ABSTRACT
Salmonellosis is one of the main bacterial infections affecting commercial poultry, causing losses to poultry production, and posing a public health concern.
Samples from internal organs (liver, cecum and spleen) of one hundred diseased broiler chickens were collected and subjected to Salmonella isolation, identification and serotyping. S. typhimurium and S. enteritidis were selected from the isolated Salmonella to prepare bacteriophages from sewage water taken at broiler farms. An experimental infection of one day old specific pathogen free (SPF) chicks followed by treatment with the prepared bacteriophages isolated from both Salmonella was performed. Caecal samples from infected chicks were subjected at intervals to bacteriophage isolation and Salmonella quantitation. The effectiveness of bacteriophage treatments on Salmonella colonization in cecum of infected chicks increased after five successive doses. At 3 day post infection (dpi), cecal contents showed a marginal decrease in Salmonella loads with more reduction at 5 dpi. From 7 dpi to the end of the experiment at 15 dpi, all the chicks were cleared for both Salmonella.
The findings of this study demonstrate that bacteriophage treatment is efficacious in reducing S. typhimurium and S. enteritidis colonization in broiler chickens within a short period and could be used as an alternative to antibiotics.
Introduction
Salmonellosis is one of the main infections affecting commercial poultry, causing losses to poultry production, and posing a public health concern [Citation1]. Salmonella, the causative agent for salmonellosis, are Gram negative, rod shaped, facultative anaerobic bacteria causing gastroenteritis [Citation2]. Fowl typhoid and pullorum disease, are widely distributed septicemic diseases, caused by S. gallinarum and S. pullorum respectively and infect primarily chickens and turkeys. These bacteria are transmitted mainly transovarially. Feces of infected birds, contaminated feed, water and litter can also be sources for infection. Clinical signs in chicks and poults include anorexia, dehydration, weakness, diarrhea and high mortality. Decreased egg production, fertility and hatchability are the most important clinical signs in mature birds. Gross and microscopic lesions include hepatitis, typhlitis, omphalitis, pneumonia, ophthalmitis salpingitis, synovitis and peritonitis [Citation3].
Poultry gastrointestinal tract is considered as a major reservoir for various pathogenic bacteria that can cause cross-contamination of poultry meat and egg products [Citation4]. For example, Salmonella can invade the intestinal epithelial cells and survive intracellularly within macrophages [Citation5] and these intracellular Salmonella are not easily controlled by antibiotics. Bacteriophage control has received much attention as a potential treatment approach for bacterial infections [Citation6] due to the emergence of antibiotic-resistant bacteria [Citation7].
Bacteriophages are bacterial viruses [Citation8], and as suggested by previous studies, possible alternatives to antibiotics for treatment of bacterial diseases [Citation9]. Bacteriophages have no adverse effects on human or animal immune systems and does not affect the normal bacterial flora [Citation10]. Phages are highly host specific, typically targetting a particular group of bacterial species [Citation11]. Bacteriophages multiply inside the infected host cell in a so called lytic infection cycle, and are released from host cells by bacteriolysis. Bacteriophages infect bacteria by injecting their DNA across the bacterial envelope into the cytoplasm of the cell [Citation12]. The injected phage DNA is replicated using the infected host cell metabolic machinery and the genes encoding the phage protein coat are expressed systematically [Citation13].
The current study aims to isolate and identify Salmonella from internal organs of diseased broilers and assessing the effectiveness of crude bacteriophage preparations in the treatment of Salmonella colonization in the birds.
Material and methods
Sample collection and identification
A total of one hundred diseased broiler chickens (different ages) from 75 farms in Dakahlia Governorate (Egypt) were collected in the end of the 2017 and in the beginning of the year 2018. Samples from internal organs (liver, spleen and cecum) from each bird were collected aseptically, labeled and transported directly in an ice box to Reference Laboratory for Veterinary Quality control on Poultry production (RLQP) (Gamasa Lab) for Salmonella isolation.
