ABSTRACT
Background
Inhibition is a critical executive control process and an established neurobiological phenotype of PTSD, yet to our knowledge, no prospective studies have examined this using a contextual cue task that enables measurement of behavioural response and neural activation patterns across proactive and reactive inhibition.
Objective
The current longitudinal study utilised functional magnetic resonance imaging (fMRI) to examine whether deficits in proactive and reactive inhibition predicted PTSD symptoms six months after trauma.
Method
Twenty-three (65% males) medical patients receiving emergency medical care from a level 1 trauma centre were enrolled in the study and invited for an MRI scan 1-2-months post-trauma. PTSD symptoms were measured using self-report at scan and 6-months post-trauma. A stop-signal anticipation task (SSAT) during an fMRI scan was used to test whether impaired behavioural proactive and reactive inhibition, and reduced activation in right inferior frontal gyrus (rIFG), ventromedial prefrontal cortex (vmPFC), and bilateral hippocampus, were related to PTSD symptoms. We predicted that lower activation levels of vmPFC and rIFG during reactive inhibition and lower activation of hippocampus and rIFG during proactive inhibition would relate to higher 6-month PTSD symptoms.
Results
No significant associations were found between behavioural measures and 6-month PTSD. Separate linear regression analyses showed that reduced rIFG activation (F1,21 = 9.97, R2 = .32, p = .005) and reduced vmPFC activation (F1,21 = 5.19, R2 = .20, p = .03) significantly predicted greater 6-month PTSD symptoms; this result held for rIFG activation controlling for demographic variables and baseline PTSD symptoms (β = −.45, p = .04) and Bonferroni correction.
Conclusion
Our findings suggest that impaired rIFG and, to a lesser extent, vmPFC activation during response inhibition may predict the development of PTSD symptoms following acute trauma exposure. Given the small sample size, future replication studies are needed.
HIGHLIGHTS
Impaired inhibition may be an important risk factor for the development of PTSD following trauma, with less right inferior frontal gyrus and ventromedial prefrontal cortex activation during response inhibition predicting PTSD development.
Antecedentes: La inhibición es un proceso de control ejecutivo crítico, y un fenotipo neurobiológico establecido del TEPT, sin embargo, en nuestro conocimiento no hay estudios prospectivos que hayan examinado esto usando una tarea con claves contextuales que permita medir la respuesta conductual y los patrones de activación neuronal en la inhibición proactiva y reactiva.
Objetivo: El siguiente estudio es de diseño longitudinal y utilizó resonancia magnética funcional (fMRI por sus siglas en inglés) para examinar si los déficit en inhibición proactiva y reactiva predijeron los síntomas de TEPT 6 meses después del trauma.
Método: 23 pacientes (65% hombres) que recibieron cuidado médico de emergencia en un centro de trauma nivel 1 se enrolaron en el estudio y se les invitó a una RNM (resonancia nuclear magnética) 1–2 meses después del trauma. Los síntomas de TEPT se midieron usando auto-reporte al momento de la exploración y 6 meses después del trauma. Se uso una tarea de anticipación de señal de parada (SSAT por sus siglas en inglés) durante la RNM funcional para evaluar si la alteración en la inhibición proactiva y reactiva, y la reducción de la activación en el giro frontal inferior derecho (rIFG por sus siglas en inglés), la corteza prefrontal ventromedial (vmPFC por sus siglas en inglés), y el hipocampo bilateral, estuvieron relacionadas a los síntomas de TEPT. Predijimos que niveles bajos de activación de vmPFC y rIFG durante la inhibición proactiva se relacionaría con mayores síntomas de TEPT a los 6 meses.
Resultados: No se encontraron asociaciones significativas entre medidas conductuales y TEPT a los 6 meses. Los análisis de regresión lineal separados mostraron que una activación reducida de rIFG (F1,21 = 9.97, R2 = .32, p = .005) y una activación reducida de vmPFC (F1,21 = 5.19, R2 = .20, p = .03) predijeron significativamente mayores síntomas de TEPT a los 6 meses; este resultado fue corroborado para la activación de rIFG controlando para variables demográficas y síntomas basales de TEPT (β = −.45, p = .04) y para la corrección de Bonferroni.
Conclusión: Nuestros hallazgos sugieren que una rIFG deficiente y, en menor grado, la activación del vmPFC durante la inhibición de la respuesta pueden predecir el desarrollo de síntomas de TEPT tras la exposición a un trauma agudo. Dado lo pequeño de la muestra, se requieren futuros estudios de replicación.
背景:抑制是一个关键的执行控制过程和 PTSD 既定神经生物学表型,但据我们所知,没有前瞻性研究使用能够测量主动和反应抑制行为反应和神经激活模式的上下文提示任务来考查这一点.
目的:本纵向研究利用功能性磁共振成像 (fMRI) 来考查主动和反应抑制不足是否可以预测创伤后六个月的 PTSD 症状.
方法:23 名(65% 男性)从 1 级创伤中心接受紧急医疗护理的内科患者参加了研究,并在创伤后 1–2 个月被邀请进行了一项 MRI 扫描。在扫描时和创伤后 6 个月使用自我报告测量了 PTSD 症状。使用 fMRI 扫描期间的停止信号预期任务 (SSAT) 来检验是否受损的行为主动和反应抑制,以及右侧额下回 (rIFG)、腹内侧前额叶皮层 (vmPFC) 和双侧海马的激活减少是否与PTSD 症状相关。我们预测,在反应抑制期间 vmPFC 和 rIFG 的较低激活水平以及在主动抑制期间较低的海马和 rIFG 激活水平与6 个月时较高的 PTSD 症状有关.
结果:在行为测量和 6 个月 PTSD 之间没有发现显著关联。单独的线性回归分析表明,减少的 rIFG 激活 (F1,21 = 9.97, R2 = .32, p = .005) 和减少的 vmPFC 激活 (F1,21 = 5.19, R2 = .20, p = .03) 显著预测更高的6个月时PTSD症状;这一结果在控制人口统计变量和基线 PTSD 症状 (β = −.45, p = .04) 和 Bonferroni 校正后对于 rIFG 激活仍成立.
结论:我们的研究结果表明,在反应抑制期间受损的 rIFG 和较小程度的 vmPFC 激活可能预测急性创伤暴露后 PTSD 症状的发展。鉴于样本量小,未来需要进行重复研究.
PALABRAS CLAVE:
1. Introduction
While the majority of individuals will be exposed to a traumatic event in their lifetime (Benjet et al., Citation2016), only 6-8% of the U.S. population goes on to develop PTSD (Kilpatrick et al., Citation2013). Thus, identifying neurobiological phenotypes that may predispose individuals to develop PTSD following acute trauma exposure is critical in efforts to improve mental health outcomes for trauma survivors. One relevant neurobiological phenotype to consider is impaired inhibition (van Rooij & Jovanovic, Citation2019).
