ABSTRACT
Background: Early life maltreatment is a risk factor for psychiatric disorders, including post-traumatic stress disorder (PTSD). Post-traumatic stress disorder is a severe and heterogeneous disorder with fluctuating states of emotional over- and undermodulation, including hypervigilance, dissociation, and emotion regulation deficits. The perception and regulation of emotions have been linked to interoception, the cortical representation and sensing of inner bodily processes. Although first therapeutic approaches targeting bodily sensations have been found effective in patients with PTSD, and deficits in interoceptive signal representation have been reported in other trauma-related disorders, such as borderline personality disorder (BPD), the role of interoception remains largely unexplored for PTSD.
Objective: The objective was to investigate the cortical representation of cardiac interoceptive signals in patients with PTSD and its associations with early life maltreatment, trait dissociation, and emotion dysregulation.
Methods: Twenty-four medication-free patients with PTSD and 31 healthy controls (HC) completed a 5-min resting electrocardiogram (ECG) with parallel electroencephalogram (EEG). Heartbeat evoked potential (HEP) amplitudes as a measure for cortical representation of cardiac interoceptive signals were compared between groups and correlated with self-report questionnaires.
Results: We did not find significantly different mean HEP amplitudes in patients with PTSD compared to HC, although HEPs of patients with PTSD were descriptively higher. No significant associations between mean HEP amplitudes and early life maltreatment, trait dissociation or emotion dysregulation were obtained within the groups.
Conclusion: The current finding does not indicate deficits in interoceptive signal representation at rest in individuals with PTSD. Whether patients with PTSD show altered HEP modulations during emotion regulation tasks and might benefit from therapeutic approaches aiming at changing the perception of bodily signals, needs to be investigated in future studies.
HIGHLIGHTS
Patients with PTSD did not show reduced, but descriptively higher, heartbeat-evoked potentials at rest which does not indicate deficits in interoceptive signal representation.
Antecedentes: El maltrato temprano es un factor de riesgo para trastornos psiquiátricos, incluyendo el Trastorno de Estrés Postraumático (TEPT). El TEPT es un desorden severo y heterogéneo con estados fluctuantes de emocionalidad sobre e infra modulada incluyendo hipervigilancia, disociación, y déficit de regulación de las emociones. La percepción y regulación de las emociones han sido ligadas con la interocepción, la representación cortical y la sensación de procesos corporales internos. Aunque los primeros enfoques terapéuticos que abordaban sensaciones corporales han sido efectivos en pacientes con TEPT, y aunque se han encontrado déficit en la representación de señales interoceptivas en otros desordenes relacionados con el trauma como el Trastorno de Personalidad Limite (TPL), el rol de la interocepción permanece vastamente inexplorado para el TEPT.
Objetivo: El objetivo fue investigar la representación cortical de las señales interoceptivas cardíacas en pacientes con TEPT y su asociación con maltrato temprano, disociación y regulación emocional.
Métodos: 24 pacientes con TEPT sin uso de medicamentos y 31 controles sanos (CS) completaron un electrocardiograma (ECG) de reposo de 5 minutos junto con un electroencefalograma (EEG) en paralelo. Se compararon las amplitudes de los potenciales evocados cardíacos (HEP por sus siglas en inglés) como medida de representación cortical de señales cardíacas interoceptivas y se correlacionaron con cuestionarios de auto-reporte en ambos grupos.
Resultados: No encontramos diferencias significativas en las amplitudes medias de HEP en pacientes con TEPT en comparación con CS, aunque las HEP en pacientes con TEPT fueron descriptivamente más altas. No se obtuvieron asociaciones significativas entre las amplitudes medias de HEP maltrato temprano, rasgos disociativos o la desregulación emocional dentro de los grupos.
Conclusión: Los hallazgos presentes no indican déficit en la representación de señales interoceptivas en reposo en individuos con TEPT. La posibilidad de que los pacientes con TEPT muestren modulaciones alteradas de HEP durante tareas de regulación emocional y que puedan beneficiarse de enfoques terapéuticos que lleven a cambiar la percepción de señales corporales, necesita ser investigada en futuros estudios.
背景: 早年虐待是包括创伤后应激障碍 (PTSD) 在内精神障碍的一个风险因素。创伤后应激障碍是一种严重的异质性障碍, 具有情绪过度调节和调节不足的波动状态, 包括高警觉、分离和情绪调节缺陷。情绪的感知和调节与内感受、皮层表征和内部身体加工感知有关。尽管针对身体感觉的首批治疗方法已经发现对 PTSD 患者有效, 并且在其他创伤相关疾病 (如边缘型人格障碍 (BPD)) 中也有报告内感受信号表征缺陷, 但内感受在 PTSD 中的作用仍然未被广泛探索。
目的: 旨在考查 PTSD 患者心脏内感受信号皮质表征及其与早期生活虐待、特质分离和情绪失调的关联
方法: 24 名未用药的 PTSD 患者和 31 名健康对照 (HC) 完成了 5 分钟带有平行脑电图 (EEG)的静息心电图 (ECG) 。心跳诱发电位 (HEP) 振幅作为心脏内感受信号皮质表征的量度, 进行了组间比较和自我报告问卷的相关。
结果: 我们未发现 PTSD 患者的平均 HEP 振幅相较于 HC 有显著差异, 尽管 PTSD 患者的 HEP 描述性更高。未发现组内平均 HEP 振幅与早期生活虐待、特质分离或情绪失调之间的显著关联
结论: 当前发现并未表明 PTSD 患者的静息态内感受信号表现存在缺陷. PTSD 患者在情绪调节任务期间是否表现出改变的 HEP 调制, 以及是否可能受益于旨在改变身体信号感知的治疗方法, 需要在未来的研究中进行考查
1. Introduction
Post-traumatic stress disorder (PTSD) is a psychiatric condition, which includes symptom domains of hyperarousal, hypervigilance, increased startle sensitivity, and re-experiencing, but also of avoidance or numbing (Colvonen et al., Citation2017; Michopoulos, Norrholm, & Jovanovic, Citation2015). Besides traumatic stress episodes as the index event of PTSD, early life maltreatment has been identified as a risk factor for this disorder (Choi et al., Citation2019). Both early life maltreatment and chronic stress are associated with a dysregulation of physiological stress axes, which affect further regulatory systems, and are considered to result in an altered representation and sensing of inner bodily signals, termed ‘interoception’ (Schulz & Vögele, Citation2015).
Interoception is defined as the processing and sensing of signals arising from the body (Khalsa & Lapidus, Citation2016). Starting with electrical and chemical impulses at receptor level, signals are transmitted finally via afferent pathways to higher cortical areas, like the insular cortex or the anterior cingulate cortex (Craig, Citation2002). An undisturbed processing of interoceptive signals is considered to be fundamental for the perception and regulation of emotions (Critchley & Garfinkel, Citation2017). As first therapeutic approaches targeting bodily sensations, such as mindfulness-based stress reduction (MBSR) (Kang, Sponheim, & Lim, Citation2020), have been found effective in PTSD (Mehling et al., Citation2018; van de Kamp et al., Citation2019), an in-depth understanding of the mechanisms underlying the processing and perception of bodily signals is required to design and to evaluate appropriate treatment strategies.
