ABSTRACT
Background: Childhood trauma (CT) is associated with altered brain anatomy. These neuroanatomical changes might be more pronounced in individuals with a psychiatric disorder. Post-traumatic stress disorder (PTSD) and borderline personality disorder (BPD) are more prevalent in individuals with a history of CT.
Objective: In this study, we examined limbic and total brain volumes in healthy women with and without a history of CT and in females with PTSD or BPD and a history of CT to see whether neuroanatomical changes are a function of psychopathology or CT.
Method: In total, 128 women (N = 70 healthy controls without CT, N = 25 healthy controls with CT, N = 14 individuals with PTSD, and N = 19 individuals with BPD) were recruited. A T1-weighted anatomical MRI was acquired from all participants for Freesurfer-based assessment of total brain, hippocampus, and amygdala volumes. Severity of CT was assessed with a clinical interview and the Childhood Trauma Questionnaire. Group differences in hippocampal and amygdala volumes (adjusted for total brain volume) and total brain volume (adjusted for height) were characterized by analysis of covariance.
Results: Volume of the total brain, hippocampus, and amygdala did not differ between the four groups (p > .05). CT severity correlated negatively with total brain volume across groups (r = −0.20; p = .029).
Conclusions: CT was associated with reduced brain volume but PTSD or BPD was not. The association between CT and reduced brain volume as a global measure of brain integrity suggests a common origin for vulnerability to psychiatric disorders later in life.
HIGHLIGHTS
No group differences (healthy women with and without CT, women with BPD and CT, women with PTSD and CT) in hippocampal, amygdala, and total brain volume.
Significant negative association between CT severity with total brain volume and depressive symptoms with hippocampal volumes.
Antecedentes: El trauma infantil (TI) se asocia con alteraciones en la anatomía cerebral. Estos cambios neuroanatómicos pueden ser más pronunciados en individuos con trastornos psiquiátricos. El trastorno de estrés postraumático (TEPT) y el trastorno de personalidad limítrofe (TPL) son más prevalentes en individuos con historia de TI.
Objetivo: En este estudio, examinamos los volúmenes límbico y cerebral total en mujeres sanas con y sin historia de TI y mujeres con TEPT o TPL e historia de TI para ver si los cambios neuroanatómicos son una función de la psicopatología o del TI.
Método: En total, 128 mujeres (N= 70 controles sanas sin TI, N= 25 controles sanas con TI, N= 14 individuos con TEPT y N= 19 individuos con TPL) fueron reclutadas. Se obtuvo una RNM anatómica ponderada en T1 de todas las participantes para la evaluación basada en Freesurfer de los volúmenes totales del cerebro, hipocampo y amígdala. La severidad del TI fue evaluada con una entrevista clínica y con el Cuestionario de Trauma Infantil. Las diferencias grupales en los volúmenes del hipocampo y amígdala (ajustadas por el volumen cerebral total) y el volumen cerebral total (ajustadas por altura) se caracterizaron mediante análisis de covarianza.
Resultados: El volumen total del cerebro, hipocampo y amígdala no difirieron entre los cuatro grupos (p > .05). La severidad del TI se correlacionó negativamente con el volumen cerebral total en todos los grupos (r = −0.20; p =.29).
Conclusiones: El TI estuvo asociado a un volumen cerebral reducido, pero el TEPT o TPL no se asociaron. La asociación entre TI y volumen cerebral disminuido como una medida global de la integridad cerebral sugiere un origen común de vulnerabilidad a los trastornos psiquiátricos más adelante en la vida.
背景:童年期创伤 (CT) 与大脑解剖结构的改变有关。这些神经解剖学变化在患有精神障碍的个体中可能更为明显。创伤后应激障碍 (PTSD) 和边缘型人格障碍 (BPD) 在有 CT 历史的个体中更为普遍。
目的:在本研究中, 我们考查了有和没有 CT 历史的健康女性以及有 CT 历史的PTSD 或 BPD 女性患者的边缘系统和总脑容量, 以确定神经解剖学变化是精神病理学还是 CT 的一个函数。
方法:总共招募了 128 名女性 (N= 70 名没有 CT 的健康对照, N= 25 名有 CT 的健康对照, N= 14 名PTSD 患者和 N= 19 名BPD 患者) 。从所有参与者处获取 T1 加权解剖 MRI, 用于基于 Freesurfer 的全脑, 海马和杏仁核体积评估。 CT 严重程度通过临床访谈和童年期创伤问卷进行评估。通过协方差分析来刻画海马和杏仁核体积 (控制总脑容量) 和总脑容量 (控制身高) 的组间差异。
结果:四组之间的总脑, 海马和杏仁核的体积没有差异 (p> .05) 。 CT 严重程度与各组的总脑容量呈负相关 (r = −0.20; p=.029) 。
结论:CT 与脑容量减少有关, 但 PTSD 或 BPD 则不然。 CT 与作为大脑完整性整体测量的脑容量减少之间的关联, 提示这是日后生活中易感精神障碍的一个常见原因
1. Introduction
During childhood, our brain is plastic and susceptible to environmental influences such as stress (Heim, Entringer, & Buss, Citation2019). Exposure to childhood trauma (CT), including neglect and sexual, physical, or emotional abuse is a risk factor for behavioural and emotional problems such as depression, suicide attempts, and drug addiction (Teicher, Samson, Anderson, & Ohashi, Citation2016). According to the latent vulnerability theory, environmental influences like CT interact with genetic, cellular, and hormonal mechanisms, creating phenotypes that increase the risk of psychiatric conditions later in life (McCrory & Viding, Citation2015). For example, CT can chronically activate the hypothalamus-pituitary-adrenal (HPA) axis, which in turn can increase glucocorticoid release. Exposure of the developing brain to elevated glucocorticoid levels can alter its development resulting in structural and functional alterations. The hippocampus and amygdala play an important role in our stress system and have a high density of glucocorticoid receptors. Preclinical and clinical studies have correlated chronic stress and elevated glucocorticoid release with impaired plasticity in the hippocampus and increased plasticity in the amygdala (Lupien, McEwen, Gunnar, & Heim, Citation2009; Sapolsky, Citation2003).