Liver, cecum and spleen from each broiler chicken were pooled together as one sample. Salmonella was isolated and identified according to ISO 6579 (2017) [Citation14] as follows: Samples were weighed and suspended in buffered peptone water (1:10 dilution) and incubated at 37ºC for 18 hours. The pre-enrichment broth after incubation was mixed and 0.1 ml of the broth was transferred into a tube containing 10 ml of Rappaport-Vassiliadis medium with soya (RVS broth). Another 1 ml of the pre-enrichment broth was transferred into a tube containing 10 ml of Muller-Kauffmann tetrathionate novobiocin broth (MKTTn broth). The inoculated RVS broth was incubated at 41.5°C for 24 hours and the inoculated MKTTn broth at 37°C for 24 hours. After incubation, a loop-full of material from the RVS broth and MKTTn was transferred and streaked separately onto the surface of Xylose Lysine Deoxycholate agar (XLD agar), Hektoen Enteric (HE agar), MacConkey’s agar and S-S agar respectively. The plates were incubated at 37°C for 24 hours then checked for growth of typical Salmonella colonies.
The isolates that were biochemically identified as Salmonella (methyl red, citrate utilization, triple sugar iron and catalase tests positive & negative to V-P, urease and indole tests) were subjected to serological identification according to Kauffman- white scheme [Citation15] for determining somatic (O) and flagellar (H) antigens [Citation16].
Bacteriophages isolation and purification
S. typhimurium and S. enteritidis isolated from diseased broiler chickens in this study were selected and used for bacteriophage preparation. According to [Citation10,Citation13]; five sewage samples were collected from broiler farms and centrifuged at low speed (8496 g) for 10 minutes to remove solid particles and then filtered with 0.22 μm pore syringe filters. For bacteriophages enrichment, isolation and purification; S. typhimurium and S. enteritidis were grown overnight at 37°C in nutrient broth to obtain pure bacterial cultures. The next day, 0.1 ml of the overnight cultures were inoculated into 10 ml nutrient broth and incubated at 37°C for 3 hours with agitation to reach exponential phase. The sewage filter sample supernatant (4.5 ml) was mixed with 0.5 ml exponential phase bacterial cultures and 0.5 ml concentrated nutrient broth and was incubated at 37°C for 24 hours. After incubation samples were centrifuged at 8496 g for 10 minutes, supernatants were filtered with a 0.22 μm filter syringe and was used as enriched phage (EP) samples.
Detection of bacteriophages: spot technique and plaque assay
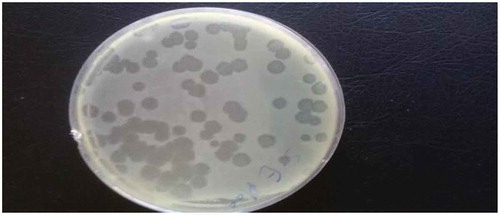
The prepared bacteriophages were tested by spot testing to ensure the presence of lytic phages in the prepared enriched phage filtrates. One ml of cultured S. typhimurium and S. enteritidis was spread separately on nutrient agar plates, the excess fluid was removed and the plates were kept at room temperature to dry. 10 μl of the prepared enriched phage filtrate was spotted on the agar and allowed to dry. The agar plates were incubated at 37°C overnight for the detection of lytic spots (clearance zones) on to the agar plates according to Rahaman et al. [Citation17].
Plaque assay was conducted according to Akhtar et al. [Citation13], with some modifications; Ten-fold serial dilution was performed using 0.1 ml of the phage suspension (as prepared above). A single colony of overnight culture of S. typhimurium and S. enteritidis was inoculated separately into 5 ml of nutrient broth and incubated at 37°C for 3 hours. Semisolid agar (0.5%) was aliquoted (3 ml) into tubes placed at 45°C in a water bath, 0.1 ml phage and 0.5 ml S. typhimurium and S. enteritidis separately were added to semisolid agar tubes, then mixed and poured onto nutrient agar plates. The plates were allowed to cool and incubated overnight at 37°C. Plaques were counted and the number of phages was determined as plaque forming unit (per ml?) (PFU).