A central parameter of early stages of interoceptive cortical signal representation in the cardiac domain represents the heartbeat-evoked potential (HEP) (Schulz, Schultchen, & Vögele, Citation2020). The HEP is an event-related potential recorded at the scalp which occurs time-locked 250–600 ms after the cardiac R-wave (Coll, Hobson, Bird, & Murphy, Citation2021; Leopold & Schandry, Citation2001). HEP amplitudes can be measured both in resting state and while performing heartbeat perception tasks (Coll et al., Citation2021; Schulz et al., Citation2020). If assessed in a resting state, HEPs are interpreted as an index of cortical representation of cardiac interoceptive signals, independent of their conscious perception (Coll et al., Citation2021; Schulz et al., Citation2013; Shao, Shen, Wilder-Smith, & Li, Citation2011), which may be seen as an early stage of signal processing (Schulz et al., Citation2020). Other interoceptive dimensions, such as interoceptive accuracy (typically measured with heartbeat perception tasks) and interoceptive sensibility (typically measured via self-report questionnaires or confidence in an interoceptive accuracy task) (Garfinkel, Seth, Barrett, Suzuki, & Critchley, Citation2015), have been shown to be independent from resting state HEP amplitudes (Baranauskas, Grabauskaité, &Griškova-Bulanova, Citation2017; Mai, Wong, Georgiou, & Pollatos, Citation2018; Verdonk et al., Citation2021). The neuronal generators of the HEP amplitude have been identified in the anterior insular and dorsal anterior cingular cortices (Müller et al., Citation2015), the two most important regions of the interoceptive network (Pollatos, Gramann, & Schandry, Citation2007). The insula is both involved in the interoceptive network and the salience network (Chong, Ng, Lee, & Zhou, Citation2017; Menon & Uddin, Citation2010), with a particular importance in PTSD due to possible misinterpretations of interoceptive stimuli in the course of an internal danger sense (Colvonen et al., Citation2017) due to traumatic experiences. While an increased anterior activation of the insula has been associated with heightened levels of interoception during states of emotional undermodulation, such as re-experiencing and hyperarousal symptoms, a decreased insula activation has been linked to emotional detachment or emotional overmodulation, hypoarousal, and dissociative symptoms (for an overview see Lanius, Frewen, Tursich, Jetly, & McKinnon, Citation2015). Since traumatized individuals often cycle between states of emotional under- and overmodulation, the cortical representation of interoceptive signals might represent a promising biomarker of (altered) insula network activity (Park et al., Citation2017; Pollatos, Herbert, Mai, & Kammer, Citation2016). Adressing this open question is essential, as interoceptive signal representation may be used as a predictor of therapy outcome and can be measured independent of any cognitive-based task at all stages of symptom burden.
Altered HEP amplitudes as a measure of interoceptive signal representation have been identified in patients with borderline personality disorder (BPD) and depersonalization-derealization disorder, two psychiatric disorders known to show high prevalence rates of early life maltreatment as well as high comorbidity with PTSD (de Aquino Ferreira, Queiroz Pereira, Neri Benevides, & Aguiar Melo, Citation2018; Frias & Palma, Citation2015; Simeon, Guralnik, Schmeidler, Sirof, & Knutelska, Citation2001; Swart, Wildschut, Draijer, Langeland, & Smit, Citation2020). BPD, depersonalization-derealization disorder, and PTSD each include symptoms such as emotional numbness and disturbed body ownership (Löffler, Kleindienst, Cackowski, Schmidinger, & Bekrater-Bodmann, Citation2020; Sierra & David, Citation2011). In individuals with BPD, lower mean HEP amplitudes, averaged across the whole scalp, were found as compared to healthy controls, and mean HEP amplitudes were negatively associated with early life maltreatment (Müller et al., Citation2015; Schmitz et al., Citation2020). However, higher HEP amplitudes were reported over frontal electrodes in individuals with BPD (Flasbeck, Popkirov, Ebert, & Brune, Citation2020; Müller et al., Citation2015). Furthermore, HEP amplitudes averaged across a two electrode setup, did not differ between patients with depersonalization-derealization disorder and healthy controls at rest, but HEPs were less affected by attention focused on heartbeats in cardiac interoception (Schulz et al., Citation2015). Despite some important differences, major symptomatic overlaps between BPD, depersonalization-derealization disorder, and PTSD are emotion dysregulation (Carpenter & Trull, Citation2013; Monde, Ketay, Giesbrecht, Braun, & Simeon, Citation2013; Pencea et al., Citation2020) and dysfunctions of the autonomic nervous system (ANS), as reflected by altered heart rate variability (HRV) (Carr, de Vos, & Saunders, Citation2018; Farina, Speranza, Imperatori, Quintiliani, & Della Marca, Citation2015; Ge, Yuan, Li, & Zhang, Citation2020). The term HRV refers to the beat-to-beat variability of heart rate (HR), which is mediated by the sympathetic and parasympathetic nervous system (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, Citation1996). The confrontation with major life stressors, such as to be expected in the aetiology of PTSD, is typically associated with dysregulations in physiological stress axes (Yehuda et al., Citation2015), for example, higher sympathetic and lower parasympathetic tone, which is reflected in reduced high-frequency HRV in PTSD (e.g. Ge et al., Citation2020). It is likely, that an altered sympathetic and parasympathetic control over the HR constitutes alterations in the cardiac signals which is, therefore, associated with an abnormal cortical representation of cardiac interoceptive signals (Flasbeck et al., Citation2020; MacKinnon, Gevirtz, McCraty, & Brown, Citation2013; Schulz et al., Citation2015, Citation2020). Since patients with BPD and PTSD show similar alterations in HRV (Carr et al., Citation2018; Ge et al., Citation2020; Koenig, Kemp, Feeling, Thayer, & Kaess, Citation2016) and high prevalence rates of early life maltreatment (Choi et al., Citation2019; de Aquino Ferreira et al., Citation2018), both might exhibit lower mean HEP amplitudes, as has been demonstrated in BPD (Müller et al., Citation2015; Schmitz et al., Citation2020).
In brief, although heart rate (HR) and HRV (Ge et al., Citation2020; Schneider & Schwerdtfeger, Citation2020) are altered in patients with PTSD, and HR reactivity may represent a pre-treatment biomarker of PTSD (Colvonen et al., Citation2017), evidence on the interoceptive signal representation in patients with PTSD is scarce. Whereas in a recent intervention study, frontal theta HEP increase during meditation mediated the treatment effect of an 8-week MBSR on PTSD symptom improvements in veterans (Kang et al., Citation2020), no study has been reported investigating interoceptive signal representation in comparison to healthy controls.
The current study thus aimed at investigating (1) the cortical representation of cardiac signals in patients with PTSD as compared to healthy controls by means of HEP amplitudes. In addition, we explored (2) its association with early life maltreatment and dissociation, as a common clinical symptom in PTSD, and emotion dysregulation as a consequence of atypical interoception. To achieve this, we assessed mean HEP amplitudes in resting state electrocardiogram (ECG) with parallel electroencephalogram (EEG) in patients with PTSD and HC. We expected lower mean HEP amplitudes in PTSD compared to healthy controls and negative correlations between HEP amplitudes and early life maltreatment, dissociation, and emotion dysregulation in the patient sample.