Inhibition reflects the ability to suppress inappropriate actions and is a critical executive control process (Walther, Goya-Maldonado, Stippich, Weisbrod, & Kaiser, Citation2010). Impaired fear inhibition and contextual cue processing are present in individuals with PTSD (Jovanovic, Kazama, Bachevalier, & Davis, Citation2012), and have been suggested to play a particular role in re-experiencing and hyperarousal PTSD symptoms (Rougemont-Bücking et al., Citation2011; Wessa & Flor, Citation2007). Moreover, greater hippocampal functioning during contextual fear conditioning has been related to greater levels of resilience in the early aftermath of trauma, whereas lower hippocampal activation during contextual cue processing in the fear extinction phase has been related to greater levels of PTSD (van Rooij et al., Citation2021). Yet, these cognitive processing deficits do not appear to be trauma specific, and instead, reflect a more general inhibition deficit in individuals with PTSD (van Rooij et al., Citation2014). Thus, inhibition deficit may be an important risk factor for the development of PTSD.
Inhibition on a cognitive level can be measured by assessing response inhibition, which reflects the suppression of initial response and an adjustment to a more appropriate behavioural response (Verbruggen & Logan, Citation2008). A Go/NoGo paradigm or the stop-signal task (SST) are two common ways to measure response inhibition, where the response to a ‘Go’ stimulus has to be inhibited when an infrequent ‘No-Go’ or ‘Stop’ signal is presented (Logan & Cowan, Citation1984). Response inhibition can be separated into two distinct categories: proactive and reactive. Proactive inhibition is the anticipation of stopping based on contextual cues. For example, in the real world, inhibition is often facilitated by contextual cues, such as inhibiting a fear response in a safe environment. Reactive inhibition is the direct stopping of a response via inhibition of the motor areas (Falconer et al., Citation2008; Zandbelt, Bloemendaal, Neggers, Kahn, & Vink, Citation2013). The stop-signal anticipation task (SSAT) (Zandbelt, Vink, & Rodriguez-Fornells, Citation2010), an adaption of the SST, enables differentiation between proactive and reactive inhibition by including contextual cues to indicate a stop chance.
Following prior research, there are three primary brain regions implicated in response inhibition in the context of PTSD that should be considered: the ventromedial prefrontal cortex (vmPFC), hippocampus, and right inferior frontal gyrus (rIFG). Both vmPFC and hippocampus are implicated in the inhibition of the fear response (Jovanovic & Ressler, Citation2010), but have also been related to PTSD using a Go/NoGo response inhibition paradigm. Lower vmPFC activation, important for prefrontal control of limbic regions, was observed in PTSD patients (Jovanovic et al., Citation2013; Stevens et al., Citation2016). Lower hippocampal activation was related to lower levels of resilience and greater PTSD symptoms in chronically and acutely traumatised civilians (van Rooij et al., Citation2016, Citation2018). Given its role in contextual cue processing and memory, reduced inhibition-related hippocampal activation in PTSD is expected to be specifically related to proactive inhibition, yet studies to support this postulation are lacking. The rIFG is a region involved in attention regulation (Hampshire, Chamberlain, Monti, Duncan, & Owen, Citation2010) and has been implicated in both reactive and proactive inhibition in healthy controls (Aron, Robbins, & Poldrack, Citation2014; Swann et al., Citation2012; Van Belle, Vink, Durston, & Zandbelt, Citation2014; Zhang and Iwaki (Citation2019)). Additionally, prior work in war veterans with and without PTSD using the SSAT showed decreased rIFG activation to contextual cue processing (i.e. proactive inhibition) in PTSD patients (van Rooij et al., Citation2014). Thus, while much is still unknown, the vmPFC appears to be more relevant for reactive inhibition and the hippocampus for proactive inhibition, and rIFG may be relevant in both when examining risk for the development of PTSD.
Prospective designs are necessary to disentangle causal pathways between impaired inhibition and PTSD (Ben-Zion et al., Citation2019; McLean et al., Citation2020). One approach is to examine change in reactive and proactive inhibition in response to treatment among PTSD patients. In studying neural mechanisms of inhibition and contextual cue processing related to treatment response in veterans with PTSD, van Rooij et al. (Citation2015) found that inhibition and contextual cue deficits remained present even in treatment responders, suggesting these may be deficits that are present prior to the development of PTSD and increase one’s risk for PTSD following trauma exposure. However, the only study that has prospectively examined whether inhibition leads to the development of PTSD following trauma exposure (van Rooij et al., Citation2018) used a Go/NoGo paradigm, not including contextual cues for proactive inhibition and not allowing for comparison of correct versus incorrect trials to assess reactive inhibition. Also, because of the high accuracy levels in the Go/NoGo task, there was no behavioural variability that could be related to PTSD development. Thus, it remains unclear whether both proactive and reactive inhibition serves as risk factors for the development of PTSD and which specific behavioural and neural activation patterns represent that risk.
In order to fill the gaps in the research identified above, the goal of the current study was to determine if deficits in proactive and reactive inhibition (measured across behavioural response and neural activation patterns) predicted the presence of PTSD symptoms 6-months post-acute trauma exposure in a sample of medical patients receiving acute medical care in the Emergency Department from a level 1 trauma centre following trauma exposure. Given the small sample size, only ROI-based and specific hypothesis-driven analyses that directly follow prior work were performed to promote direct comparison and increase consistency. We hypothesised that (1) impaired proactive and reactive inhibition behavioural measures would be related to greater levels of PTSD at 6-months; (2) lower activation levels of vmPFC and rIFG during reactive inhibition would be related to greater levels of PTSD at 6-months; and (3) lower activation levels of hippocampus and rIFG during proactive inhibition would be related to greater levels of PTSD at 6-months. Lastly, given that previous studies have suggested that impaired inhibition and contextual cue processing play a particular role in re-experiencing and hyperarousal PTSD symptoms, follow up exploratory analyses were conducted to better understand associations between 6-month PTSD symptom clusters and outcome measures.