Accordingly, multiple studies have focused on the hippocampus and amygdala in adults with experiences of CT. In a meta-analysis, Calem et al. (Calem, Bromis, McGuire, Morgan, & Kempton, Citation2017) analysed results from 15 studies including 783 individuals with CT and 998 individuals without CT. First, they compared healthy controls with and without CT and found slightly reduced hippocampal volumes in healthy controls with CT. Second, they compared healthy controls without CT with participants with CT and additional psychopathology. Here, they found the hippocampal volume was more strongly reduced in individuals with CT and psychopathology, indicating that the relationship between CT and reduced hippocampal volume was more pronounced in individuals with additional psychopathology. Regarding the amygdala, findings are mixed and volume alterations seem to be related to comorbid psychopathology (Teicher et al., Citation2016). In their meta-analysis, Calem et al. found no association between CT and amygdala volume (Calem et al., Citation2017).
Importantly, stress and glucocorticoids do not only affect limbic brain areas. Glucocorticoids bind to two different receptors in the brain: glucocorticoid receptors which are ubiquitously distributed in the brain and mineralocorticoid receptors which are highly dense in the limbic system (Mifsud & Reul, Citation2018; Wang et al., Citation2013). Thus, CT exposure may have global effects on the developing brain. However, the few studies investigating the relationship between CT and total brain volume found no significant differences in brain volume between maltreated and non-maltreated individuals (Cohen et al., Citation2006; Schmahl, Vermetten, Elzinga, & Bremner, Citation2003).
Of note, several psychiatric disorders such as major depressive disorder (MDD) and post-traumatic stress disorder (PTSD) are associated with brain volume reductions (Thompson et al., Citation2020). CT and reduced brain volume play an especially important role in the aetiology of PTSD and borderline personality disorder (BPD).
With prevalence rates up to 63%, PTSD is a frequent sequela of CT (Hart & Rubia, Citation2012) and many studies have found reduced hippocampus and amygdala volumes in these individuals (Logue et al., Citation2018; O’Doherty, Chitty, Saddiqui, Bennett, & Lagopoulos, Citation2015). A meta-analysis found bilateral reduction of hippocampal and amygdala volumes in individuals with PTSD and CT compared with healthy controls (Ahmed-Leitao, Spies, van den Heuvel, & Seedat, Citation2016), but these group differences were not confirmed in other studies (Bremner et al., Citation1997; Pederson et al., Citation2004; Veer et al., Citation2015). This heterogeneity between studies may be due to variations in PTSD symptom severity (Bremner et al., Citation2003; Weniger, Lange, Sachsse, & Irle, Citation2008) or type and duration of CT (Teicher & Samson, Citation2016).
Most studies have found no association between CT-related PTSD and total brain volume (Bremner et al., Citation2003; Weniger et al., Citation2008). However, a recent meta-analysis reported lower total brain volumes in individuals with PTSD than in traumatized and non-traumatized healthy controls, but the role of CT was not analysed (Bromis, Calem, Reinders, Williams, & Kempton, Citation2018). Studies with monozygotic twins have shown that reduced hippocampal volumes may constitute a genetic risk factor for developing PTSD (Gilbertson et al., Citation2002). Both individuals with combat-related trauma-exposed PTSD and their healthy unexposed twins had smaller hippocampal volumes than trauma-exposed and unexposed twin pairs without PTSD.
It remains unclear whether alterations in brain volume are primarily a function of CT or a biomarker for PTSD later in life, and whether brain anatomy further changes as a function of psychopathology. Previous studies either used a non-traumatized healthy control group or a traumatized control group and did not control for current comorbid MDD, which has consistently been associated with smaller hippocampal volumes (Lorenzetti, Allen, Fornito, & Yücel, Citation2009; Schmaal et al., Citation2016).
BPD has a prevalence of up to 90% among individuals with CT (Battle et al., Citation2004; Zanarini, Frankenburg, Hennen, Reich, & Silk, Citation2006). Most studies on BPD have reported significantly reduced hippocampal volumes in individuals with BPD compared with healthy controls (Brambilla et al., Citation2004; Cattane, Rossi, Lanfredi, & Cattaneo, Citation2017; Driessen et al., Citation2000; Irle, Lange, & Sachsse, Citation2005; Nunes et al., Citation2009; Ruocco, Amirthavasagam, & Zakzanis, Citation2012; Schmahl et al., Citation2003), but there have been exceptions. For example, one study showed that smaller hippocampal volumes were only present in individuals with BPD who had lifetime PTSD (Kreisel et al., Citation2015). Another study showed that hippocampal volume was more reduced in individuals with BPD and CT (Brambilla et al., Citation2004) while another linked smaller hippocampal size to stronger trauma-related clinical symptoms (Irle et al., Citation2005). Results are also mixed regarding amygdala volumes in individuals with BPD; some studies have reported lower amygdala volumes in individuals with BPD than in healthy controls (Schmahl et al., Citation2003; van Elst et al., Citation2003, Citation2007; Weniger, Lange, Sachsse, & Irle, Citation2009) whereas others have reported no group differences (Brambilla et al., Citation2004; New et al., Citation2007). Schmahl et al. (Schmahl et al., Citation2003) found no differences in total brain volumes between females with BPD and healthy individuals. Importantly, individuals with BPD typically have various comorbid mental disorders, of which PTSD and MDD are the most prominent. No study has investigated a BPD cohort without comorbid disorders or compared BPD individuals with and without CT. Therefore, it is not possible to distinguish the effects of CT, BPD, and other mental disorders on brain volume.
Based on the existing literature, it is difficult to draw conclusions on neuroanatomical changes in individuals with PTSD or BPD and whether these changes are a function of psychopathology or preceding CT. Limitations of the existing literature are: 1) the inclusion of only one comparison group without psychopathology, either with CT or without CT and 2) the inclusion of various comorbid disorders, most importantly MDD, that may account for variation in neuroanatomy.
The aim of the current study was to investigate the association between neuroanatomical changes (i.e. total brain, hippocampal, and amygdala volumes) and CT as well as CT-related psychiatric disorders. We compared healthy women without CT and without mental disorders with a) women with CT but without any mental disorder, b) women with CT-related PTSD and c) women with BPD who reported CT. We decided to only include pre-menopausal women to ensure homogeneity as there seem to be significant gender differences regarding the biological stress response, e.g. the neuroprotective role of oestrogen in relation to stress (McEwen, Citation2002). We excluded participants with current comorbid MDD in all groups. We expected individuals with PTSD and BPD to have smaller hippocampal and amygdala volumes than healthy women with and without CT. In addition, we expected healthy women with CT to have smaller hippocampal volumes than healthy controls without CT (PTSD=BPD<HC+CT<HC). We did not formulate hypotheses on the association between amygdala volumes and CT without psychopathology or on group differences in total brain volume because earlier findings were inconsistent.