2. Method
2.1. Participants
Twenty-four medication-free participants with a current DSM-IV-diagnosis of PTSD (PTSD; 23 women, 1 man; mean age = 36.21 years, SD = 11.09 years) and 31 age- and sex-matched HC (29 women, 2 men; mean age = 31.06, SD = 9.19 years) were enrolled in the study. Demographic and clinical information are presented in (). The two groups were matched in body-mass-index (BMI), heart rate, and age. Healthy controls had never received a psychiatric diagnosis or undergone psychological/psychiatric treatment. Group allocation was based on clinical interviews. Participants with PTSD had all faced repeated traumatic experiences before the age of 18 years (see early life maltreatment scores in ).
Table 1. Comparison of patients with post-traumatic stress disorder and healthy controls in terms of sex, education, and the prevalence of comorbid psychiatric diagnoses
Table 2. Clinical and self-reported data of patients with post-traumatic stress disorder and healthy controls
General exclusion criteria were (a) neurological disorders; (b) current substance use, assessed via urine toxicology screening; (c) substance abuse in the last two months; (d) a current or lifetime diagnosis of BPD; (e) severe medical illness; and (f) regular psychotropic medication. Additional exclusion criteria for patients with PTSD comprised: Lifetime diagnosis of schizophrenia, schizoaffective or bipolar disorder, and substance dependence in the last 12 months. Current comorbid psychiatric diagnoses of patients with PTSD included: affective disorders (N = 6), other anxiety disorders (N = 11), eating disorders (N = 5), somatic symptom disorders (N = 3). Healthy controls did not have any current or lifetime psychiatric disorders.
Recruitment was done by the central project of the KFO-256, a Clinical Research Unit funded by the German Research Foundation, dedicated to investigating mechanisms of disturbed emotion processing in BPD (Schmahl et al., Citation2014) and the GRK2350, a Research Training Group funded by the German Research Foundation, dedicated to investigating the impact of adverse childhood experiences on psychosocial and somatic conditions across the lifespan (Cackowski & Schmahl, Citation2019). Participants were recruited via advertisements and from the pool of in- and outpatients of the Department of Psychosomatic Medicine and Psychotherapy at the Central Institute of Mental Health and of the Department of General Psychiatry at the University of Heidelberg. HC were recruited through advertisements and the local residents’ registration office. The Ethics Committee of the Medical Faculty of the University of Heidelberg approved the study. All participants provided written informed consent and were paid for their participation.
2.2. Procedure
2.2.1. Data acquisition
A 5-min resting state EEG and ECG (closed eyes) was recorded. Participants sat in a comfortable upright position in a sound-attenuated, dimly lit room. The EEG was measured from 60 Ag/AgCl electrodes (equidistant reference system; EasyCap GmbH, Herrsching, Germany), using an average reference (<10 kΩ, 72-channel QuickAmp amplifier; Brain Products, GmbH, Gilching, Germany; with a band-pass filter of 0.01–200 Hz and a sampling rate of 1000 Hz). The signal was recorded via BrainVision Recorder 1.20. Vertical and horizontal electrooculogram were recorded from the epicanthus of each eye and from the supra- and infra-orbital positions of the left eye. In addition, the ECG was recorded according to Einthoven II, using two monitoring electrodes with micropore tape and solid gel (3 M Healthcare).
2.2.2. Data processing
A band-pass filter was set to 0.01–200 Hz, signals were digitized at 1,000 Hz (Brain Vision Analyser 2.0 software, Brain Products GmbH), filtered digital (band-pass: 0.1–35 Hz, 24 dB/octave rolloff), corrected for eye-movement artefacts, rejected semiautomatic of trials with artefacts, and segmented into 1,200-ms periods (−200 ms to 1,000 ms of the ECG R-wave; see Gray et al., Citation2007) with baseline correction (−200 ms to R-peak). On average, the analysed number of segments were 301.88 in the PTSD group (SD = 49.34) and 296.97 in the HC group (SD = 50.23) (U = 365.0, z = −0.12, p = .905). The number of rejected segments due to artefacts did not differ between both groups (PTSD group: 3.53%; HC group: 4.45%; U = 409.0, z = 0.63, p = .530). For semiautomatic detection of the R-peaks in the filtered ECG data (down sampling 250 Hz) and subsequent calculation of heart rate, we used ARTiiFACT software (Kaufmann, Sütterlin, Schulz, & Vögele, Citation2011). Separate HEP-averages were computed for each electrode and individual. Statistical analyses were conducted on the mean voltage from 455 to 595 ms after the R-wave, since influences of the electrocardiac field-artefact are less than 1% in this time window (Gray et al., Citation2007). For mean HEP amplitudes, we calculated the average across all 60 head electrodes.
2.3. Measures
2.3.1. Psychiatric disorders
Qualified and trained diagnosticians (i.e. at least a master’s degree in clinical psychology) assessed PTSD and other psychiatric diagnoses with the semi-structured interviews Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, Citation1995) and International Personality Disorder Examination (IPDE; Loranger, Janca, & Norman, Citation1997) for selected Axis II disorders in both patients and healthy controls.
2.3.2. Early life maltreatment
Early life maltreatment was measured using 25 of the 28 items on the German version of the Childhood Trauma Questionnaire (CTQ; Klinitzke, Romppel, Hauser, Brahler, & Glaesmer, Citation2012). Participants rated the frequency of traumatic experiences on a 5-point Likert scale ranging from 1 (not at all) to 5 (very often). A total score was calculated ranging from 25 to 125 (overall internal consistency in the present study α = .92 [PTSD] and α = .85 [HC]).
2.3.3. Emotion dysregulation
Emotion dysregulation was assessed with the Difficulties in Emotion Regulation Scale (DERS; Gratz & Roemer, Citation2004). Participants rated each item on a 5-point Likert scale ranging from 1 (almost never) to 5 (almost always). A total score was calculated ranging from 36 to 180 (overall internal consistency in the present study α = .88 [PTSD] and α = .87 [HC]).
2.3.4. Trait dissociation
Trait dissociation was assessed with the ‘Fragebogen zur Erfassung Dissoziativer Symptome’ (FDS), the German adaptation of the Dissociative Experiences Scale (Spitzer et al., Citation1998). The FDS consists of 44 items measuring the frequency of dissociative experiences. Participants rated the frequency of each item in 10% increments, ranging from 0% (never) to 100% (always). A mean score was calculated ranging from 0 to 100 (overall internal consistency in the present study α = .93 [PTSD] and α = .84 [HC]).
2.3.5. General psychopathology
General psychopathology was measured using the German version of the Brief Symptom Inventory (BSI; Franke, Citation2000). The BSI assesses past-week clinically relevant symptoms. Participants rated each of the 53 items on a 5-point Likert scale ranging from 0 (not at all) to 4 (extremely). The Global Severity Index (GSI), defined as the mean of all items, was calculated ranging from 0 to 4 (overall internal consistency in the present study α = .92 [PTSD] and α = .86 [HC]).