In vivo assay of bacteriophage treatment in experimentally infected chicks
Ethical approval: The animal experiment was performed according to the legally approved protocol [AHRI (29) 23/11/2017] of the Animal Health and Research Institute (AHRI), Giza, Egypt. Isolation experiments were performed in separated cages at a biosecurity level- two (BSL-2) animal facility.
Experimental design: One hundred and twenty SPF chicks (one day old) were housed in air-filtered isolation cabinets in Reference Laboratory for Veterinary Quality Control on Poultry production (RLQP). From , the chicks under experiment (120 chicks) were divided into four groups, each group contained 30 chicks. Each group of birds was placed individually, provided feed and water ad libitum. The chicks were maintained at an age-appropriate temperature until the end of the experiment.
Table 1. experimental design of bacteriophage treatments in (SPF) chicks challenged with S. typhimurium and S. enteritidis.
Bacteriophages of S. typhimurium that were prepared in this study were administered orally on several occasions; in all chicks of group 2 at days 1, 2, 3, 6, 8, 10, 13 and 15 with a 0.1 ml solution containing 1.18 × 1011 PFU phages/chick in 0.1 ml. Subsequently, all chicks in this group were challanged orally at day two with a single dose of 105 CFUs S. typhimurium in a total volume of 0.1 ml. Group 3 followed a similar regime, except that the orally administered bacteriophages (1.03 × 1012 PFU/chick) were amplified and isolated from S. enteritidis cultures and the chicks were challenged orally at day two with a single dose of 105 CFUs S. enteritidis.
Group 4 was a positive control for both Salmonella. Fifteen chicks were inoculated orally at day two with a single dose of 0.1 ml culture of S. typhimurium at a concentration of 105 CFU/chick. The remaining fifteen chicks were kept in another separate isolator and inoculated orally with a single dose of 0.1 ml culture of S. enteritidis at a concentration of 105 CFU/chick. Group 1 was left as a negative control and not inoculated with bacteriophages or Salmonella.
Five chicks from group 1, 2, 3 and 4 respectively, were euthanized at days 5, 7, 9, 11, 14 and 17. The experiment was terminated on day 17.
Isolation of Salmonella and bacteriophages from chicks during experiment
Liver, spleen and cecum of the necropsied chicks were subjected to Salmonella isolation and identification according to ISO 6579 (2017) [Citation17]; the representative colonies of S. typhimurium and S. enteritidis were confirmed by slide agglutination tests with poly (O), poly (H), and serotype-specific antisera. The Bacteriophages were isolated from caecal contents of group 2 and 3; a suspension of cecal contents (1 gram caecal content: 9 ml physiological saline, pH 7.4) was centrifuged at (10,000 rpm) for 10 minutes to remove any debris. The supernatant was then filtered with 0.22 μm filter syringe to remove any remaining bacteria. Finally 10 μl of the filtrate was used for spot testing to detect the presence of bacteriophages according to Rahaman et al. [Citation17].