2. Methods and materials
2.1. Participants
The study sample consisted of 128 women who had participated in two functional neuroimaging studies (Golde et al., Citation2020; Metz et al., Citation2019). We recruited 70 healthy women without CT, 25 healthy women with CT, 14 individuals with PTSD and CT, and 19 individuals with BPD and CT. CT as an inclusion criterium was defined as a minimum of three traumatic events (sexual or physical) before the age of 18 and was thoroughly assessed with pre-screenings via phone and a clinical interview, i.e. the PTSD section of the German version of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (SCID-I) (Wittchen, Zaudig, & Fydrich, Citation1997). We then quantified self-reported severity of CT for correlational analyses with the Childhood Trauma Questionnaire (CTQ) (Bernstein, Fink, Handelsman, & Foote, Citation1998). The CTQ has five subscales: emotional abuse, physical abuse, emotional neglect, physical neglect, and sexual abuse. We measured the severity of PTSD symptoms with the Posttraumatic Diagnostic Scale (PDS) (Foa, Citation1995) and self-reported depressive symptoms with the Beck Depression Inventory II (BDI-II) (Beck, Steer, & Brown, Citation1996). Women with a current episode of MDD were excluded from the study. All participants were unmedicated.
Trained psychologists assessed current psychiatric disorders in all participants with the German version of the SCID-I and SCID-II (Wittchen et al., Citation1997). Any healthy controls with and without CT who had a current or lifetime diagnosis of any psychiatric disorder were excluded. Individuals with PTSD or BPD who had a current episode of MDD, schizophrenia, schizoaffective disorder, bipolar disorder, or anorexia disorder were also excluded. Further exclusion criteria for all participants were severe somatic diseases, diseases of the central nervous, endocrine, metabolic, or autoimmune system, current infections, pregnancy, a body mass index (BMI) above 30, left-handedness, intake of psychotropic medicine, and MRI contraindications.
Participants were recruited at the Charité Universitätsmedizin Berlin, Department of Psychiatry and Psychotherapy, Campus Benjamin Franklin. Healthy controls with and without CT were recruited via advertisement and received financial reimbursement. All participants provided informed written consent prior to participation. The study was in accordance with the latest version of the Declaration of Helsinki and approved by the local ethics committee.
2.2. MRI acquisition
We acquired T-1 weighted high-resolution magnetization prepared gradient-echo scans (MPRAGE) using a 3 Tesla Siemens Magnetron TrioTim scanner with a 12-channel radiofrequency head coil. Sagittal images were acquired with the following parameters: 176 slices, repetition time = 1900 ms, flip angle 9°, echo time = 2.52 ms, field of view = 256 mm, matrix size = 256 × 256, and voxel size = 1 × 1 × 1 mm3.
2.3. Image preprocessing
Image preprocessing and segmentation were performed with the freely available FreeSurfer ‘recon-all’ pipeline (v.6.0.0; https://surfer.nmr.mgh.harvard.edu). Recon-all processing incorporates all parts of the FreeSurfer reconstruction process, including motion correction, intensity normalization, removal of non-brain tissue, and automated Talairach transformation. The procedure uses a-priori acquired knowledge of spatial relationships between different brain structures to parcellate the brain into different cortical and subcortical structures as precisely as possible. The whole method was described in detail by Fischl et al. (Fischl et al., Citation2002). All recorded images were visually inspected. Data quality was evaluated by comparing asymmetries of the structures with findings in the literature (Woolard & Heckers, Citation2012). The current analyses focused on whole brain (grey and white matter) volume as well as hippocampal and amygdala volumes.
2.4. Statistical analysis
We computed one-way analyses of covariance (ANCOVA) to investigate group differences in hippocampal volume (total, left, right), amygdala volume (total, left, right), and total brain volume. Analyses of hippocampal and amygdala volumes were adjusted for total brain volume and analyses of total brain volume were controlled for height.
Partial correlations were calculated between self-reported severity of childhood trauma (CTQ score), PTSD symptoms (PDS score), depressive symptoms (BDI-II score), and hippocampal and amygdala volume (both adjusted for total brain volume) and total brain volume (adjusted for height).
All statistical analyses were performed using IBM SPSS statistics version 25.
3. Results
3.1. Sample characteristics
Sociodemographic and clinical data are presented in . The four groups did not differ in age, BMI, level of education, family status, smoking habits, and intake of hormonal contraceptives. All participants were unmedicated. As expected, the groups differed on clinical questionnaire scores (CTQ, PDS, and BDI-II). Healthy controls without CT had lower CTQ scores than healthy controls with CT, individuals with PTSD, and individuals with BPD did. Healthy controls without CT had a significantly lower PDS and BDI-II scores than healthy controls with CT did. Both patient groups reported significantly more severe PTSD and depressive symptoms than healthy controls with and without CT did (see ).
Table 1. Sample characteristics
The SCID interview revealed the following current comorbid diagnoses: bulimia nervosa (n = 1) and panic disorder (n = 2) in individuals with PTSD; and PTSD (n = 6), bulimia nervosa (n = 2), social phobia (n = 2), and substance abuse (n = 1) in individuals with BPD. Individuals with PTSD also reported that they were previously diagnosed with MDD (n = 6), alcohol abuse (n = 2), alcohol dependency (n = 1), substance abuse (n = 1), and eating disorder (n = 1). Individuals with BPD reported that they were previously diagnosed with MDD (n = 7), bulimia nervosa (n = 2), substance abuse (n = 5), anorexia nervosa (n = 1), alcohol abuse (n = 3), panic disorder (n = 1), alcohol dependency (n = 2), adjustment disorder (n = 1), agoraphobia (n = 1), and substance dependency (n = 1).
3.2. Hippocampus, amygdala, and total brain volume
We logarithmized the volumetric data and used these normally distributed data for the ANOVA analysis. We calculated standardized scores to detect outliers (± 3 SD) and excluded three healthy controls without CT. Therefore, data of 67 healthy controls were included in the final analyses.