2. Method
2.1. Procedure
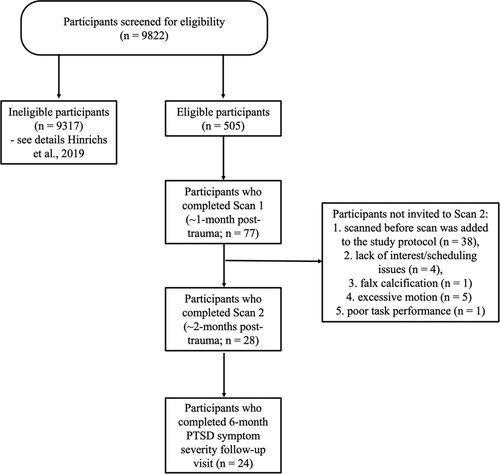
Participants were recruited between October 2015 and April 2017 from the Emergency Department at Grady Memorial Hospital in Atlanta, GA, a level 1 urban trauma centre. To be eligible for study inclusion, participants had to be aged 18–65 years and have experienced a DSM-IV criterion A trauma within the last 24 hours (American Psychological Association, Citation2013). Following initial medical evaluation and clearance, trained evaluators approached eligible trauma survivors in the Emergency Department and obtained informed consent. Participants underwent a bedside assessment lasting approximately 1.5 hours, which included questions related to the index trauma, psychological symptoms, and demographics. All individuals were able to participate, regardless of the level of psychological symptoms. Participants were asked to return for follow-up visits to assess PTSD symptom development and were compensated $50 at each of these visits. PTSD symptom severity at 6-months post-trauma was used as the outcome measure in this study. Participants who were eligible, were invited for an MRI scan ∼1 month post-trauma, and individuals who successfully completed the first MRI scan ((Stevens et al., Citation2017; van Rooij et al., Citation2018), data not included in the current study) and who were willing and able to return were invited for an additional scan visit. This additional scan visit occurred around two months (mean = 66 days, SD = 24) after the trauma. See for a breakdown of participants at each study timepoint. All study procedures were reviewed and approved by the Emory University Institutional Review Board and the Grady Research Oversight Committee.
2.2. Participants
Inclusion criteria included being able to provide informed consent, understand and speak English, and have a phone to allow contact for follow-up appointment scheduling. Individuals with a current or past history of mania, schizophrenia, other psychoses or prominent suicidal ideation in the last month, intoxication, severe pain, active labour, respiratory distress, intensive care unit admission or surgery, medical instability, loss of consciousness for more than five minutes, or hemodynamic compromise were ineligible for the study. Participants with falx calcification or excessive motion during this first scan visit were not invited for the second scan. Out of the 24 individuals that completed 6-month PTSD assessment, n = 23 had usable fMRI data.Footnote1 Thus, the present sample included 23 adults. Demographic and clinical characteristics are presented in .
Table 1. Demographic characteristics of study sample.
2.3. Measures
Standardised Trauma Interview (STI). Participants were administered an STI in the ED to collect sociodemographic information (e.g. sex, age, race, income) and characteristics of the index trauma exposure, including the type of trauma exposure (Kessler, Chiu, Demler, & Walters, Citation2005).
Posttraumatic Stress Disorder Symptom Scale (PSS) (Foa, Riggs, Dancu, & Rothbaum, Citation1993). The PSS is a well-validated 17-item self-report measure that was used to assess PTSD symptoms following trauma, based on DSM-IV-TR criteria, at the time of scan and 6-month time points. This study was initiated before the release of DSM-5 and therefore only included DSM-IV-TR PTSD symptoms. We used a count of overall PTSD symptom severity and a probable diagnosis based on DSM-IV-TR diagnosis, including the presence of at least one re-experiencing symptom, three avoidance/numbing symptoms, and two hyperarousal symptoms. Twenty-two percent (n = 5) of the participants met for probable PTSD diagnosis at 6-months.
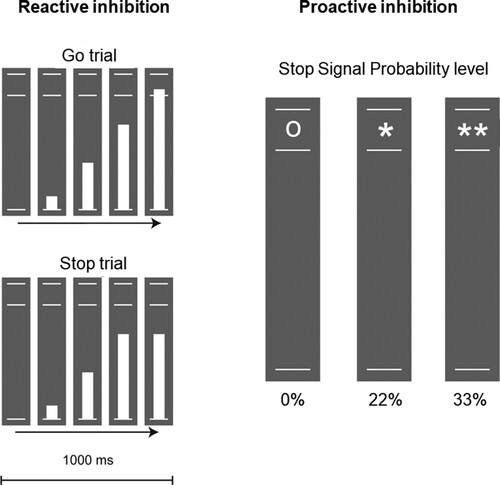
Stop Signal Anticipation Task. The stop signal anticipation task (SSAT) has been used widely to assess reactive and proactive inhibition (e.g.Pas, Hulshoff Pol, Raemaekers, & Vink, Citation2021; van Rooij et al., Citation2014, Citation2015; Zandbelt et al., Citation2010). Test-retest of the SSAT has been shown in prior research (Buimer et al., Citation2020). In this task, three parallel horizontal lines were displayed and a bar moved at a constant speed from the lower to the upper in 1000 ms, reaching the middle line at 800 ms. The participant was instructed to stop the bar as close to the middle line as possible by pressing a button on the button box using their thumb (Go trials); however, on some trials, the bar stopped on its own before reaching the middle line and the participant had to withhold their response (Stop trials). The chance that the bar stopped on its own was indicated with a symbol below the bottom line, ‘o’ for 0% stop signal probability level, ‘*’ for 22% and ‘**’ for 33% probability, similar to (Pas et al., Citation2021). A step-wise approach was used to adjust task difficulty based on the success of the previous trial, thereby keeping the number of failed and successful trials comparable between participants and sessions. The total number of trials was 256 equally distributed across stop signal probability levels and presented in pseudorandom order. shows visualisation of the task overview.
2.4. Behavioural analysis
Proactive inhibition was assessed as the increase in reaction time with increasing stop-signal probability (0%, 22%, 33%). A steeper increase, or greater slope, indicated better proactive inhibition, and this slope was calculated for analysis of proactive inhibition. For behavioural assessment of reactive inhibition, the stop signal reaction time (SSRT) was calculated across the two stop-signal probability levels (22 and 33%) using the integration method. The SSRT measures the inhibition of a response that was already initiated and reflects the latency of the inhibition process (Logan & Cowan, Citation1984). Better reactive inhibition is indicated by a smaller SSRT. The SSRT and not accuracy was used as the behavioural outcome in this study, because the task was designed to adjust task difficulty resulting in similar number of correct and incorrect responses across participants.
Descriptive statistics were run on all variables of interest and skewness and kurtosis fell within the normal range. First, to confirm proactive inhibition, a repeated measures analysis of variance (ANOVA) was run to evaluate whether mean reaction time increased with increasing stop signal probability. Then bivariate correlation analyses were run between overall PTSD symptom severity and the slope (for proactive inhibition) and the SSRT (for reactive inhibition). Exploratory follow-up correlation analyses were repeated for PTSD symptom clusters separately only if overall PTSD symptom severity was significantly associated with slope and/or SSRT.