2.3.6. Depressiveness
Depressiveness was measured using the Beck Depression Inventory (BDI; Hautzinger, Bailer, Worall, & Keller, Citation1994). Each of the 21 items represents an affective or somatic symptom related to depression and is composed of four statements reflecting symptom severity. Participants rated each item on a statement scale ranging from 0 (no disturbance) to 3 (maximal disturbance). A total score was calculated ranging from 0 to 63 (overall internal consistency in the present study α = .90 [PTSD] and α = .63 [HC]).
2.3.7. Trait anxiety
Trait anxiety was measured using the State-Trait-Anxiety-Inventory (STAI; Laux, Glanzmann, Schaffner, & Spielberger, Citation1981). This inventory assesses state anxiety and trait anxiety with 40 items. Participants rated each of the items on a 4-point Likert Scale ranging from 1 (not at all) to 4 (very much so). In the current study, only the trait anxiety score was used ranging from 20 to 80 (overall internal consistency in the present study α = .89 [PTSD] and α = .86 [HC]).
2.4. Data analysis
Statistical Analyses were conducted using SPSS (Version 25.0). BMI and clinical self-reports were missing in up to four cases (as indicated in ) and imputed by group mean values.
To investigate group differences in cortical representation of cardiac interoceptive signals, we performed three successive analyses:
First, a t-test for independent samples was used to investigate group differences in mean HEP amplitudes over all 60 electrodes.
Second, to further investigate the spatial distribution of HEP amplitudes, a 2 × 8 × 2 mixed design analysis of variance (ANOVA) was used to investigate the between-subject factor group (PTSD, HC) and the within-subject factors scalp location (frontal, frontocentral, central, centroparietal, parietal, temporal, parietooccipital, occipital) and laterality (left, right) on mean HEP-amplitudes (consistent to Mai et al., Citation2018). This resulted in the following clusters: frontal left (FP1, AF3, F1, F5, F9, FT7, FT9, F9/FT9), frontal right (FP2, AF4, F2, F6, F10, FT10, FT8, F10/FT10), frontocentral left (FC3, FC5), frontocentral right (FC4, FC6), central left (C3, C5), central right (C4, C6), centroparietal left (CP1, CP3, CP5), centroparietal right (CP2, CP4, CP6), parietal left (P1, P7, P9), parietal right (P2, P8, P10), temporal left (T7, TP7, TP9), temporal right (T8, TP8, TP10), parietooccipital left (PO1), parietooccipital right (PO2), occipital left (O1, O9), and occipital right (O2, O10).
Third, to increase the comparability of the current study with previous findings in BPD (Müller et al., Citation2015), we performed a second 2 × 4 mixed design ANOVA, which included the between-subject factor group and the midline electrodes as within-subjects factor (electrode position: Fz, Cz, Pz, and Oz; consistent with Müller et al., Citation2015).
Associations between HEPs, early life maltreatment, trait dissociation, and emotion dysregulation were analysed with Spearman correlations for each group separately. Correlation coefficients were compared using Fisher’s z transformation.
Finally, a Mann-Whitney-U-test was used for comparison of ECG mean amplitudes between groups, since ECG data were not normally distributed in the HC group. Further Mann-Whitney-U-tests were used for comparison of clinical and self-reported data between groups in .
For the two ANOVAs, Greenhouse-Geisser corrected results are reported. Post-hoc analyses were conducted using t-tests for dependent samples, and Bonferroni correction was applied. The normality distribution of the data was investigated using the Shapiro–Wilk test.
A significance threshold of p < .05 two-tailed was employed for all analyses. For t-tests, Cohen’s d is reported as an effect size index, while for the ANOVAs partial η2 is used as an effect size index. According to Cohen (Citation1988), small, moderate, and large effects are defined as Cohen’s d of 0.20, 0.50 and 0.80, partial η2 of .01, .06, and 0.14, and correlation coefficients r of .10, .30 and .50, respectively.
Sensitivity analyses (1-β ≥ .80) showed that the sample size was adequate to detect medium-sized correlations of r = .48 in the PTSD group and medium group differences of d = 0.68.
3. Results
3.1. T-test
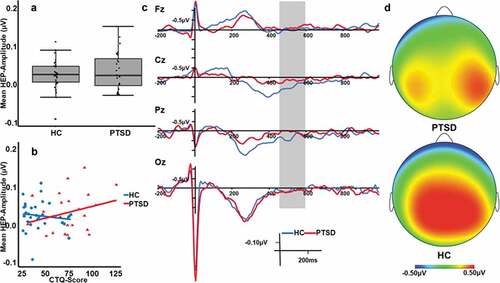
As shown in , HEP amplitudes of patients with PTSD did not differ significantly from those of HC individuals (t[53] = 0.74, p = .462, d = 0.20). Patients with PTSD showed descriptively higher HEP amplitudes (M = 0.03 µV, SD = 0.05 µV) compared to HC individuals (M = 0.02 µV, SD = 0.04 µV). Furthermore, we tested if the variance in the PTSD group was higher than in the HC group, but the difference in variance was only significant at trend level (F[1, 53] = 3.92, p = .053).
Figure 1. Mean heartbeat-evoked potential amplitudes of current PTSD patients and healthy controls
3.2. ANOVA for clusters with factors scalp position and laterality
At parietal and temporal electrodes, HEP amplitudes were higher over the right than over the left hemisphere (interaction effect of scalp location by laterality: F[4, 215] = 4.67, p = .001, η2 = .08; corresponding post-hoc tests: parietal left vs. right t[54] = −4.51, pBonf < .001, d = 0.54 and temporal left vs. right t[54] = −5.15, pBonf < .001, d = 0.75), as shown in . Overall, activity was lower for frontal electrodes compared to central, centroparietal, parietal, and parietooccipital (all pBonf < .024, d > 0.60) electrodes and higher for centroparietal electrodes compared to all other electrodes (all pBonf < .026, d > 0.37). However, no significant main or interaction effect of group emerged in this analysis (main effect of group: F[1, 53] = 0.80, p = .375, η2 = .02, interaction effect of group by scalp location: F[3, 160] = 0.86, p = .463, η2 = .02, interaction effect of group by laterality: F[1, 53] = 0.27, p = .607, η2 = .01, interaction effect of group by scalp location by laterality: F[4, 215] = 0.34, p = .854, η2 = .01).
3.3. ANOVA for midline electrode positions
HEP amplitudes were lower at the frontal midline electrode compared to the central midline electrode (t[54] = −3.71, pBonf = .003, d = 0.85; see also ) with no significant interaction effect of group by electrode position (F[3, 159] = 0.64, p = .591, η2 = .01).
3.4. Correlations
As shown in , no significant correlations were obtained between mean HEP-amplitudes and early life maltreatment (CTQ score; PTSD group: ρ = .23, p = .289; HC group ρ = −.10, p = .584). Likewise, the correlations between mean HEP-amplitudes and trait dissociation (FDS total score; PTSD group: ρ = .23, p = .273; HC group: ρ = −.21, p = .254), and between mean HEP amplitudes and emotion dysregulation (DERS total score; PTSD group: ρ = .33, p = .112; HC group: ρ = .18, p = .347) did not reach significance. Comparisons between the correlation coefficients of the two groups yielded no significant differences (all z < 1.55, p > .061).