Quantitative detection of Salmonella in caecal contents of chicks
Total DNA was extracted from caecal contents of all chicks in groups 2 & 3 and one chick from group 1 and 4 at each time point using QIAamp DNA Mini kit (Qiagen, Germany, GmbH) with modifications from the manufacturer’s recommendations (the rest of chicks in group 1 and 4 were examined by plating on XLD agar [Citation18]). Briefly, 200 µl of the caecal content suspension was incubated with 10 µl of proteinase K and 200 µl of lysis buffer at 56°C for 10 min. After incubation, 200 µl of 100% ethanol was added to the lysate. The sample was then washed and centrifuged following the manufacturer’s recommendations. DNA was eluted with 100 µl of elution buffer provided in the kit. Oligonucleotide primers and probes that used were provided from Metabion (Germany) listed in . DNA amplifications were performed in a final volume of 25 µl containing 3 µl of DNA template, 12.5 µl of 2 × QuantiTect Probe RT-PCR Master Mix, 8.875 µl PCR grade water, 0.25 µl of each primer (50 pmol conc.) and 0.125 µl of each probe (30 pmol conc.). Primary denaturation was performed at 94°C for 15 min, followed by 40 cycles of denaturation at 94°C for 15 s, annealing at 49°C for 30 s and extension at 72°C for 10 s. The bacterial concentration of samples was determined with cfu/g. A known standard (of Known CFU/g) was ten-fold serially diluted, then DNA was extracted separately from the last 5 dilutions and tested by rt-PCR with the same conditions of the unknown samples. The standard was included in all PCR runs. The reaction was done in Stratagene MX3005P real time PCR machine (that can show the standard quantity with 3 decimals).
Table 2. Oligonucleotide primers and probes used in this study.
Results
Incidence of Salmonella isolation
Eight Salmonella clones were isolated from 100 diseased broiler chickens in Dakahlia Governorate with an incidence of 8%. The serotyping of the isolated Salmonella revealed (2) S. typhimurium, (2) S. kentucky, (1) S. enteritidis, (1) S. molade, (1) S. inganda and (1) S. apyeme.
Bacteriophage isolation, detection and enumeration
Bacteriophages of S. typhimurium and S. enteritidis were prepared from environmental sewage samples from broiler farms. Spot testing confirmed the presence of specific lytic bacteriophages through the appearance of clearance zones (lytic spots) in plates coated with respective Salmonella species. Bacteriophages were also enumerated using plaque assay for both Salmonella species to prepare the phage samples needed for the experiment () and the results revealed that the concentration of bacteriophages infecting S. typhimurium was 118 × 1010 PFU/ml and 103 × 1011 PFU/ml for S. enteritidis.
Clinical signs and bacteriophage isolation
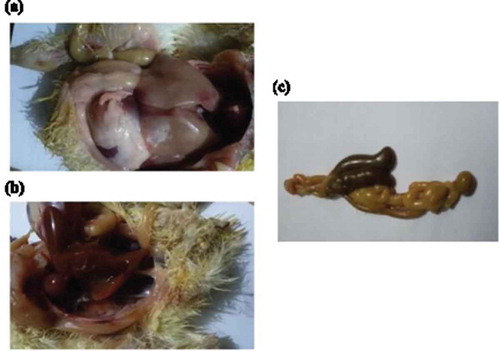
All chicks in group 1 (negative control) showed no clinical signs and no post mortem findings. The chicks in group 4 part A and part B (positive controls for S. typhimurium and S. enteritidis infections respectively) showed depression, loss of appetite, inability to stand, diarrhea and pasting vent. Congestion of internal organs, hemorrhages in liver, unabsorbed yolk sac in some chicks and enlargement of two caeci with diarrhea were observed (). The mortalities appeared after 3 days of Salmonella and the incidence of mortalities at the end of the experiment were 30% for S. enteritidis and 20% for S. typhimurium.
Figure 2. (a) hemorrhagic patches in liver with distention of intestine with diarrhea (b) congestion of internal organs with pasting vent (c) enlargement and distention of the two ceci with diarrhea.
In the infected groups 2 and 3 that were treated with bacteriophages at different time points (days 1, 2, 3, 6, 8, 10, 13 and 15) showed depression and diarrhea after 2 days of infection. These signs disappeared gradually and the postmortem findings at 3 dpi were enlargement of two ceci with diarrhea, congestion in liver and few chicks showed hemorrhages in heart. At 7 dpi until the end of experiment, the activity of the chicks increased and the diarrhea stopped.
Bacteriophages were isolated from caecal contents of sacrificed chicks in group 2 and 3; spot tests confirmed the presence of Salmonella specific bacteriophages, producing distinct clearance zones on plates with the two different Salmonella species respectively.