2.5. Brain imaging acquisition and analysis
MRI scans were collected on a Siemens 3.0-Tesla Magnetom Trio TIM whole-body MR scanners (Siemens, Malvern, PA) using a 12-channel head coil. Functional images were acquired using 2D echo-planar imaging (ep2d_bold). Volumes contained 44 slices (slice thickness = 2.5 mm, interslice gap = 0.5 mm) acquired in a descending sequential slice order parallel to the anterior-posterior commissure line with TR = 2360 ms, TE = 30 ms, flip angle = 70°, voxel size = 3 × 3×2.5 mm3. A 3D T1-weighted MP-RAGE image (176 slices, TR = 2250 ms, TE = 4.18 ms, voxel size 1 × 1 × 1 mm) was used for within-subject registration.
Functional data were processed and analyzed with SPM12 (http://www.fil.ion.ucl.ac.uk/spm) following earlier studies using the SSAT (Zandbelt et al., Citation2010). In brief, preprocessing included realignment to correct for head motion (least-square approach and rigid-body transformation), slice timing correction (interpolation of slices to centre slice in time), spatial normalisation (to Montreal Neurological Institute, MNI, template brain), and smoothing (8mm full width at half maximum).
First level analyses were performed for each individual to create contrast maps for proactive and reactive inhibition. For proactive inhibition, the parametric contrast (slope) for the three stop signal probability levels (0, 22 and 33%) was calculated. For reactive inhibition, the contrast between stop success (correct stop trials) and stop failure (incorrect stop trials) was calculated. Region of interest (ROI) analyses were conducted extracting the contrast values for the reactive inhibition for the rIFG and vmPFC and for proactive inhibition for the rIFG and bilateral hippocampus. The ROIs were chosen following prior work in PTSD by this group to allow for direct comparisons and increase consistency. The rIFG was used in van Rooij et al. (Citation2015, Citation2014) and was defined by the average response of an independent sample performing the SSAT (Zandbelt et al., Citation2010). The vmPFC and bilateral hippocampus ROI were used in van Rooij et al. (Citation2016, Citation2018). The vmPFC was defined based on a 6mm spherical ROI centred around the peak voxel of decreased activation during the Go/NoGo task in PTSD patients, shown in Jovanovic et al. (Citation2013). The bilateral hippocampus ROI was defined using the AAL atlas.
First, for neural correlates of inhibition, contrast values in the vmPFC and rIFG ROIs for reactive inhibition and rIFG and hippocampus ROIs for proactive inhibition were correlated with PTSD symptoms at 6-months post-trauma. Bonferroni correction was used for the four correlation analyses that were conducted: p < .0125. Then, statistically significant correlations between ROIs and 6-month PTSD were further analyzed by performing linear regression analyses. For ease of interpretation, Model 1 included only the ROI contrast values for rIFG and vmPFC (separate models). Model 2 included age and gender as covariates in both regression models as an observed difference in response inhibition has been found for both (Kleerekooper et al., Citation2016; Li, Huang, Constable, & Sinha, Citation2006); PTSD symptoms at time of scan was added as an additional covariate in the models if there was a significant correlation with the ROI. Finally, exploratory correlation analyses with the three DSM-IV-TR PTSD symptom clusters were conducted for any ROI that was significantly associated with overall PTSD symptom severity. Bonferroni correction for the six exploratory correlations conducted was p < .008. A table showing potential, probable, and extreme outlier potential for variables of interest is included in Supplemental Table 1.
3. Results
3.1. Behavioural response
Repeated measures ANOVA showed a significant main effect for reaction time (F1.2 = 9.12, p = .004), such that there was an increase in reaction time across the three trials, indicating proactive inhibition. As shown in , examination of bivariate correlations between overall PTSD symptom severity with proactive inhibition slope and SSRT showed no significant correlations (slope: r = .01, p = .95; SSRT: r = −.13, p = .55).
Table 2. Bivariate correlations between proactive inhibition slope and stop chance reaction time (SSRT) during stop signalling anticipation task with PTSD symptoms at six months post-trauma.
3.2. Neural activation
PTSD symptoms 6-months post-trauma correlated significantly with less rIFG (r = −0.57, p = .005) and vmPFC (r = −0.45, p = .033) activation during reactive inhibition (see ). No significant correlations with proactive inhibition in the hippocampus or rIFG were observed (r = .08, p = .70 and r = .08, p = .72, respectively).Footnote2 Only the correlation with rIFG activation during reactive inhibition survived Bonferroni correction. Follow-up analyses showed that there was a significant correlation between rIFG activation and PTSD symptoms at time of scan (r = −.47, p = .024), but not vmPFC activation (r = −.31, p = .15). The rIFG and vmPFC were not significantly correlated in this sample: r = .33, p = .121.
Figure 3. Region of Interest correlation analyses. Left, the two regions of interest are displayed in red. Right, activation results of region response during reactive inhibition with PTSD symptoms six months post-trauma, p < .05. Scatter plot graph shows the correlation between mean contrast estimate across voxels in the rIFG and vmPFC clusters and PTSD symptoms (rIFG: r = −0.57, p = .005; vmPFC: r = −0.45, p = .033).
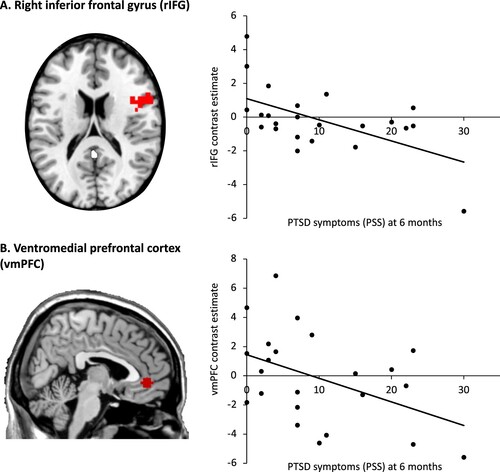
Next, linear regression analyses based on significant correlational results were run. As shown in , linear regression Model 1 showed that reduced rIFG activation accounted for 32% of the variance in 6-month PTSD symptoms (F1,21 = 9.97, p = .005); when age, gender and PTSD symptoms at time of scan were included in Model 2, the model remained significant (F3,19 = 4.06, p = .016) and only rIFG was a significant predictor of 6-month PTSD symptoms (β = −.45, t = −2.27, p = .04). The linear regression Model 1 with vmPFC predicting 6-month PTSD symptoms was also significant (F1,21 = 5.19, p = .03), and reduced vmPFC activation explained 20% of the variance in 6-month PTSD symptoms (see and ). Model 2 including age, gender and vmPFC was not significant (F2,20 = 2.84, p = .07). Exploratory analyses for symptom clusters with neural activation during reactive inhibition showed that the rIFG negatively correlated significantly with all clusters,Footnote3 whereas the vmPFC specifically correlated with the PTSD avoidance and numbing cluster (r = −.51, p = .012; see ). However, when correcting for multiple comparisons, only rIFG activation remained significantly negatively associated with PTSD re-experiencing symptoms.