3.5. Additional analyses of ECG amplitudes
Mean ECG amplitudes did not differ between the two groups (U = 435.0, z = 1.07, p = .285). There was no significant association with mean HEP amplitudes (ρ = −.17, p = .205).
4. Discussion
To our knowledge, this is the first study to investigate the cortical representation of cardiac interoceptive signals by means of HEP amplitudes, in patients with PTSD compared to healthy controls. We found no significant differences between both groups and no significant associations between mean HEP amplitudes, early life maltreatment, trait dissociation, and emotion dysregulation. Inconsistent with our hypotheses, we did not found reduced HEPs in a resting state in the PTSD group, and found non-significant positive associations between early life maltreatment, trait dissociation, and emotion dysregulation.
Altered HEP amplitudes have previously been found in psychiatric disorders known to show high prevalence rates of early life maltreatment, high comorbidity as well as symptomatologic overlap with PTSD, such as BPD (Müller et al., Citation2015; Schmitz et al., Citation2020) and depression (Terhaar, Viola, Bär, & Debener, Citation2012). In the current study, however, we did not find significantly altered resting state HEP amplitudes at any electrode position or cluster in a medication-free sample of patients with PTSD. Similar null findings for resting state HEP amplitudes have also been reported in patients with depersonalization-derealization disorder (Schulz et al., Citation2015) and panic disorder (Yoris et al., Citation2017). Although PTSD is not classified as an anxiety disorder anymore in DSM-5, the current finding of a unaltered HEP amplitude is in line with the comparable finding in patients with panic disorder. As panic disorder is characterized by hypervigilance for inner bodily changes, one might speculate that patients with PTSD might exhibit similar bodily hypervigilance. However, contrary to the notion of elevated symptoms due to heightened awareness of physical sensations in models of anxiety (Domschke, Stevens, Pfleiderer, & Gerlach, Citation2010; Paulus & Stein, Citation2010), higher levels of interoceptive accuracy in heartbeat perception have been negatively associated with PTSD symptoms in female sexual trauma survivors (Reinhardt et al., Citation2020). Although the current sample consisted of patients with a clinical diagnosis of PTSD and might therefore exhibit different mechanisms, heightened awareness might pose a functional approach – rather than a hypervigilant avoidance-behaviour. Studies are needed that incorporate interoceptive attentional states, e.g. while performing an interoceptive task (e.g. Fittipaldi et al., Citation2020; Smith et al., Citation2020), in order to investigate HEP modulation in PTSD and to shed light on to the question whether cortical representation of interoceptive signals is associated with higher or lower interoceptive accuracy in PTSD and further early trauma-related disorders, such as BPD.
A possible explanation of the current findings of comparable HEP amplitudes as healthy controls, is that pathogenic processes might not significantly influence interoceptive signal representation in resting state in patients with PTSD. Instead, both emotional under- and overmodulation have been associated with insula activity patterns in PTSD (Lanius et al., Citation2015). As the HEP amplitude is modulated by affective predictions (Marshall, Gentsch, & Schutz-Bosbach, Citation2020), investigating HEP modulation during emotion regulation tasks might shed light on to the question, how interoceptive and neural mechanisms during dynamic states of hyperarousal and dissociation might differ in PTSD. Furthermore, self-reported body dissociation, which includes experiences of emotional disconnection and may be seen as a coping style and inner attitude towards one’s own body as opposed to general dissociative symptoms, has been shown to be higher in trauma-exposed individuals (Price, Citation2007; Price & Thompson, Citation2007). The current finding of comparable HEP amplitudes at rest as healthy controls in PTSD, might reflect a stronger interoceptive signal as compared to findings in other mental disorders, but this signal might also be blurred by more noise and affect higher order mechanisms, such as body dissociation. Whether interoceptive signal representation in PTSD contributes to maladaptive beliefs about the importance of bodily signals, needs to be addressed in further studies. The dissociative subtype of PTSD is common (Swart et al., Citation2020) and has been reported to show distinct functional connectivity patterns in the insula (Harricharan et al., Citation2020), an interoceptive core region (Pollatos et al., Citation2007). Thus, interoceptive signal representation might differ for patients with the dissociative subtype of PTSD. Patients with depersonalization-derealization disorder have been reported to show comparable mean HEP amplitudes at rest as healthy controls but a lack of attentional modulation of HEP during cardiac interoception (Schulz et al., Citation2015). Furthermore, patients with dissociative disorder showed deficits in heartbeat perception (Schäflein, Sattel, Pollatos, & Sack, Citation2018), which might be indicative of an avoidance of bodily signals and might further hinder functional approach-behaviour in patients with comorbid PTSD (see also Reinhardt et al., Citation2020). In the current study, half of the patients with PTSD scored above the FDS cut-off of 13 (Rodewald, Ursula, & Hinderk, Citation2006), according to which pathological dissociative experiences may be assumed. Due to the small sample size, exploratory analyses comparing patients with PTSD below and above the cut-off score and HC were not computed. However, the dissociative subtype needs to be investigated in future studies.
Besides comparing mean HEP amplitudes between patients with PTSD and HC, we also investigated the relationship between the mean HEP amplitude and early life maltreatment. Studies investigating the impact of early life maltreatment on interoception are scarce, with first studies indicating that early life maltreatment may enhance stress-induced and psychopathology-related malfunctions in interoceptive domains (Müller et al., Citation2015; Schaan et al., Citation2019; Schmitz et al., Citation2020; Schulz et al., Citation2021). In the current study, however, the association between early life maltreatment and HEP in patients with PTSD did not reach significance. Since an association between early life maltreatment and heartbeat perception after an acute stressor but not at resting state has been reported in healthy individuals (Schaan et al., Citation2019), states of emotional under- and overmodulation in patients with PTSD need to be investigated.
Before strong conclusions can be drawn, the following limitations of the current study need to be considered: First, although the sample of patients with PTSD was free of medication and carefully matched with the healthy controls, the sample size is only moderate. Whereas statistical power was adequate for detecting medium group differences and medium-sized correlations in the two samples, the obtained group difference between PTSD and HC for mean HEP amplitudes was smaller than expected on the basis of previously reported medium effect sizes in similar studies with clinical and healthy control groups (Flasbeck et al., Citation2020; Müller et al., Citation2015; Schmitz et al., Citation2020). Second, the sample was almost all-female. Since sex effects on HEPs have been reported (MacKinnon et al., Citation2013), associations between HEPs, sex and type of trauma need to be investigated in further studies. Third, information about psychotherapeutic interventions and treatment status were not collected and could thus not be taken into account. Since preliminary evidence indicates a possible mediation effect of HEP changes on the relationship between a mindfulness-based stress reduction on PTSD symptom improvements (Kang et al., Citation2020), future studies might investigate the impact of therapeutic interventions on HEP amplitudes in patient samples. Fourth, a number of comorbid disorders were diagnosed in the PTSD group. Although this represents a typical pattern of clinical samples, it cannot be ruled out that these comorbidities, such as somatic symptom and eating disorders, may have affected the results. More and larger studies including data of different clinical groups with trauma-related disorders are needed, before strong conclusions regarding disorder-specific dysfunctions in interoceptive signal processing (or a lack thereof) can be drawn.
