111/j.1532-5415.2000.tb03032.x.
- Josephs, K. A., Dickson, D. W., Tosakulwong, N., Weigand, S. D., Murray, M. E., Petrucelli, L., … Whitwell, J. L. (2017). Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer’s disease: A longitudinal retrospective study. Lancet Neurology, 16, 917–924. doi:10.1016/S1474-4422(17)30284-3.
- Jurica, P. J., Leitten, C. L., & Mattis, S. (2001). Dementia Rating Scale-2. Lutz, FL: Psychological Assessment Resources.
- Kang, B., Xu, H., & McConnell, E. S. (2019). Neurocognitive and psychiatric comorbidities of posttraumatic stress disorder among older veterans; a systemic review. International Journal of Geriatric Psychiatry, 34, 522–538. doi:10.1002/gps.5055.
- Kay, S. R., Opler, L. A., & Fiszbein, A. (1986). Significance of positive and negative syndromes in chronic schizophrenia. British Journal of Psychiatry, 149, 439–448. doi:10.1192/bjp.149.4.439.
- Kessler, R. C., Aguilar-Gaxiola, S., Alonso, J., Benjet, C., Bromet, E. J., Cardoso, G., … Koenen, K. C. (2017). Trauma and PTSD in the WHO World Mental Health Surveys. European Journal of Psychotraumatology, 8(sup5), 1353383. doi:10.1080/20008198.2017.1353383.
- Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., & Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry, 62, 593–602. doi:10.1001/archpsyc.62.6.593.
- Khouzam, H. R. (2008). Posttraumatic stress disorder and aging. Postgraduate Medicine, 120(3), 122–129. doi:10.3810/pgm.2008.09.1913.
- Lange, I., Goossens, L., Michielse, S., Bakker, J., Lissek, S., Papalini, S., … Schruers, K. (2017). Behavioral pattern separation and its link to the neural mechanisms of fear generalization. . Social Cognitive and Affective Neuroscience, 12(11), 1720–1729. doi:10.1093/scan/nsx104.
- Lanius, R. A., Frewen, P. A., Tursich, M., Jetly, R., & McKinnon, M. C. (2015, March). Restoring large-scale brain networks in PTSD and related disorders: A proposal for neuroscientifically-informed treatment interventions. European Journal of Psychotraumatology, 6(1), 27313. doi:10.3402/ejpt.v6.27313.
- Lee, L. (2019). PTSD and aging. PTSD Research Quarterly, 30(4), 1–15.
- Lohr, J. B., Palmer, B. W., Eidt, C. A., Aailaboyina, S., Mausbach, B. T., Wolkowitz, O. M., … Jeste, D. V. (2015). Is post-traumatic stress disorder associated with premature senescence? A review of the literature. American Journal of Geriatric Psychiatry, 23(7), 709–725. doi:10.1016/j.jagp.2015.04.001.
- Lopresto, D., Schipper, P., & Homberg, J. (2016). Neural circuits and mechanisms involved in fear generalization: Implications for the pathophysiology and treatment of posttraumatic stress disorder. Neuroscience & Biobehavioral Reviews, 60, 31–42. doi:10.1016/j.neubiorev.2015.10.009.
- Majer, R., Simon, V., Csiba, L., Kardos, L., Frecska, E., & Hortobágyi, T. (2019). Behavioural and psychological symptoms in neurocognitive disorders: Specific patterns in dementia subtypes. Open Medicine, 14(1), 307–316.
- Maren, S., & Holmes, A. (2015). Stress and fear extinction. Neuropsychopharmacology, 41, 58–79. doi:10.1515/med-2019-0028.
- Martinez-Clavera, C., James, S., Bowditch, E., & Kuruvilla, T. (2017). Delayed-onset post-traumatic stress disorder symptoms in dementia. Progress in Neurology and Psychiatry, 21, 26–31. doi:10.1002/pnp.477.
- Mawanda, F., Wallace, R. B., McCoy, K., & Abrams, T. E. (2017). PTSD, psychotropic medication use, and the risk of dementia among US Veterans: A retrospective cohort study. Journal of the American Geriatrics Society, 65(5), 1043–1050. doi:10.1111/jgs.14756.
- McEwen, B. (2013). The brain on stress: Toward an integrative approach to brain, body and behavior. Perspectives on Psychological Science, 1(6), 673–675. doi:10.1177/1745691613506907.
- Mellotte, H., Murphy, D., Rafferty, L., & Greenberg, N. (2017). Pathways into mental health care for UK veterans: A qualitative study. European Journal of Psychotraumatology, 8(1), 1389207. doi:10.1080/20008198.2017.1389207.
- Miller, M. W., Lin, A. P., Wolf, E. J., & Miller, D. R. (2017). Oxidative stress, inflammation, and neuroprogression in Chronic PTSD. Harvard Review of Psychiatry, 25, 1–13. doi:10.1097/HRP.0000000000000140.