Table 3. Linear regression model predicting PTSD symptoms at six months following index trauma from rIFG (Model 1) and age, gender, PTSD at time of MRI, and rIFG (Model 2).
Table 4. Linear regression model predicting PTSD symptoms at 6 months following index trauma from vmPFC (Model 1) and age, gender, and vmPFC (Model 2).
Table 5. Exploratory bivariate correlations between reactive inhibition neural activation of rIFG and vmPFC during stop signalling anticipation task with PTSD symptom clusters at six months post-trauma.
4. Discussion
The current study was the first to use an fMRI scan to examine unique roles of reactive (outright stopping) and proactive (anticipation of stopping based on contextual cues) inhibition in predicting the development of PTSD symptoms following acute trauma exposure. In line with our hypothesis, we found that less activation in rIFG and vmPFC during reactive inhibition was related to higher PTSD symptoms at 6-months. Our hypotheses regarding behavioural responses and proactive inhibition neural activation patterns with bilateral hippocampus and rIFG were not supported. While the sample was small, this longitudinal study supports and extends earlier findings suggesting that impaired neural response patterns in rIFG and vmPFC during reactive inhibition may help to identify those at risk for the development of PTSD symptoms.
Our finding that reduced activation in vmPFC during reactive inhibition trials was related to the development of PTSD symptoms is supported by previous cross-sectional results showing decreased activation in PFC regions during inhibition trials in PTSD+ individuals compared to controls using a Go/NoGo paradigm (Falconer et al., Citation2008; Jovanovic et al., Citation2013). More generally, the vmPFC is implicated in response to emotional conflict Etkin, Citation2006, Citation2007) and inhibition of the fear response (Jovanovic et al., Citation2013; Milad et al., Citation2007), and has consistently been found to be impaired in PTSD using different fMRI paradigms. For example, PTSD patients show reduced vmPFC activation during fear extinction (Rougemont-Bücking et al., Citation2011) and reduced functional connectivity with the amygdala in response to threat cues (Stevens et al., Citation2013). It is critical to note that our vmPFC findings did not hold after controlling for age and gender or Bonferroni correction and thus was not as strong as rIFG findings and should be taken with caution.
The rIFG has less often been implicated in PTSD, but our finding that reduced activation in rIFG during reactive inhibition trials was related to later PTSD symptoms supports a prior cross-sectional study using the SSAT. Veterans with PTSD, but not combat or healthy controls, showed reduced activation in rIFG during proactive inhibition trials (van Rooij et al., Citation2014), and this impairment did not improve with successful treatment suggesting a more trait-like or pre-existing risk factor. The current study further supports the hypothesis that reduced rIFG functioning is a risk factor for PTSD development; however, here we observed lower activation during reactive and not proactive inhibition suggesting a somewhat different impairment in the development of PTSD versus maintenance of PTSD. These results withstood Bonferroni correction. Exploratory follow-up analyses between rIFG and vmPFC activation and severity of symptoms across the three PTSD symptom clusters demonstrated a robust association between less rIFG activation and PTSD re-experiencing symptoms at six months. None of the other exploratory results remained after the Bonferroni correction. Future larger-scale studies are necessary to further evaluate potential differential effects across symptom clusters.
Links between pre-trauma cognitive control deficits and the development of PTSD have been found in twin studies (Gilbertson et al., Citation2006). Worse neuropsychological performance on response inhibition was related to reduced functional connectivity between mPFC and rostral anterior cingulate cortex (ACC) and IFG regions in veterans with PTSD, suggesting potential widespread dysregulation across both cognitive and emotional processing in PTSD patients that includes dysfunction in the IFG region (Clausen et al., Citation2017). Additionally, a study comparing individuals with recent trauma exposure to non-trauma controls found increased white matter volume near rIFG, potentially reflecting the response to pre-trauma inhibitory control deficits (Wermuth et al., Citation2021). Evidence of increased rIFG-parahippocampal connectivity in PTSD patients compared to no-PTSD controls during a memory suppression task suggests the potential for a compensatory mechanism in PTSD trying to gain inhibitory input in the memory retrieval process (Steward, Das, Malhi, Bryant, & Felmingham, Citation2020). Similar findings were shown during an intrusive memory suppression task with trauma-exposed civilians, where PTSD- individuals showed reduced IFG-parahippocampal functional connectivity but not PTSD+ individuals, supporting a potential disruption of the regulation signal that helps to control activation of unwanted memories in individuals with PTSD (Mary et al., Citation2020). Importantly, there is evidence that rIFG activation and connectivity with other fear-related brain regions can improve with trauma-focused treatment (Rousseau et al., Citation2019) and may improve emotion and fear regulation when targeted using transcranial direct current stimulation (tDCS) (Herrmann, Beier, Simons, & Polak, Citation2016), although findings are mixed (Smits, Geuze, Schutter, van Honk, & Gladwin, Citation2021). A recent resilience model of PTSD has been suggested whereby post-trauma biomarkers of cognitive control, specifically dorsolateral PFC structural integrity, promotes resilience (Roeckner, Oliver, Lebois, van Rooij, & Stevens, Citation2021). Our findings suggest that the rIFG may be another cognitive control region likely implicated in resilience.
We did not find evidence of altered neural activation patterns during proactive inhibition predicting PTSD symptoms in this sample. This was contrary to our hypothesis and prior evidence suggesting that rIFG activation during proactive inhibition is associated with PTSD (van Rooij et al., Citation2014). Furthermore, given the hippocampus’ role in context processing we expected to see differences in hippocampal functioning to be related to PTSD development. There are a couple of possible explanations. First, the current version of the task differs from prior versions such that only three levels of stop chances are indicated by signs (0, *, **) compared to five levels indicated by the colour of the line. This difference may be less noticeable to participants and therefore more subtle. Yet, we did observe the overall expected effect of proactive inhibition on the behavioural level. Second, given that the change in stop signal probability between the three levels is subtle, we may be underpowered to detect differences in neural activation related to the development of PTSD. Finally, it is possible that proactive inhibition is not a risk factor for the development of PTSD, but instead reflects a deficit present in the context of PTSD; however, due to our small sample size, clear conclusions regarding this cannot be determined at this time.