In sum, this is the first study to report interoceptive signal processing in individuals with PTSD in a resting state. Contrary to our hypothesis, mean HEP amplitudes did not significantly differ from those of healthy controls and were not significantly associated with early life maltreatment, trait dissociation, and emotion dysregulation. The current finding thus indicate differences in interoceptive processes between PTSD and other trauma-associated mental disorders, such as BPD and depression, where significantly reduced HEP amplitudes have been reported. Further studies are needed, to investigate and compare different dimensions of interoceptive processes, including heartbeat perception and the subjective sensibility for interoceptive signals, in addition to the cortical representation of cardiac signals in PTSD as well as other trauma-associated disorders.
Acknowledgments
We thank K. Herwig for supporting the EEG measurement and analysis, L. Kramer, V. Sebold, and F. Mancke for their help with data collection, and the team of the Clinical Research Group on Mechanisms of Disturbed Emotion Processing in Borderline Personality Disorder (KFO 256) for participant recruitment and organization.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, M.S. The data are not publicly available due to privacy and ethical restrictions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Baranauskas, M., Grabauskaité, A., & Griškova-Bulanova, I. (2017). Brain responses and self-reported indices of interoception: Heartbeat evoked potentials are inversely associated with worrying about body sensations. Physiology and Behavior, 180, 1–12. doi:10.1016/j.physbeh.2017.07.032.
- Cackowski, S., & Schmahl, C. (2019). Research Training Group (RTG)/Graduiertenkolleg (GRK) 2350. Impact of Adverse Childhood Experiences on Psychosocial and Somatic Conditions Across the Lifespan. Neuroforum, 25(4), 265–266. doi:10.1515/nf-2019-0022.
- Carpenter, R. W., & Trull, T. J. (2013). Components of emotion dysregulation in borderline personality disorder: A review. Current Psychiatry Reports, 15(1), 335. doi:10.1007/s11920-012-0335-2.
- Carr, O., de Vos, M., & Saunders, K. E. A. (2018). Heart rate variability in bipolar disorder and borderline personality disorder: A clinical review. Evidence-Based Mental Health, 21(1), 23–30. doi:10.1136/eb-2017-102760.
- Choi, K. R., Ford, J. D., Briggs, E. C., Munro-Kramer, M. L., Graham-Bermann, S. A., & Seng, J. S. (2019). Relationships between maltreatment, posttraumatic symptomatology, and the dissociative subtype of PTSD among adolescents. Journal of Trauma & Dissociation, 20(2), 212–227. doi:10.1080/15299732.2019.1572043.
- Chong, J. S. X., Ng, G. J. P., Lee, S. C., & Zhou, J. (2017). Salience network connectivity in the insula is associated with individual differences in interoceptive accuracy. Brain Structure & Function, 222(4), 1635–1644. doi:10.1007/s00429-016-1297-7.
- Cohen, J. (1988). Statistical Power analysis for the behavioral sciences. Hove: Psychology Press.
- Coll, M. P., Hobson, H., Bird, G., & Murphy, J. (2021). Systematic review and meta-analysis of the relationship between the heartbeat-evoked potential and interoception. Neuroscience and Biobehavioral Reviews, 122, 190–200. doi:10.1016/j.neubiorev.2020.12.012.
- Colvonen, P. J., Glassman, L. H., Crocker, L. D., Buttner, M. M., Orff, H., Schiehser, D. M., … Afari, N. (2017). Pretreatment biomarkers predicting PTSD psychotherapy outcomes: A systematic review. Neuroscience and Biobehavioral Reviews, 75, 140–156. doi:10.1016/j.neubiorev.2017.01.027.
- Craig, A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews: Neuroscience, 3(8), 655–666. doi:10.1038/nrn894.
- Critchley, H. D., & Garfinkel, S. N. (2017). Interoception and emotion. Current Opinion in Psychology, 17, 7–14. doi:10.1016/j.copsyc.2017.04.020.
- de Aquino Ferreira, L. F., Queiroz Pereira, F. H., Neri Benevides, A. M. L., & Aguiar Melo, M. C. (2018). Borderline personality disorder and sexual abuse: A systematic review. Psychiatry Research, 262, 70–77. doi:10.1016/j.psychres.2018.01.043.
- Domschke, K., Stevens, S., Pfleiderer, B., & Gerlach, A. L. (2010). Interoceptive sensitivity in anxiety and anxiety disorders: An overview and integration of neurobiological findings. Clinical Psychology Review, 30(1), 1–11. doi:10.1016/j.cpr.2009.08.008.
- Farina, B., Speranza, A. M., Imperatori, C., Quintiliani, M. I., & Della Marca, G. (2015). Change in heart rate variability after the adult attachment interview in dissociative patients. Journal of Trauma & Dissociation, 16(2), 170–180. doi:10.1080/15299732.2014.975309.
- First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (1995). Structured clinical interview for DSM-IV (SCID-I). New York: New York Biometrics research department.
- Fittipaldi, S., Abrevaya, S., Fuente, A., Pascariello, G. O., Hesse, E., Birba, A., … Ibanez, A. (2020). A multidimensional and multi-feature framework for cardiac interoception. Neuroimage, 212, 116677. doi:10.1016/j.neuroimage.2020.116677.
- Flasbeck, V., Popkirov, S., Ebert, A., & Brune, M. (2020). Altered interoception in patients with borderline personality disorder: A study using heartbeat-evoked potentials. Borderline Personality Disorder and Emotion Dysregulation, 7(1), 24. doi:10.1186/s40479-020-00139-1.
- Franke, G. H. (2000). Brief symptom inventory von L. R.Derogatis (Kurzform der SCL-90-R) - Deutsche version. Manual [Brief Symptom Inventory by L. R. Derogatis (Short form of the SCL-90-R) – German version. Manual]. Göttingen: Beltz Test.
- Frias, A., & Palma, C. (2015). Comorbidity between post-traumatic stress disorder and borderline personality disorder: A review. Psychopathology, 48(1), 1–10. doi:10.1159/000363145.
- Garfinkel, S. N., Seth, A. K., Barrett, A. B., Suzuki, K., & Critchley, H. D. (2015). Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biological Psychology, 104, 65–74. doi:10.1016/j.biopsycho.2014.11.004.
- Ge, F., Yuan, M., Li, Y., & Zhang, W. (2020). Posttraumatic stress disorder and alterations in resting heart rate variability: A systematic review and meta-analysis. Psychiatry Investigation, 17(1), 9–20. doi:10.30773/pi.2019.0112.
- Gratz, K. L., & Roemer, L. (2004). Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment, 26(1), 41–54. doi:10.1023/B:JOBA.0000007455.08539.94.
- Gray, M. A., Taggart, P., Sutton, P. M., Groves, D., Holdright, D. R., Bradbury, D., … Critchley, H. D. (2007). A cortical potential reflecting cardiac function. Proceedings of the National Academy of Sciences of the United States of America, 104(16), 6818–6823. doi:10.1073/pnas.0609509104.
- Harricharan, S., Nicholson, A. A., Thome, J., Densmore, M., McKinnon, M. C., Theberge, J., … Lanius, R. A. (2020). PTSD and its dissociative subtype through the lens of the insula: Anterior and posterior insula resting-state functional connectivity and its predictive validity using machine learning. Psychophysiology, 57(1), e13472. doi:10.1111/psyp.13472.