- Mohlenhoff, B. S., O’Donovan, A., Weiner, M. W., & Neylan, T. C. (2017). Dementia risk in posttraumatic stress disorder: The relevance of sleep-related abnormalities in brain structure, amyloid, and inflammation. Current Psychiatry Reports, 19(11), 89. doi:10.1007/s11920-017-0835-1.
- Nelson, M. D., & Tumpap, A. M. (2017). Posttraumatic stress disorder symptom severity is associated with left hippocampal volume reduction: A meta-analytic study. CNS Spectrums, 22(4), 363–372. doi:10.1017/S1092852916000833.
- Nunes, P. V., Schwarzer, M. C., Leite, R. E. P., Ferretti-Rebustini, R. E. L., Pasqualucci, C. A., Nitrini, R., … Suemoto, C. K. (2019). Neuropsychiatric inventory in community-dwelling older adults with mild cognitive impairment and dementia. Journal of Alzheimer’s Disease, 68(2), 669–678. doi:10.3233/JAD-180641.
- Overall, J. E., & Gorham, D. R. (1962). The brief psychiatric rating scale. Psychological Reports, 10, 799–812.
- Patterson, C. (2018). World Alzheimer report 2018. London: Alzheimer’s Disease International.
- Pietrzak, R. H., Goldstein, R. B., Southwick, S. M., & Grant, B. F. (2012). Psychiatric comorbidity of full and partial posttraumatic stress disorder among older adults in the USA: Results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. American Journal of Geriatric Psychiatry, 20(5), 380–390. doi:10.1097/JGP.0b013e31820d92e7.
- Pinciotti, C. M., Bass, D. M., McCarthy, C. A., Judge, K. S., Wilson, N. L., Morgan, R. O., … Kunik, M. E. (2017). Negative consequences of family caregiving for veterans with PTSD and dementia. Journal of Nervous and Mental Disease, 205(2), 106–111. doi:10.1097/NMD.0000000000000560.
- Prince, M., Brycea, R., Albanese, A., Wimo, A., Ribeiro, W., & Ferri, C. P. (2013). The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s & Dementia, 9(1), 63–75.e62. doi:10.1016/j.jalz.2012.11.007.
- Qureshi, S. U., Kimbrell, T., Pyne, J. M., Magruder, K. M., Hudson, T. J., Petersen, N. J., … Kunik, M. E. (2010). Greater prevalence and incidence of dementia in older veterans with posttraumatic stress disorder. Journal of the American Geriatrics Society, 58(9), 1627–1633. doi:10.1111/j.1532-5415.2010.02977.x.
- Reynolds, K., Pietrzak, R. H., Mackenzie, C. S., Chou, K. L., & Sareen, J. (2016). Post-traumatic stress disorder across lifespan: Findings from a nationally representative survey. American Journal of Geriatric Psychiatry, 24(24), 81–93. doi:10.1016/j.jagp.2015.11.001.
- Richardson, L. K., Frueh, B. C., & Acierno, R. (2010). Prevalence estimates of combat-related PTSD: A critical review. Australian & New Zealand Journal of Psychiatry, 44, 4–19. doi:10.3109/00048670903393597.
- Roberts, A. L., Koenen, K., Chen, Q., Gilsanz, P., Mason, S. M., Prescott, J., … Kubzansky, L. D. (2017, May). Posttraumatic stress disorder and accelerated aging: PTSD and leukocyte telomere length in a sample of civilian women. Depression and Anxiety, 34(5), 391–400. doi:10.1002/da.22620.
- Rutten, B. P. F., Vermetten, E., Vinkers, C. H., Ursini, G., Daskalakis, N. P., Pishva, E., … Boks, M. P. M. (2018). Longitudinal analyses of the DNA methylome in deployed military servicemen identify susceptibility loci for post-traumatic stress disorder. Molecular Psychiatry, 23(5), 1145–1156. doi:10.1038/mp.2017.120.
- Scott, J. C., Matt, G. E., Wrocklage, K. M., Crnich, C., Jordan, J., Southwick, S. M., … Schweinsburg, B. C. (2015). A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bulletin, 141(1), 105–140. doi:10.1037/a0038039.
- Senanarong, V., Cummings, J. L., Fairbanks, L., Mega, M., Masterman, D. M., O’Connor, S. M., & Strickland, T. L. (2004). Agitation in Alzheimer’s disease is a manifestation of frontal lobe dysfunction. Dementia and Geriatric Cognitive Disorders, 17(1–2), 14–20. doi:10.1159/000074080.