The four groups (healthy controls without CT, healthy controls with CT, individuals with PTSD, and individuals with BPD) did not differ in total brain volume (p = .256). There were also no group differences in hippocampal volume (total: p= .922, left: p = .737, and right: p = .979) or amygdala volume (total: p = .809, left: p = .930, and right: p = .728). Data are presented in .
Table 2. Volumes of hippocampus, amygdala, total and intracranial brain
3.3. Dose-response relationship between CT severity and clinical symptoms and brain volumes
The CTQ total score, which reflects the severity of CT, was significantly associated with total brain volume (r = −0.20; p = .029; see ) across groups. Exploratory analyses of the CTQ subscales showed significant negative correlations between total brain volume and physical abuse (r = −0.20; p = .025) and emotional neglect (r = −0.18; p = .048), and a negative trend with sexual abuse (r = −0.17; p = .065) and physical neglect (r = −0.16; p = .086). Hippocampal and amygdala volumes did not correlate with CTQ scores (see Table S1).
Figure 1. Correlation of CTQ score and total brain volume
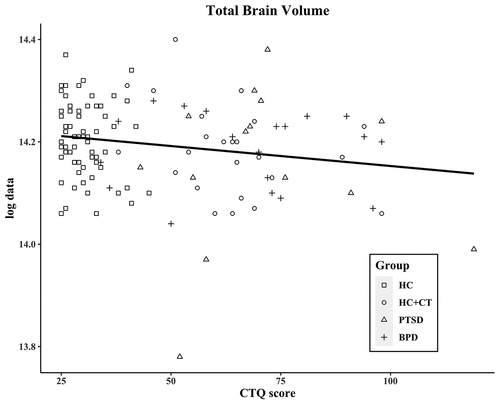
The PDS scores, which reflect the severity of PTSD, did not correlate significantly with brain volumes across groups.
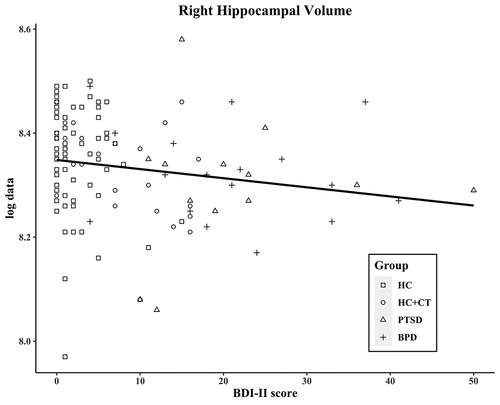
The BDI-II total score measures the severity of depressive symptoms and correlated negatively with total hippocampal volume (r = −0.19; p = .033), left hippocampal volume (r = −0.18; p = .050; see ), and right hippocampal volume (r = −0.20; p = .030; see ). These trends remained after controlling for total brain volume (total: r = −0.18; p = .053; left: r = −0.16; p = .088; right: r = −0.18; p = .051; see Table S2).
Figure 2. Correlation of BDI-II score and of left hippocampal volume
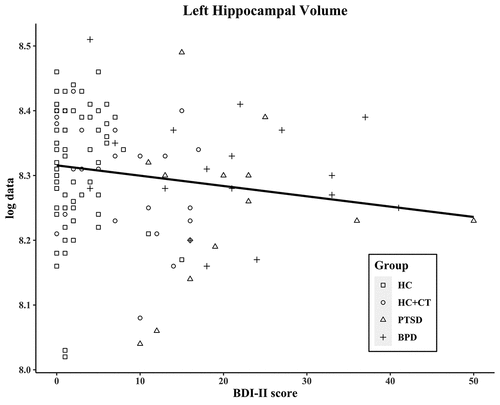
Figure 3. Correlation of BDI-II score and of right hippocampal volume
We conducted a linear regression analysis to test whether CT moderates the association between depressive symptoms and hippocampal volume. The results showed no significant interaction effect of CTQ and BDI-II scores on left, right, and total hippocampal volumes.
There were no significant correlations between CTQ, PDS, and BDI-II scores and amygdala volumes. As CTQ, BDI-II, and PDS scores were not normally distributed, we reran our correlational analyses using Spearman’s rho correlation coefficient (De Winter, Gosling, & Potter, Citation2016). The results did not change. All correlational analyses are presented Tables S1 and S2.
4. Discussion
In the present study, we examined whether women with and without PTSD or BPD who experienced CT had reduced limbic brain volumes and total brain volumes compared with healthy women who did not experience CT. We found no differences in hippocampal, amygdala, and total brain volumes between individuals with PTSD and CT, individuals with BPD and CT, healthy controls with CT, and healthy controls without CT. The self-reported severity of CT (CTQ score) associated negatively with total brain volume across groups, while self-reported depressive symptoms (BDI-II score) associated negatively with left, right, and total hippocampal volumes. Here, we discuss our two main findings: the lack of differences in brain volumes between the clinical groups and the dose-response relationships between CT severity and total brain volume and between severity of depressive symptoms and hippocampal volumes.
4.1. No differences in limbic and total brain volumes
We observed no group differences in hippocampal, amygdala, and total brain volumes. We did not confirm our hypothesis that hippocampal and amygdala volumes would be lower in individuals with PTSD and BPD than in healthy women with and without CT, nor our hypothesis that hippocampal volumes would be lower in healthy women with CT than in healthy women without CT.
In contrast with our findings, previous research has revealed reduced hippocampal volumes in healthy individuals with CT compared with healthy individuals without CT (Calem et al., Citation2017). Furthermore, meta-analyses focusing on associations between CT, psychopathology (PTSD, BPD), and neuroanatomy have found reduced hippocampal and amygdala volumes in individuals with CT-related PTSD (Ahmed-Leitao et al., Citation2016) and in individuals with BPD (Ruocco et al., Citation2012). However, these earlier studies included individuals with various comorbid psychiatric disorders, including MDD. It is possible that we did not observe similar significant group differences in hippocampal and amygdala volumes between our groups because we excluded individuals with current comorbid MDD from all groups. This explanation is in line with our finding that a dose-response relationship exists between self-reported depressive symptoms and hippocampal volumes. Future studies should investigate whether comorbid MDD explains the reported relationship between CT, psychopathology, and reduced limbic brain volume. In agreement with our findings, previous studies have also reported no associations between CT, PTSD, BPD and total brain volume (Bremner et al., Citation2003; Cohen et al., Citation2006; Schmahl et al., Citation2003; Weniger et al., Citation2008).