- Hautzinger, M., Bailer, M., Worall, H., & Keller, F. (1994). Beck-Depressionsinventar (BDI): Bearbeitung der deutschen Ausgabe. Testhandbuch Bern: Verlag Hans Huber (pp. Hans–Huber).
- Kang, S. S., Sponheim, S. R., & Lim, K. O. (2020). Interoception underlies the therapeutic effects of mindfulness meditation for post-traumatic stress disorder: A randomized clinical trial. arXiv: Neurons and Cognition.
- Kaufmann, T., Sütterlin, S., Schulz, S. M., & Vögele, C. (2011). ARTiiFACT: A tool for heart rate artifact processing and heart rate variability analysis. Behavior Research Methods, 43(4), 1161–1170. doi:10.3758/s13428-011-0107-7.
- Khalsa, S. S., & Lapidus, R. C. (2016). Can Interoception improve the pragmatic search for biomarkers in psychiatry? Frontiers in Psychiatry, 7, 121. doi:10.3389/fpsyt.2016.00121.
- Klinitzke, G., Romppel, M., Hauser, W., Brahler, E., & Glaesmer, H. (2012). The German version of the Childhood Trauma Questionnaire (CTQ): Psychometric characteristics in a representative sample of the general population. Psychotherapie, Psychosomatik, Medizinische Psychologie, 62(2), 47–51. doi:10.1055/s-0031-1295495.
- Koenig, J., Kemp, A. H., Feeling, N. R., Thayer, J. F., & Kaess, M. (2016). Resting state vagal tone in borderline personality disorder: A meta-analysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 64, 18–26. doi:10.1016/j.pnpbp.2015.07.002.
- Lanius, R. A., Frewen, P. A., Tursich, M., Jetly, R., & McKinnon, M. C. (2015). Restoring large-scale brain networks in PTSD and related disorders: A proposal for neuroscientifically-informed treatment interventions. European Journal of Psychotraumatology, 6(1), 27313. doi:10.3402/ejpt.v6.27313.
- Laux, L., Glanzmann, P., Schaffner, P., & Spielberger, C. D. (1981). State‐Trait‐angstinventar[The state trait anxiety inventory]. Weinheim, Germany: Beltz.
- Leopold, C., & Schandry, R. (2001). The heartbeat-evoked brain potential in patients suffering from diabetic neuropathy and in healthy control persons. Clinical Neurophysiology, 112(4), 674–682. doi:10.1016/S1388-2457(01)00480-1.
- Löffler, A., Kleindienst, N., Cackowski, S., Schmidinger, I., & Bekrater-Bodmann, R. (2020). Reductions in whole-body ownership in borderline personality disorder - A phenomenological manifestation of dissociation. Journal of Trauma & Dissociation, 21(2), 264–277. doi:10.1080/15299732.2019.1678213.
- Loranger, A. W., Janca, A., and Norman, S. (Eds.). (1997). Assessment and diagnosis of personality disorders: The ICD-10 international personality disorder examination (IPDE). Cambridge: Cambridge University Press.
- MacKinnon, S., Gevirtz, R., McCraty, R., & Brown, M. (2013). Utilizing heartbeat evoked potentials to identify cardiac regulation of vagal afferents during emotion and resonant breathing. Applied Psychophysiology and Biofeedback, 38(4), 241–255. doi:10.1007/s10484-013-9226-5.
- Mai, S., Wong, C. K., Georgiou, E., & Pollatos, O. (2018). Interoception is associated with heartbeat-evoked brain potentials (HEPs) in adolescents. Biological Psychology, 137, 24–33. doi:10.1016/j.biopsycho.2018.06.007.
- Marshall, A. C., Gentsch, A., & Schutz-Bosbach, S. (2020). Interoceptive cardiac expectations to emotional stimuli predict visual perception. Emotion, 20(7), 1113–1126. doi:10.1037/emo0000631.
- Mehling, W. E., Chesney, M. A., Metzler, T. J., Goldstein, L. A., Maguen, S., Geronimo, C., … Neylan, T. C. (2018). A 12-week integrative exercise program improves self-reported mindfulness and interoceptive awareness in war veterans with posttraumatic stress symptoms. Journal of Clinical Psychology, 74(4), 554–565. doi:10.1002/jclp.22549.
- Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5–6), 655–667. doi:10.1007/s00429-010-0262-0.
- Michopoulos, V., Norrholm, S. D., & Jovanovic, T. (2015). Diagnostic biomarkers for posttraumatic stress disorder: Promising Horizons from translational neuroscience research. Biological Psychiatry, 78(5), 344–353. doi:10.1016/j.biopsych.2015.01.005.
- Monde, K. M., Ketay, S., Giesbrecht, T., Braun, A., & Simeon, D. (2013). Preliminary physiological evidence for impaired emotion regulation in depersonalization disorder. Psychiatry Research, 209(2), 235–238. doi:10.1016/j.psychres.2013.02.020.
- Müller, L. E., Schulz, A., Andermann, M., Gäbel, A., Gescher, D. M., Spohn, A., … Bertsch, K. (2015). Cortical representation of afferent bodily signals in borderline personality disorder: neural correlates and relationship to emotional dysregulation. JAMA Psychiatry, 72(11), 1077–1086. doi:10.1001/jamapsychiatry.2015.1252.
- Park, H. D., Bernasconi, F., Salomon, R., Tallon-Baudry, C., Spinelli, L., Seeck, M., … Blanke, O. (2017). Neural sources and underlying mechanisms of neural responses to heartbeats, and their role in bodily self-consciousness: An intracranial EEG study. Cerebral Cortex, 1–14. doi:10.1093/cercor/bhx136.
- Paulus, M. P., & Stein, M. B. (2010). Interoception in anxiety and depression. Brain Structure & Function, 214(5–6), 451–463. doi:10.1007/s00429-010-0258-9.
- Pencea, I., Munoz, A. P., Maples-Keller, J. L., Fiorillo, D., Schultebraucks, K., Galatzer-Levy, I., … Powers, A. (2020). Emotion dysregulation is associated with increased prospective risk for chronic PTSD development. Journal of Psychiatric Research, 121, 222–228. doi:10.1016/j.jpsychires.2019.12.008.
- Pollatos, O., Gramann, K., & Schandry, R. (2007). Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping, 28(1), 9–18. doi:10.1002/hbm.0258.
- Pollatos, O., Herbert, B. M., Mai, S., & Kammer, T. (2016). Changes in interoceptive processes following brain stimulation. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 371(1708). doi:10.1098/rstb.2016.0016.
- Price, C. J., & Thompson, E. A. (2007). Measuring dimensions of body connection: Body awareness and bodily dissociation. Journal of Alternative and Complementary Medicine, 13(9), 945–953. doi:10.1089/acm.2007.0537.
- Price, C. (2007). Dissociation reduction in body therapy during sexual abuse recovery. Complementary Therapies in Clinical Practice, 13(2), 116–128. doi:10.1016/j.ctcp.2006.08.004.