- Sperling, W., Kreil, S. K., & Biermann, T. (2011). Posttraumatic stress disorder and dementia in Holocaust survivors. Journal of Nervous and Mental Disease, 199(3), 196–198. doi:10.1097/NMD.0b013e31820c71e0.
- van Achterberg, M. E., Rohrbaugh, R. M., & Southwick, S. M. (2001). Emergence of PTSD in trauma survivors with dementia. Journal of Clinical Psychiatry, 62(3), 206–207. doi:10.4088/JCP.v62n0312c.
- van Zelst, W. H., de Beurs, E., Beekman, A. T., van Dyck, R., & Deeg, D. D. (2006). Well-being, physical functioning, and use of health services in the elderly with PTSD and subthreshold PTSD. International Journal of Geriatric Psychiatry, 21(2), 180–188. doi:10.1002/gps.1448.
- Verma, S., Orengo, C. A., Maxwell, R., Kunik, M. E., Molinari, V. A., Vasterling, J. J., & Hale, D. D. (2001). Contribution of PTSD/POW history to behavioral disturbances in dementia. International Journal of Geriatric Psychiatry, 16(4), 356–360. doi:10.1002/gps.333.
- Wang, T. Y., Wei, H. T., Liou, Y. J., Su, T. P., Bai, Y. M., Tsai, S. J., … Chen, M. H. (2016). Risk for developing dementia among patients with posttraumatic stress disorder: A nationwide longitudinal study. Journal of Affective Disorders, 205, 306–310. doi:10.1016/j.jad.2016.08.013.
- Wells, G., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M., & Tugwell, P. (2000). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute.
- West, M. J. (1993). Regionally specific loss of neurons in the aging human hippocampus. Neurobiology of Aging, 14(4), 287–293. doi:10.1016/0197-4580(93)90113-P.
- Wilson, I. A., Gallagher, M., Eichenbaum, H., & Tanila, H. (2006). Neurocognitive aging: Prior memories hinder new hippocampal encoding. Trends in Neurosciences, 29, 662–670. doi:10.1016/j.tins.2006.10.002.
- Yaffe, K., Vittinghoff, E., Lindquist, K., Barnes, D., Covinsky, K. E., Neylan, T., … Marmar, C. (2010). Posttraumatic stress disorder and risk of dementia among US veterans. Archives of General Psychiatry, 67(6), 608–613. doi:10.1001/archgenpsychiatry.2010.61.
- Yassa, M. A., & Stark, C. E. L. (2011, July). Pattern separation in the hippocampus. Trends in Neurosciences, 34(10), 515–525. doi:10.1016/j.tins.2011.06.006.
Appendices
Appendix A.
Complete search strategy
PubMEd
S1: (dement* OR Alzheimer*).af
S2: (PTSD OR Post Traumatic Stress Disorder).af
S3: S1 AND S2
S4: limit S3 to ’Humans’ and ‘English language’ (filters)
25/11/2019 → 261 hits
PsycINFO
S1: dement# OR alzheimer
S2: PTSD OR Post Traumatic Stress Disorder
S3: S1 AND S2
S4: limit S3 to ’Human’ and ‘English language’ (limit to)
25/11/2019 → 117 hits
CINAHL
S1: dement# OR alzheimer
S2: PTSD OR Post Traumatic Stress Disorder
S3: S1 AND S2
S4: limit S3 to ‘English language’
25/11/2019 → 63 hits
Embase
S1: dement* OR Alzheimer*
S2: PTSD OR Post Traumatic Stress Disorder
S3: S1 AND S2
S4: limit S3 to ‘Human’ and ‘English language’
25/11/2019 → 419 hits
Appendix B
Rating of The Newcastle-Ottawa Scale (NOS):
* = 1 point
Ball (2009) (cohort)
Representativeness:
1 c (veterans- cohort selected at VA med center Houston)
2 * a (same cohort)
3 * b (RAS, SMAI)
Comparability:
1 * a (prior aggression), * b (antipsychotics)
Outcome:
1 d
2 * a (1 year)
3 * a (all completed)
Total : 6 points
Verma (2001) (cross-sectional)
Representativeness:
1 c (all patients admitted at VA – because of BPSD)
2 d (sample size not justified)
3 * a (same setting)
4 * b (partially validated: CMAI, the others not)
Comparability:
1 * a (matching MMSE), * b (age)
Outcome:
1 ** a (blinded to hypothesis)
2 * a (statistical tests is clearly described and appropriate)
Total : 6 points
Pinchiotti (2017) (cross-sectional):
Representativeness:
1 c (veterans, 60+, PDC-VA- living with partners)
2 d (sample size not justified)
3 * a (same setting)
4 * b (validated: in previous work)
Comparability:
1 * a, * b (all variables in a logistic regression model)
Outcome:
1 ** a (blinded to hypothesis)
2 * a (statistical tests is clearly described and appropriate)
Total : 7 points