4.2. Dose-response relationships between self-reported CT severity and total brain volumes
Overall, we found a negative association between self-reported CT severity (CTQ scores) and total brain volume. Across groups, total brain volume was smaller in individuals who reported more severe CT. However, no association between self-reported CT severity and hippocampal and amygdala volumes was observed.
Previous studies have reported an association between CT and reduced hippocampal volumes (Calem et al., Citation2017); however, the present results suggest a dose-response relationship between CT and total brain volume. Preclinical and clinical studies have shown dose-response relationships between stress in early life and biological stress markers, including corticotrophin-releasing hormone in cerebral spinal fluid, cortisol, and immune mediators (Heim & Nemeroff, Citation2001; Plotsky et al., Citation2005; Syed & Nemeroff, Citation2017). Research has shown that CT can modify the expression of specific genes, such as the serotonin transporter gene (5-HTTLPR; Caspi et al., Citation2003; Karg, Burmeister, Shedden, & Sen, Citation2011) and variants of FKBP5 (Binder et al., Citation2008; Klengel et al., Citation2013). As a result, a vulnerable phenotype can develop that includes an altered response of the HPA axis, our body’s main stress system (Frodl & O’Keane, Citation2013; Hornung & Heim, Citation2014). Consequently, elevated levels of glucocorticoids are released that can modify brain functions by binding to mineralocorticoid and glucocorticoid receptors.
The vulnerability hypothesis suggests that neuroplasticity is high during childhood, which makes the developing brain more vulnerable to prolonged endocrine and immune stress responses (Lupien et al., Citation2009). Distinct periods of sensitivity have been identified during which specific brain structures are particularly vulnerable to CT (Heim & Binder, Citation2012; Teicher & Samson, Citation2016). In our cohort, CT occurred from birth until early adulthood. This wide age range of CT exposure could explain the significant association between CT severity and reduction in total brain volume, as CT may have affected different brain regions depending on when the trauma occurred. Interestingly, we can relate our findings to the debatable distinction between BPD and PTSD (Cloitre, Garvert, Weiss, Carlson, & Bryant, Citation2014; Giourou et al., Citation2018). Amad and colleagues (Amad, Radua, Vaiva, Williams, & Fovet, Citation2019; Amad, Ramoz, Thomas, & Gorwood, Citation2016) suggested describing the relationship between PTSD and BPD based on an age-dependent neuroplasticity framework. It postulates that identical traumatic experiences differentially affect brain plasticity based on age of exposure. They propose that CT during early childhood increases the risk for BPD whereas trauma later in life increases the risk for PTSD.
Our results support the idea that reduced brain volume is a function of CT rather than psychopathology. Future research should further investigate exact relationships between age of exposure to CT and volume reductions in particular brain areas as well as distinct psychiatric diagnoses.
4.3. Dose-response relationships between self-reported depressive symptoms and hippocampal volumes
We observed a negative correlation between self-reported depressive symptoms (BDI-II score) and hippocampal volume reduction, with more severe depressive symptoms being associated with smaller hippocampal volumes. This was still a trend after controlling for total brain volume, indicating a relationship between depressive symptoms and hippocampal volume reductions, even though we excluded individuals with current comorbid MDD.
The neurotoxicity hypothesis (Lupien et al., Citation2009) and glucocorticoid vulnerability hypothesis (Amad et al., Citation2019) suggest that prolonged exposure to chronic stress increases the vulnerability of hippocampal neurons, eventually resulting in hippocampal atrophy. The hippocampus has a high density of glucocorticoid receptors and is exposed to elevated glucocorticoid levels during prolonged stress (Lupien et al., Citation2009; Sapolsky, Citation2003). This makes the hippocampus more vulnerable to neurotoxic or metabolic challenges (Conrad, Citation2008). CT alters the HPA axis and elevates glucocorticoid levels (Frodl & O’Keane, Citation2013; Hornung & Heim, Citation2014); however, we found no correlation between CT and hippocampal volume and no significant interaction effect of CT and depressive symptoms on hippocampal volume. Depression has also been linked to HPA-axis dysregulation and elevated glucocorticoid levels (Dean & Keshavan, Citation2017). Our results may suggest that a subgroup of individuals who were exposed to CT are more vulnerable to developing depressive symptoms and reductions in hippocampal volume. Heim et al. (Heim, Newport, Mletzko, Miller, & Nemeroff, Citation2008) proposed that CT might interact with genetic factors (such as the s allele of 5-HTTLPR and polymorphisms in BDNF) to create a vulnerable phenotype with neuroanatomical changes such as hippocampal volume reductions. Under chronic stress, individuals with this phenotype are assumed to be more vulnerable to developing depressive symptoms. This is in accordance with findings from a landmark twin study suggesting that smaller hippocampal volume may be a risk factor for developing PTSD after trauma exposure (Gilbertson et al., Citation2002).
4.4. Strengths and limitations
A strength of our study is that we carefully selected our participants and included individuals with and without psychopathology who were exposed to severe, repeated early life trauma. All participants underwent structured clinical interviews to exclude current comorbid MDD and healthy controls were excluded if there was a history of lifetime psychopathology. Also, all participants were unmedicated. This carefully selected, homogenous study population enabled us to compare CT-related neuroanatomical alterations and psychopathology-related neuroanatomical alterations. We only included pre-menopausal women to ensure homogeneity. Because of sex differences in the stress response and the possible neuroprotective effects of oestrogen (McEwen, Citation2002), the present results may not be transferable to males or to post-menopausal females.
Although we excluded individuals with current comorbid MDD, other comorbidities such as substance abuse and lifetime comorbidities (including lifetime MDD) may have influenced the results (Thompson et al., Citation2020). The major limitation of the current study is that our psychopathology subgroups were small and six participants with BPD had comorbid PTSD. The small sample size may explain the negative results as our sample may have been underpowered to detect small differences between groups. Furthermore, we have to be cautious with interpretations of our correlational analyses as we conducted multiple comparisons. Another limitation is that we used a retrospective self-report to quantify CT. Therefore, biases such as imperfect memory and participants’ motivation to report CT may have influenced the results (Danese, Citation2020). Future research should aim to replicate the findings with a larger sample size and apply a multi-source approach to assess CT more reliably (Sierau et al., Citation2017). Future research should also aim to collect data on other psychological constructs (e.g. severity of emotional dysregulation and self-harm) to investigate the relationship between certain symptom clusters and neuroanatomical correlates.