- Reinhardt, K. M., Zerubavel, N., Young, A. S., Gallo, M., Ramakrishnan, N., Henry, A., & Zucker, N. L. (2020). A multi-method assessment of interoception among sexual trauma survivors. Physiology and Behavior, 226, 113108. doi:10.1016/j.physbeh.2020.113108.
- Rodewald, F., Ursula, G., & Hinderk, M. E. (2006). Screening for major dissociative disorders with the FDS, the German version of the dissociative experience scale. Psychotherapie Psychosomatik Medizinische Psychologie, 56(6), 249–258. doi:10.1055/s-2006-932590.
- Schaan, V. K., Schulz, A., Rubel, J. A., Bernstein, M., Domes, G., Schachinger, H., & Vogele, C. (2019). Childhood trauma affects stress-related interoceptive accuracy. Frontiers in Psychiatry, 10, 750. doi:10.3389/fpsyt.2019.00750.
- Schäflein, E., Sattel, H. C., Pollatos, O., & Sack, M. (2018). Disconnected - impaired interoceptive accuracy and its association with self-perception and cardiac vagal tone in patients with dissociative disorder. Frontiers in Psychology, 9, 897. doi:10.3389/fpsyg.2018.00897.
- Schmahl, C., Herpertz, S. C., Bertsch, K., Ende, G., Flor, H., Kirsch, P., … Bohus, M. (2014). Mechanisms of disturbed emotion processing and social interaction in borderline personality disorder: State of knowledge and research agenda of the German Clinical Research Unit. Borderline Personality Disorder and Emotion Dysregulation, 1(1), 12. doi:10.1186/2051-6673-1-12.
- Schmitz, M., Müller, L. E., Schulz, A., Kleindienst, N., Herpertz, S. C., & Bertsch, K. (2020). Heart and brain: Cortical representation of cardiac signals is disturbed in borderline personality disorder, but unaffected by oxytocin administration. Journal of Affective Disorders, 264, 24–28. doi:10.1016/j.jad.2019.11.139.
- Schneider, M., & Schwerdtfeger, A. (2020). Autonomic dysfunction in posttraumatic stress disorder indexed by heart rate variability: A meta-analysis. Psychological Medicine, 50(12), 1937–1948. doi:10.1017/S003329172000207X.
- Schulz, A., Deuter, C. E., Breden, I. H., Vogele, C., Wingenfeld, K., Otte, C., & Kuehl, L. K. (2021). Noradrenergic activation induced by yohimbine decreases interoceptive accuracy in healthy individuals with childhood adversity. Development and Psychopathology, 1–12. doi:10.1017/S0954579420001613.
- Schulz, A., Köster, S., Beutel, M. E., Schächinger, H., Vögele, C., Rost, S., … Michal, M. (2015). Altered patterns of heartbeat-evoked potentials in depersonalization/derealization disorder: Neurophysiological evidence for impaired cortical representation of bodily signals. Psychosomatic Medicine, 77(5), 506–516. doi:10.1097/PSY.0000000000000195.
- Schulz, A., Schultchen, D., & Vögele, C. (2020). Interoception, stress, and physical symptoms in stress-associated diseases. European Journal of Health Psychology, 27(4), 132–153. doi:10.1027/2512-8442/a000063.
- Schulz, A., Strelzyk, F., Ferreira de Sá, D. S., Naumann, E., Vögele, C., & Schächinger, H. (2013). Cortisol rapidly affects amplitudes of heartbeat-evoked brain potentials–implications for the contribution of stress to an altered perception of physical sensations? Psychoneuroendocrinology, 38(11), 2686–2693. doi:10.1016/j.psyneuen.2013.06.027.
- Schulz, A., & Vögele, C. (2015). Interoception and stress. Frontiers in Psychology, 6, 993. doi:10.3389/fpsyg.2015.00993.
- Shao, S., Shen, K., Wilder-Smith, E. P., & Li, X. (2011). Effect of pain perception on the heartbeat evoked potential. Clinical Neurophysiology, 122(9), 1838–1845. doi:10.1016/j.clinph.2011.02.014.
- Sierra, M., & David, A. S. (2011). Depersonalization: A selective impairment of self-awareness. Consciousness and Cognition, 20(1), 99–108. doi:10.1016/j.concog.2010.10.018.
- Simeon, D., Guralnik, O., Schmeidler, J., Sirof, B., & Knutelska, M. (2001). The role of childhood interpersonal trauma in depersonalization disorder. American Journal of Psychiatry, 158(7), 1027–1033. doi:10.1176/appi.ajp.158.7.1027.
- Smith, R., Kuplicki, R., Feinstein, J., Forthman, K. L., Stewart, J. L., Paulus, M. P., … Khalsa, S. S. (2020). A Bayesian computational model reveals a failure to adapt interoceptive precision estimates across depression, anxiety, eating, and substance use disorders. PLoS Computational Biology, 16(12), e1008484. doi:10.1371/journal.pcbi.1008484.
- Spitzer, C., Freyberger, H. J., Stieglitz, R. D., Carlson, E. B., Kuhn, G., Magdeburg, N., & Kessler, C. (1998). Adaptation and psychometric properties of the German version of the Dissociative Experience Scale. Journal of Traumatic Stress, 11(4), 799–809. doi:10.1023/A:1024457819547.
- Swart, S., Wildschut, M., Draijer, N., Langeland, W., & Smit, J. H. (2020). Dissociative subtype of posttraumatic stress disorder or PTSD with comorbid dissociative disorders: Comparative evaluation of clinical profiles. Psychological Trauma: Theory, Research, Practice and Policy, 12(1), 38–45. doi:10.1037/tra0000474.
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996). Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. European Heart Journal, 17(3), 354–381. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8737210
- Terhaar, J., Viola, F. C., Bär, K.-J., & Debener, S. (2012). Heartbeat evoked potentials mirror altered body perception in depressed patients. Clinical Neurophysiology, 123(10), 1950–1957. doi:10.1016/j.clinph.2012.02.086.
- van de Kamp, M. M., Scheffers, M., Hatzmann, J., Emck, C., Cuijpers, P., & Beek, P. J. (2019). Body- and movement-oriented interventions for posttraumatic stress disorder: A systematic review and meta-analysis. Journal of Traumatic Stress, 32(6), 967–976. doi:10.1002/jts.22465.
- Verdonk, C., Trousselard, M., Di Bernardi Luft, C., Medani, T., Billaud, J. B., Ramdani, C., … Vialatte, F. (2021). The heartbeat evoked potential does not support strong interoceptive sensibility in trait mindfulness. Psychophysiology, e13891. doi:10.1111/psyp.13891.
- Yehuda, R., Hoge, C. W., McFarlane, A. C., Vermetten, E., Lanius, R. A., Nievergelt, C. M., … Hyman, S. E. (2015). Post-traumatic stress disorder. Nature Reviews Disease Primers, 1, 15057. doi:10.1038/nrdp.2015.57.
- Yoris, A., Garcia, A. M., Traiber, L., Santamaria-Garcia, H., Martorell, M., Alifano, F., … Sedeno, L. (2017). The inner world of overactive monitoring: Neural markers of interoception in obsessive-compulsive disorder. Psychological Medicine, 47(11), 1957–1970. doi:10.1017/S0033291717000368.