Behaviourally, our results showed a positive effect of stop-signal probability levels on reaction time across trials, demonstrating that proactive inhibition did occur in this sample. However, in contrast to our hypothesis and prior cross-sectional findings of associations between PTSD and response inhibition (Falconer et al., Citation2008; Swick, Honzel, Larsen, Ashley, & Justus, Citation2012; van Rooij et al., Citation2014), behavioural responses of both reactive and proactive inhibition were not related to later development of PTSD symptoms in this sample. It is critical to replicate this study in a larger prospective sample to further clarify the unique roles of reactive and proactive inhibition in risk for PTSD following trauma exposure.
There are a number of limitations to consider in interpreting the results of this study. First, 22% of participants (n = 5) met for a probable diagnosis of PTSD and so we were primarily looking at subthreshold PTSD symptom severity. Second, PTSD symptoms and probable diagnosis were assessed using a self-report measure of PTSD and it would be beneficial for future studies to include clinician-administered scales (e.g. Clinician Administered PTSD Scale). Third, our measure only included DSM-IV-TR PTSD symptoms and so could not look at the four symptom clusters and additional symptoms now included in DSM-5. Fourth, reactive and proactive inhibition were measured after the trauma occurred, and so causality regarding if inhibition deficits were present prior to the trauma or was a result of exposure to the trauma cannot be disentangled. We did find that activation of rIFG during reactive inhibition was related to PTSD symptoms at the time of scan, and so how the presence of trauma or PTSD symptoms may influence inhibition versus the other way around remains unclear. Fifth, we did not find significant associations between behavioural responses of both reactive and proactive inhibition to be significantly associated with 6-month PTSD symptoms and therefore, the theoretical basis for conducting additional neural results is lacking. Sixth, because the main goal of this study is to replicate and extend prior findings, we used ROIs that were previously defined (Jovanovic et al., Citation2012; van Rooij et al., Citation2016; Zandbelt et al., Citation2010) and used (Stevens et al., Citation2021; Van Rooij et al., Citation2015, Citation2014, Citation2016, Citation2018) in earlier studies. One limitation with this approach is that these ROIs were constructed using different methods in the different prior studies (i.e. task-based mask for rIFG, anatomical ROI for the hippocampus, creating a sphere around the peak voxel for vmPFC). This approach allows for direct comparisons, however, we acknowledge that using the same method of ROI definition in one study would be more straightforward. Moreover, our findings may be excessively conservative, as whole brain analyses were not conducted and we may have missed a potential association using this hypothesis driven approach following prior ROIs. Finally, the sample size for this study was small, not only contributing to a lack of power to find an effect but also potentially inflating the significant effects found. Although data did not include extreme outliers, potential, and probable outliers were identified and those may have influenced study results (see Supplemental Table 1). In spite of this, the current findings are in line with previous neuroimaging studies examining response inhibition in PTSD.
In conclusion, results from this study showed that impaired rIFG activation to response inhibition measured two months after trauma was related to higher levels of PTSD symptoms in recently traumatised adults. This effect was also found to a lesser extent with impaired vmPFC response. Neural activation patterns during reactive inhibition may serve as one important indicator to consider when identifying trauma patients most at risk for the development of PTSD and who would likely benefit from post-trauma intervention. Since deficits in inhibitory control can have substantial detrimental effects on daily functioning in addition to the development of PTSD (Aupperle, Melrose, Stein, & Paulus, Citation2012), offering interventions that are best suited to help trauma patients directly address these deficits warrants further consideration. One such option could be behavioural interventions to improve mechanisms related to response inhibition. Another approach is the use of non-invasive neurostimulation such as tDCS or transcranial magnetic simulation (TMS) to directly target brain regions of interest. In addition to the vmPFC, this is one of the first studies showing the rIFG as a potential target for (early) interventions. The rIFG has been targeted in non-invasive neurostimulation studies, and was shown to reduce skin conductance responses to threatening stimuli (Herrmann et al., Citation2016), suggesting its importance for fear-related emotion regulation. Thus, rIFG is a potential interesting target for neurostimulation interventions for the development of PTSD and addressing deficits in response inhibition.
Ethics approval and consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Emory University Institutional Review Board and the Grady Research Oversight Committee.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Supplemental Material
Download MS Word (20.9 KB)Acknowledgements
The authors would like to acknowledge Lauren Hudak, M.D., Debra Houry, M.D., and Abigail HankinWei, M.D., for their collaborative efforts on this study. They would also like to thank Rebecca Hinrichs, Vasiliki Michopoulos, Alex O. Rothbaum, Sterling Winters, Jessica Maples-Keller, Yvonne Ogbonmwan, Thomas Crow, Heather Grinstead, Devika Fiorillo, Renuka Reddy, Zachary Clifford, Adam Munoz, Erin Lightman-Renner, Lydia Odenat, Loren M. Post, Liza C. Zwiebach, Kathryn Breazeale, Jessica Goodnight, Natasha Mehta, Elicia D. Skelton, Taleesha S. Booker, Jonathan Zebrowski, Siddharta Kosaraju, and Ariella Dagi for their work in the Emergency Department recruiting and assessing participants.
Data Availability
Data from this study is fully available online in a repository and can be found on the osf.io platform at https://osf.io/3q7ze/. Doi:10.17605/OSF.IO/3Q7ZE
Disclosure statement
Dr. Ressler has received consulting income from Alkermes, research support from NIH, Genomind, and Brainsway, and he is on scientific advisory boards for Janssen and Verily, all of which is unrelated to the present work. Dr. Rothbaum is a consultant to and owns equity in Virtually Better, Inc. that creates virtual environments. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies, and is unrelated to the present work. Other authors report no potential conflicts of interest.
Additional information
Funding
Notes
1 One participant did not understand the directions of the task, resulting in unusable data.
2 When one probable outlier from reactive rIFG was removed, the correlation with 6-month PTSD symptoms remained significant (see Supplemental Table 2).
3 When one probable outlier from reactive rIFG and one probable outlier from re-experiencing symptoms were removed, the correlation between rIFG and PTSD symptom clusters were significant for avoidance/numbing and hyperarousal but no longer for re-experiencing symptoms (see Supplemental Table 2).
References
- American Psychological Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Pub.