4.5. Conclusion
The aim of the current study was to investigate the relationship between CT, trauma-related psychiatric disorders, and brain volume. We found no group differences in volume of the hippocampus, amygdala, and total brain. However, CT severity was negatively associated with total brain volume and depressive symptoms were negatively related to hippocampal volumes. Based on our results, we have two conclusions. First, the association between CT and total brain volume (as a measure of brain integrity in various clinical conditions) suggests a common origin for vulnerability to trauma-related psychiatric disorders such as PTSD and BPD during early life. Second, depressive symptoms were associated with a smaller hippocampal volume, suggesting that reductions in hippocampal volume may be a component of a vulnerable depressive phenotype. This phenotype may be a consequence of CT and may include comorbid PTSD and BPD symptoms. In light of missing group differences, our findings indicate that brain volume alterations are a cross-diagnostic feature that are related to aetiological (CT) and symptomatic factors. The finding adds to the existing debate over common origins and common underlying structures of distinct psychiatric disorders (Wigman et al., Citation2015).
Supplemental Material
Download ()Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request (please contact Catarina Rosada; [email protected]). The data are not publicly available due to legal and ethical restrictions.
Disclosure statement
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ahmed-Leitao, F., Spies, G., van den Heuvel, L., & Seedat, S. (2016). Hippocampal and amygdala volumes in adults with posttraumatic stress disorder secondary to childhood abuse or maltreatment: A systematic review. Psychiatry Research: Neuroimaging, 256, 33–12. doi:10.1016/j.pscychresns.2016.09.008
- Amad, A., Radua, J., Vaiva, G., Williams, S. C., & Fovet, T. (2019). Similarities between borderline personality disorder and post traumatic stress disorder: Evidence from resting-state meta-analysis. Neuroscience & Biobehavioral Reviews, 105, 52–59. doi:10.1016/j.neubiorev.2019.07.018
- Amad, A., Ramoz, N., Thomas, P., & Gorwood, P. (2016). The age-dependent plasticity highlights the conceptual interface between borderline personality disorder and PTSD. European Archives of Psychiatry and Clinical Neuroscience, 266(4), 373–375. doi:10.1007/s00406-015-0648-3
- Battle, C. L., Shea, M. T., Johnson, D. M., Yen, S., Zlotnick, C., Zanarini, M. C., & Morey, L. C. (2004). Childhood maltreatment associated with adult personality disorders: Findings from the Collaborative Longitudinal Personality Disorders Study. Journal of Personality Disorders, 18(2), 193–211. doi:10.1521/pedi.18.2.193.32777
- Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation.
- Bernstein, D. P., & Fink, L. (1998). Childhood trauma questionnaire: A retrospective self-report manual. San Antonio, TX: The Psychological Corporation.
- Binder, E. B., Bradley, R. G., Liu, W., Epstein, M. P., Deveau, T. C., Mercer, K. B., … & Ressler, K. J. (2008). Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama, 299(11), 1291–1305. doi:10.1001/jama.299.11.1291
- Brambilla, P., Soloff, P. H., Sala, M., Nicoletti, M. A., Keshavan, M. S., & Soares, J. C. (2004). Anatomical MRI study of borderline personality disorder patients. Psychiatry Research: Neuroimaging, 131(2), 125–133. doi:10.1016/j.pscychresns.2004.04.003
- Bremner, J. D., Randall, P., Vermetten, E., Staib, L., Bronen, R. A., Mazure, C., & Charney, D. S. (1997). Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biological Psychiatry, 41(1), 23–32. doi:10.1016/S0006-3223(96)00162-X
- Bremner, J. D., Vythilingam, M., Vermetten, E., Southwick, S. M., McGlashan, T., Nazeer, A., & Charney, D. S. (2003). MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. American Journal of Psychiatry, 160(5), 924–932. doi:10.1176/appi.ajp.160.5.924
- Bromis, K., Calem, M., Reinders, A. A., Williams, S. C., & Kempton, M. J. (2018). Meta-analysis of 89 structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. American Journal of Psychiatry, 175(10), 989–998. doi:10.1176/appi.ajp.2018.17111199
- Calem, M., Bromis, K., McGuire, P., Morgan, C., & Kempton, M. J. (2017). Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. NeuroImage: Clinical, 14, 471–479. doi:10.1016/j.nicl.2017.02.016
- Caspi, A., Sugden, K., Moffitt, T. E., Taylor, A., Craig, I. W., Harrington, H., … & Poulton, R. (2003). Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science, 301(5631), 386–389. doi:10.1126/science.1083968
- Cattane, N., Rossi, R., Lanfredi, M., & Cattaneo, A. (2017). Borderline personality disorder and childhood trauma: Exploring the affected biological systems and mechanisms. BMC Psychiatry, 17(1), 1–14. doi:10.1186/s12888-017-1383-2
- Cloitre, M., Garvert, D. W., Weiss, B., Carlson, E. B., & Bryant, R. A. (2014). Distinguishing PTSD, complex PTSD, and borderline personality disorder: A latent class analysis. European Journal of Psychotraumatology, 5(1), 25097. doi:10.3402/ejpt.v5.25097
- Cohen, R. A., Grieve, S., Hoth, K. F., Paul, R. H., Sweet, L., Tate, D., & Williams, L. M. (2006). Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry, 59(10), 975–982. doi:10.1016/j.biopsych.2005.12.016
- Conrad, C. D. (2008). Chronic stress-induced hippocampal vulnerability: The glucocorticoid vulnerability hypothesis. Reviews in the Neurosciences, 19(6), 395–411. doi:10.1515/REVNEURO.2008.19.6.395
- Danese, A. (2020). Annual Research Review: Rethinking childhood trauma‐new research directions for measurement, study design and analytical strategies. Journal of Child Psychology and Psychiatry, 61(3), 236–250. doi:10.1111/jcpp.13160
- De Winter, J. C., Gosling, S. D., & Potter, J. (2016). Comparing the Pearson and Spearman correlation coefficients across distributions and sample sizes: A tutorial using simulations and empirical data. Psychological Methods, 21(3), 273. doi:10.1037/met0000079
- Dean, J., & Keshavan, M. (2017). The neurobiology of depression: An integrated view. Asian Journal of Psychiatry, 27, 101–111. doi:10.1016/j.ajp.2017.01.025
- Driessen, M., Herrmann, J., Stahl, K., Zwaan, M., Meier, S., Hill, A., … Petersen, D. (2000). Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry, 57(12), 1115–1122. doi:10.1001/archpsyc.57.12.1115
- Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., & Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. doi:10.1016/S0896-6273(02)00569-X
- Foa, E. B. (1995). The posttraumatic diagnostic scale (PDS) manual. Minneapolis, MN: National Computer Systems. 1-5.