- Aron, A. R., Robbins, T. W., & Poldrack, R. A. (2014). Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences, 18(4), 177–185. doi:10.1016/j.tics.2013.12.003
- Aupperle, R. L., Melrose, A. J., Stein, M. B., & Paulus, M. P. (2012). Executive function and PTSD: Disengaging from trauma. Neuropharmacology, 62(2), 686–694. doi:10.1016/j.neuropharm.2011.02.008
- Ben-Zion, Z., Fine, N. B., Keynan, N. J., Admon, R., Halpern, P., Liberzon, I., … Shalev, A. Y. (2019). Neurobehavioral moderators of post-traumatic stress disorder (PTSD) trajectories: Study protocol of a prospective MRI study of recent trauma survivors. European Journal of Psychotraumatology, 10(1), 1683941. doi:10.1080/20008198.2019.1683941
- Benjet, C., Bromet, E., Karam, E. G., Kessler, R. C., McLaughlin, K. A., Ruscio, A. M., … Koenen, K. C. (2016). The epidemiology of traumatic event exposure worldwide: Results from the world mental health Survey consortium. Psychological Medicine, 46(2), 327–343. doi:10.1017/S0033291715001981
- Buimer, E. E., Pas, P., Brouwer, R. M., Froeling, M., Hoogduin, H., Leemans, A., … Mandl, R. C. W. (2020). The YOUth cohort study: MRI protocol and test-retest reliability in adults. Developmental Cognitive Neuroscience, 45, 100816. doi:10.1016/j.dcn.2020.100816
- Clausen, A. N., Francisco, A. J., Thelen, J., Bruce, J., Martin, L. E., McDowd, J., … Aupperle, R. L. (2017). PTSD and cognitive symptoms relate to inhibition-related prefrontal activation and functional connectivity. Depression and Anxiety, 34(5), 427–436. doi:10.1002/da.22613
- Etkin, A., Egner, T., Peraza, D. M., Kandel, E. R., & Hirsch, J. (2006). Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51, 871–882. doi:10.1016/j.neuron.2006.07.029
- Etkin, A., & Wager, T. D. (2007). Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–1488. doi:10.1176/appi.ajp.2007.07030504
- Falconer, E., Bryant, R., Felmingham, K. L., Kemp, A. H., Gordon, E., Peduto, A., … Williams, L. M. (2008). The neural networks of inhibitory control in posttraumatic stress disorder. Journal of Psychiatry & Neuroscience: Jpn, 33(5), 413.
- Foa, E. B., Riggs, D. S., Dancu, C. V., & Rothbaum, B. O. (1993). Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress, 6(4), 459–473. doi:10.1002/jts.2490060405
- Gilbertson, M. W., Paulus, L. A., Williston, S. K., Gurvits, T. V., Lasko, N. B., Pitman, R. K., & Orr, S. P. (2006). Neurocognitive function in monozygotic twins discordant for combat exposure: Relationship to posttraumatic stress disorder. Journal of Abnormal Psychology, 115(3), 484–495. doi:10.1037/0021-843X.115.3.484
- Hampshire, A., Chamberlain, S. R., Monti, M. M., Duncan, J., & Owen, A. M. (2010). The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage, 50(3), 1313–1319. doi:10.1016/j.neuroimage.2009.12.109
- Herrmann, M. J., Beier, J. S., Simons, B., & Polak, T. (2016). Transcranial direct current stimulation (tDCS) of the right inferior frontal gyrus attenuates skin conductance responses to unpredictable threat conditions. Frontiers in Human Neuroscience, 10, 352. doi:10.3389/fnhum.2016.00352
- Jovanovic, T., Ely, T., Fani, N., Glover, E. M., Gutman, D., Tone, E. B., … Ressler, K. J. (2013). Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex, 49(7), 1884–1891. doi:10.1016/j.cortex.2012.08.011
- Jovanovic, T., Kazama, A., Bachevalier, J., & Davis, M. (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 62(2), 695–704. doi:10.1016/j.neuropharm.2011.02.023
- Jovanovic, T., & Ressler, K. J. (2010). How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. American Journal of Psychiatry, 167(6), 648–662. doi:10.1176/appi.ajp.2009.09071074
- Kessler, R. C., Chiu, W. T., Demler, O., & Walters, E. E. (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National comorbidity Survey replication. Archives of General Psychiatry, 62(6), 617–627. doi:10.1001/archpsyc.62.6.617
- Kilpatrick, D. G., Resnick, H. S., Milanak, M. E., Miller, M. W., Keyes, K. M., & Friedman, M. J. (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of Traumatic Stress, 26(5), 537–547. doi:10.1002/jts.21848
- Kleerekooper, I., van Rooij, S. J. H., van den Wildenberg, W. P. M., de Leeuw, M., Kahn, R. S., & Vink, M. (2016). The effect of aging on fronto-striatal reactive and proactive inhibitory control. Neuroimage, 132, 51–58. doi:10.1016/j.neuroimage.2016.02.031
- Li, C. R., Huang, C., Constable, R. T., & Sinha, R. (2006). Gender differences in the neural correlates of response inhibition during a stop signal task. Neuroimage, 32(4), 1918–1929. doi:10.1016/j.neuroimage.2006.05.017
- Logan, G. D., & Cowan, W. B. (1984). On the ability to inhibit thought and action: A theory of an act of control. Psychological Review, 91, 295–327. doi:10.1037/0033-295X.91.3.295
- Mary, A., Dayan, J., Leone, G., Postel, C., Fraisse, F., Malle, C., … Gagnepain, P. (2020). Resilience after trauma: The role of memory suppression. Science, 367, 6479. doi:10.1126/science.aay8477
- McLean, S. A., Ressler, K., Koenen, K. C., Neylan, T., Germine, L., Jovanovic, T., … Kessler, R. (2020). The AURORA study: A longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Molecular Psychiatry, 25(2), 283–296. doi:10.1038/s41380-019-0581-3
- Milad, M. R., Wright, C. I., Orr, S. P., Pitman, R. K., Quirk, G. J., & Rauch, S. L. (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry, 62(5), 446–454. doi:10.1016/j.biopsych.2006.10.011
- Pas, P., Hulshoff Pol, H. E., Raemaekers, M., & Vink, M. (2021). Self-regulation in the Pre-adolescent brain. Developmental Cognitive Neuroscience, 51, 101012. doi:10.1016/j.dcn.2021.101012
- Roeckner, A. R., Oliver, K. I., Lebois, L. A. M., van Rooij, S. J. H., & Stevens, J. S. (2021). Neural contributors to trauma resilience: A review of longitudinal neuroimaging studies. Translational Psychiatry, 11(1), 1–17. doi:10.1038/s41398-021-01633-y
- Rougemont-Bücking, A., Linnman, C., Zeffiro, T. A., Zeidan, M. A., Lebron-Milad, K., Rodriguez-Romaguera, J., … Milad, M. R. (2011). Altered processing of contextual information during fear extinction in PTSD: An fMRI study. CNS Neuroscience & Therapeutics, 17(4), 227–236. doi:10.1111/j.1755-5949.2010.00152.x
- Rousseau, P.-F., El Khoury-Malhame, M., Reynaud, E., Boukezzi, S., Cancel, A., Zendjidjian, X., … Khalfa, S. (2019). Fear extinction learning improvement in PTSD after EMDR therapy: An fMRI study. European Journal of Psychotraumatology, 10(1), 1568132. doi:10.1080/20008198.2019.1568132
- Smits, F. M., Geuze, E., Schutter, D. J. L. G., van Honk, J., & Gladwin, T. E. (2021). Effects of tDCS during inhibitory control training on performance and PTSD, aggression and anxiety symptoms: A randomized-controlled trial in a military sample. Psychological Medicine, 1–11. doi:10.1017/S0033291721000817
- Stevens, J. S., Ely, T. D., Sawamura, T., Guzman, D., Bradley, B., Ressler, K. J., & Jovanovic, T. (2016). Childhood maltreatment predicts reduced inhibition-related activity in the rostral anterior cingulate in PTSD, but not trauma-exposed controls. Depression and Anxiety, 33(7), 614–622. doi:10.1002/da.22506
- Stevens, J. S., Harnett, N. G., Lebois, L. A. M., van Rooij, S. J. H., Ely, T. D., Roeckner, A., … Ressler, K. J. (2021). Brain-based biotypes of psychiatric vulnerability in the acute aftermath of trauma. American Journal of Psychiatry, 178(11), 1037–1049. doi:10.1176/appi.ajp.2021.20101526
- Stevens, J. S., Jovanovic, T., Fani, N., Ely, T. D., Glover, E. M., Bradley, B., & Ressler, K. J. (2013). Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research, 47(10), 1469–1478. doi:10.1016/j.jpsychires.2013.05.031
- Stevens, J. S., Kim, Y. J., Galatzer-Levy, I. R., Reddy, R., Ely, T. D., Nemeroff, C. B., … Ressler, K. J. (2017). Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress Disorder Symptom maintenance after acute civilian trauma. Biological Psychiatry, 81(12), 1023–1029. doi:10.1016/j.biopsych.2016.11.015
- Steward, T., Das, P., Malhi, G. S., Bryant, R. A., & Felmingham, K. L. (2020). Dysfunctional coupling of the parahippocampal cortex and inferior frontal gyrus during memory suppression in posttraumatic stress disorder. European Neuropsychopharmacology, 41, 146–151. doi:10.1016/j.euroneuro.2020.09.634
- Swann, N. C., Cai, W., Conner, C. R., Pieters, T. A., Claffey, M. P., George, J. S., … Tandon, N. (2012). Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. Neuroimage, 59(3), 2860–2870. doi:10.1016/j.neuroimage.2011.09.049
- Swick, D., Honzel, N., Larsen, J., Ashley, V., & Justus, T. (2012). Impaired response inhibition in veterans with post-traumatic stress disorder and mild traumatic brain injury. Journal of the International Neuropsychological Society, 18(5), 917–926. doi:10.1017/S1355617712000458
- Van Belle, J., Vink, M., Durston, S., & Zandbelt, B. B. (2014). Common and unique neural networks for proactive and reactive response inhibition revealed by independent component analysis of functional MRI data. Neuroimage, 103, 65–74. doi:10.1016/j.neuroimage.2014.09.014
- Van Rooij, S. J., Geuze, E., Kennis, M., Rademaker, A. R., & Vink, M. (2015). Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology, 40(3), 667–675. doi:10.1038/npp.2014.220
- van Rooij, S. J., & Jovanovic, T. (2019). Impaired inhibition as an intermediate phenotype for PTSD risk and treatment response. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 89, 435–445. doi:10.1016/j.pnpbp.2018.10.014
- van Rooij, S. J., Rademaker, A. R., Kennis, M., Vink, M., Kahn, R. S., & Geuze, E. (2014). Impaired right inferior frontal gyrus response to contextual cues in male veterans with PTSD during response inhibition. Journal of Psychiatry and Neuroscience, 39(5), 330–338. doi:10.1503/jpn.130223
- van Rooij, S. J., Ravi, M., Ely, T. D., Michopoulos, V., Winters, S. J., Shin, J., … Stevens, J. S. (2021). Hippocampal activation during contextual fear inhibition related to resilience in the early aftermath of trauma. Behavioural Brain Research, 408, 113282. doi:10.1016/j.bbr.2021.113282
- van Rooij, S. J., Stevens, J. S., Ely, T. D., Fani, N., Smith, A. K., Kerley, K. A., … Jovanovic, T. (2016). Childhood trauma and COMT genotype interact to increase hippocampal activation in resilient individuals. Frontiers in Psychiatry, 7, 156. doi:10.3389/fpsyt.2016.00156
- van Rooij, S. J., Stevens, J. S., Ely, T. D., Hinrichs, R., Michopoulos, V., Winters, S. J., … Jovanovic, T. (2018). The role of the hippocampus in predicting future posttraumatic stress disorder symptoms in recently traumatized civilians. Biological Psychiatry, 84(2), 106–115. doi:10.1016/j.biopsych.2017.09.005
- van Rooij, S., Kennis, M., Sjouwerman, R., van den Heuvel, M. P., Kahn, R. S., & Geuze, E. (2015). Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychological Medicine, 45(13), 2737–2746. doi:10.1017/S0033291715000707
- Verbruggen, F., & Logan, G. D. (2008). Response inhibition in the stop-signal paradigm. Trends in Cognitive Sciences, 12(11), 418–424. doi:10.1016/j.tics.2008.07.005
- Walther, S., Goya-Maldonado, R., Stippich, C., Weisbrod, M., & Kaiser, S. (2010). A supramodal network for response inhibition. Neuroreport, 21(3), 191–195. doi:10.1097/WNR.0b013e328335640f
- Wermuth, K., Ülsmann, D., Borngräber, J., Gallinat, J., Schulte-Herbrüggen, O., & Kühn, S. (2021). Structural signature of trauma: White matter volume in right inferior frontal gyrus is positively associated with use of expressive suppression in recently traumatized individuals. European Journal of Psychotraumatology, 12(1), 1837512. doi:10.1080/20008198.2020.1837512
- Wessa, M., & Flor, H. (2007). Failure of extinction of fear responses in posttraumatic stress disorder: Evidence from second-order conditioning. American Journal of Psychiatry, 164(11), 1684–1692. doi:10.1176/appi.ajp.2007.07030525
- Zandbelt, B. B., Bloemendaal, M., Neggers, S. F. W., Kahn, R. S., & Vink, M. (2013). Expectations and violations: Delineating the neural network of proactive inhibitory control. Human Brain Mapping, 34(9), 2015–2024. doi:10.1002/hbm.22047
- Zandbelt, B., Vink, M., & Rodriguez-Fornells, A. (2010). On the role of the striatum in response inhibition. PLoS One, 5, e13848. doi:10.1371/journal.pone.0013848
- Zhang, F., & Iwaki, S. (2019). Common neural network for different functions: An investigation of proactive and reactive inhibition. Frontiers in Behavioral Neuroscience, 13, 124. doi:10.3389/fnbeh.2019.00124