- Frodl, T., & O’Keane, V. (2013). How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiology of Disease, 52, 24–37. doi:10.1016/j.nbd.2012.03.012
- Gilbertson, M. W., Shenton, M. E., Ciszewski, A., Kasai, K., Lasko, N. B., Orr, S. P., & Pitman, R. K. (2002). Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience, 5(11), 1242–1247. doi:10.1038/nn958
- Giourou, E., Skokou, M., Andrew, S. P., Alexopoulou, K., Gourzis, P., & Jelastopulu, E. (2018). Complex posttraumatic stress disorder: The need to consolidate a distinct clinical syndrome or to reevaluate features of psychiatric disorders following interpersonal trauma? World Journal of Psychiatry, 8(1), 12. doi:10.5498/wjp.v8.i1.12
- Golde, S., Wingenfeld, K., Riepenhausen, A., Schröter, N., Fleischer, J., Prüssner, J., … Otte, C. (2020). Healthy women with severe early life trauma show altered neural facilitation of emotion inhibition under acute stress. Psychological Medicine, 50(12), 2075–2084. doi:10.1017/S0033291719002198
- Hart, H., & Rubia, K. (2012). Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience, 6(52). doi:10.3389/fnhum.2012.00052
- Heim, C. M., & Binder, E. B. (2012). Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental Neurology, 233(1), 102–111. doi:10.1016/j.expneurol.2011.10.032
- Heim, C. M., Entringer, S., & Buss, C. (2019). Translating basic research knowledge on the biological embedding of early-life stress into novel approaches for the developmental programming of lifelong health. Psychoneuroendocrinology, 105, 123–137. doi:10.1016/j.psyneuen.2018.12.011
- Heim, C. M., & Nemeroff, C. B. (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry, 49(12), 1023–1039. doi:10.1016/S0006-3223(01)01157-X
- Heim, C. M., Newport, D. J., Mletzko, T., Miller, A. H., & Nemeroff, C. B. (2008). The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology, 33(6), 693–710. doi:10.1016/j.psyneuen.2008.03.008
- Hornung, O. P., & Heim, C. M. (2014). Gene-environment interactions and intermediate phenotypes: Early trauma and depression. Frontiers in Endocrinology, 5, 14. doi:10.3389/fendo.2014.00014
- Irle, E., Lange, C., & Sachsse, U. (2005). Reduced size and abnormal asymmetry of parietal cortex in women with borderline personality disorder. Biological Psychiatry, 57(2), 173–182. doi:10.1016/j.biopsych.2004.10.004
- Karg, K., Burmeister, M., Shedden, K., & Sen, S. (2011). The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry, 68(5), 444–454. doi:10.1001/archgenpsychiatry.2010.189
- Klengel, T., Mehta, D., Anacker, C., Rex-Haffner, M., Pruessner, J. C., Pariante, C. M., & Binder, E. B. (2013). Allele-specific FKBP5 DNA demethylation mediates gene–childhood trauma interactions. Nature Neuroscience, 16(1), 33–41. doi:10.1038/nn.3275
- Kreisel, S. H., Labudda, K., Kurlandchikov, O., Beblo, T., Mertens, M., Thomas, C., … Driessen, M. (2015). Volume of hippocampal substructures in borderline personality disorder. Psychiatry Research: Neuroimaging, 231(3), 218–226. doi:10.1016/j.pscychresns.2014.11.010
- Logue, M. W., van Rooij, S. J., Dennis, E. L., Davis, S. L., Hayes, J. P., Stevens, J. S., & Morey, R. A. (2018). Smaller hippocampal volume in posttraumatic stress disorder: A multisite ENIGMA-PGC study: Subcortical volumetry results from posttraumatic stress disorder consortia. Biological Psychiatry, 83(3), 244–253. doi:10.1016/j.biopsych.2017.09.006
- Lorenzetti, V., Allen, N. B., Fornito, A., & Yücel, M. (2009). Structural brain abnormalities in major depressive disorder: A selective review of recent MRI studies. Journal of Affective Disorders, 117(1–2), 1–17. doi:10.1016/j.jad.2008.11.021
- Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. M. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. doi:10.1038/nrn2639
- McCrory, E. J., & Viding, E. (2015). The theory of latent vulnerability: Reconceptualizing the link between childhood maltreatment and psychiatric disorder. Development and Psychopathology, 27(2), 493–505. doi:10.1017/S0954579415000115
- McEwen, B. S. (2002). Sex, stress and the hippocampus: Allostasis, allostatic load and the aging process. Neurobiology of Aging, 23(5), 921–939. doi:10.1016/S0197-4580(02)00027-1
- Metz, S., Fleischer, J., Gärnter, M., Golde, S., Duesenberg, M., Roepke, S., … Wingenfeld, K. (2019). Effects of hydrocortisone on autobiographical memory retrieval in patients with posttraumatic stress disorder and borderline personality disorder: The role of childhood trauma. Neuropsychopharmacology, 44(12), 2038–2044. doi:10.1038/s41386-019-0459-8
- Mifsud, K. R., & Reul, J. M. (2018). Mineralocorticoid and glucocorticoid receptor-mediated control of genomic responses to stress in the brain. Stress, 21(5), 389–402. doi:10.1080/10253890.2018.1456526
- New, A. S., Hazlett, E. A., Buchsbaum, M. S., Goodman, M., Mitelman, S. A., Newmark, R., & Siever, L. J. (2007). Amygdala–prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology, 32(7), 1629–1640. doi:10.1038/sj.npp.1301283
- Nunes, P. M., Wenzel, A., Borges, K. T., Porto, C. R., Caminha, R. M., & De Oliveira, I. R. (2009). Volumes of the hippocampus and amygdala in patients with borderline personality disorder: A meta-analysis. Journal of Personality Disorders, 23(4), 333–345. doi:10.1521/pedi.2009.23.4.333
- O’Doherty, D. C., Chitty, K. M., Saddiqui, S., Bennett, M. R., & Lagopoulos, J. (2015). A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Research: Neuroimaging, 232(1), 1–33. doi:10.1016/j.pscychresns.2015.01.002
- Pederson, C. L., Maurer, S. H., Kaminski, P. L., Zander, K. A., Peters, C. M., Stokes‐Crowe, L. A., & Osborn, R. E. (2004). Hippocampal volume and memory performance in a community‐based sample of women with posttraumatic stress disorder secondary to child abuse. Journal of Traumatic Stress: Official Publication of the International Society for Traumatic Stress Studies, 17(1), 37–40. doi:10.1023/B:JOTS.0000014674.84517.46
- Plotsky, P. M., Thrivikraman, K. V., Nemeroff, C. B., Caldji, C., Sharma, S., & Meaney, M. J. (2005). Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology, 30(12), 2192–2204. doi:10.1038/sj.npp.1300769
- Ruocco, A. C., Amirthavasagam, S., & Zakzanis, K. K. (2012). Amygdala and hippocampal volume reductions as candidate endophenotypes for borderline personality disorder: A meta-analysis of magnetic resonance imaging studies. Psychiatry Research: Neuroimaging, 201(3), 245–252. doi:10.1016/j.pscychresns.2012.02.012
- Sapolsky, R. M. (2003). Stress and plasticity in the limbic system. Neurochemical Research, 28(11), 1735–1742. doi:10.1023/A:1026021307833
- Schmaal, L., Veltman, D. J., van Erp, T. G., Sämann, P. G., Frodl, T., Jahanshad, N., & Hibar, D. P. (2016). Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA Major Depressive Disorder working group. Molecular Psychiatry, 21(6), 806–812. doi:10.1038/mp.2015.69
- Schmahl, C. G., Vermetten, E., Elzinga, B. M., & Bremner, J. D. (2003). Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Research: Neuroimaging, 122(3), 193–198. doi:10.1016/S0925-4927(03)00023-4
- Sierau, S., Brand, T., Manly, J. T., Schlesier-Michel, A., Klein, A. M., Andreas, A., … White, L. O. (2017). A multisource approach to assessing child maltreatment from records, caregivers, and children. Child Maltreatment, 22(1), 45–57. doi:10.1177/1077559516675724
- Syed, S. A., & Nemeroff, C. B. (2017). Early life stress, mood, and anxiety disorders. Chronic Stress, 1, 1–16. doi:10.1177/2470547017694461
- Teicher, M. H., & Samson, J. A. (2016). Annual research review: Enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry, 57, 241–266. doi:10.1111/jcpp.12507
- Teicher, M. H., Samson, J. A., Anderson, C. M., & Ohashi, K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17(10), 652–666. doi:10.1038/nrn.2016.111
- Thompson, P. M., Jahanshad, N., Ching, C. R., Salminen, L. E., Thomopoulos, S. I., Bright, J., … Zelman, V. (2020). ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Translational Psychiatry, 10(1), 1–28. doi:10.1038/s41398-020-0705-1
- van Elst, L. T., Hesslinger, B., Thiel, T., Geiger, E., Haegele, K., Lemieux, L., … Ebert, D. (2003). Frontolimbic brain abnormalities in patients with borderline personality disorder: A volumetric magnetic resonance imaging study. Biological Psychiatry, 54(2), 163–171. doi:10.1016/S0006-3223(02)01743-2
- van Elst, L. T., Ludaescher, P., Thiel, T., Büchert, M., Hesslinger, B., Bohus, M., … Lieb, K. (2007). Evidence of disturbed amygdalar energy metabolism in patients with borderline personality disorder. Neuroscience Letters, 417(1), 36–41. doi:10.1016/j.neulet.2007.02.071
- Veer, I. M., Oei, N. Y., Van Buchem, M. A., Spinhoven, P., Elzinga, B. M., & Rombouts, S. A. (2015). Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Research: Neuroimaging, 233(3), 436–442. doi:10.1016/j.pscychresns.2015.07.016
- Wang, Q., Van Heerikhuize, J., Aronica, E., Kawata, M., Seress, L., Joels, M., & Lucassen, P. J. (2013). Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiology of Aging, 34(6), 1662–1673. doi:10.1016/j.neurobiolaging.2012.11.019
- Weniger, G., Lange, C., Sachsse, U., & Irle, E. (2008). Amygdala and hippocampal volumes and cognition in adult survivors of childhood abuse with dissociative disorders. Acta Psychiatrica Scandinavica, 118(4), 281–290. doi:10.1111/j.1600-0447.2008.01246.x
- Weniger, G., Lange, C., Sachsse, U., & Irle, E. (2009). Reduced amygdala and hippocampus size in trauma-exposed women with borderline personality disorder and without posttraumatic stress disorder. Journal of Psychiatry & Neuroscience: JPN, 34, 383–388. doi:10.5167/uzh-28387
- Wigman, J. T. W., Van Os, J., Borsboom, D., Wardenaar, K. J., Epskamp, S., Klippel, A., … Wichers, M. (2015). Exploring the underlying structure of mental disorders: Cross-diagnostic differences and similarities from a network perspective using both a top-down and a bottom-up approach. Psychological Medicine, 45(11), 2375–2387. doi:10.1017/S0033291715000331
- Wittchen, H. U., Zaudig, M., & Fydrich, T. (1997): Skid. Strukturiertes klinisches Interview für DSM-IV. Achse I und II. Handanweisung.
- Woolard, A. A., & Heckers, S. (2012). Anatomical and functional correlates of human hippocampal volume asymmetry. Psychiatry Research: Neuroimaging, 201(1), 48–53. doi:10.1016/j.pscychresns.2011.07.016
- Zanarini, M. C., Frankenburg, F. R., Hennen, J., Reich, D. B., & Silk, K. R. (2006). Prediction of the 10-year course of borderline personality disorder. American Journal of Psychiatry, 163(5), 827–832. doi:10.1176/ajp.2006.163.5.827