Salmonella isolation, identification and quantitative detection
All chicks in group 1 (uninfected negative control) were negative for Salmonella colonization as determined by plating on XLD agar. All chicks in group 4-A and 7 chicks in group 2 till the end of the experiment were positive for S. typhimurium and the species was confirmed using serotype-specific antisera.
Similarily, all chicks in group 4-B and 7 chicks in group 3 were positive for S. enteritidis and again, the species was confirmed using serotype-specific antisera. All chicks in group 4 (infection control) were tested positive for Salmonella by plating on XLD agar.
Quantitative Real time PCR (RT- PCR) was used as a rapid and accurate technique to determine Salmonella loads in caecum of necropsied chicks in groups 2 and 3 and to investigate the significance and the effectiveness of bacteriophage treatments on Salmonella colonization. & show that bacteriophage treatments start to reduce S. typhimurium (group 2) and S. enteritidis (group 3) colonization in cecum after four successive doses. At the 3 dpi of age, cecal contents of all sacrificed chicks showed a slight decrease in Salmonella colonization in comparison to positive control groups. In the 5 dpi colonization of both Salmonella reduced in comparison with the 3 dpi. From the beginning of the 7 dpi till the end of the experiment at 15 dpi(after five successive doses of bacteriophage treatments) all the chicks showed no colonization for both Salmonella in caecum which suggest that the bacteriophage treatment is successful in treatment of Salmonella..
Table 3. Quantitative detection of S. typhimurium colonization in cecum of chicks under experiment.
Table 4. Quantitative detection of S. enteritidis colonization in cecum of chicks under experiment.
Discussion
In the current study the incidence of Salmonella was similar to that previously reported by Abd El-Ghany et al. [Citation20], who isolated Salmonella from diseased broilers in Kalubia Governorate- Egypt with an incidence of (7.03%). Serotyping in that study revealed the presence of S. typhimurium, S. kentucky and S. enteritidis, species that were also present in the current study. Also, some authors such as Jafari et al. [Citation21], and Boonmara et al. [Citation22], previously reported similar incidence levels of Salmonella, which were (5.8%) and (6.65%) respectively. In a previous study performed by Mohamed et al. [Citation23], in Kafr Elsheik Governorate- Egypt, Salmonella was isolated from broiler chickens with an incidence of (2.5%) and this percentage was lower than the current study. A higher percentage of Salmonella isolation was recorded and isolated with an incidence of (12%).
Bacteriophages in this study were isolated and prepared from sewage of poultry farms. Presence of Salmonella specific bacteriophages has previously been reported from excretion sewage of commercial broiler houses [Citation24]. Meanwhile Andreatti Filho et al. [Citation18], isolated bacteriopgages from environmental drag swabs in commercial broiler houses. Spot tests were used in this study to confirm the presence of specific bacteriophages in the preparations, other studies such as Rahaman et al. [Citation17], have used similar strategies to test for the presence of specific bacteriophages. Further, the presence of bacteriophages during the experiment was verified by isolation of phages from caecal contents of chicks treated with bacteriophages, a similar method used in previous studies [Citation25,Citation26].
In this study bacteriophages were administrated to chicks before oral Salmonella infections followed by 4 successive phage treatments after the bacterial challenge. Although Salmonella were still able to colonize the chicks, bacterial loads decreased after four successive phage treatments. After the 5th dose no bacteria were detected indicating that the chicks treated with phages were cured of Salmonella. This effect is most likely due to the lytic effect of the administered bacteriophages against Salmonella. Moreover, bacteriophages have previously been used to control intracellular pathogens [Citation27]. Some bacteriophages can be efficacious in reducing S. enteritidis colonization in poultry during a short period [Citation18]. In our study, we were able to clear Salmonella from infected chicks after successive phage treatments applied within a short time period after infection. A previous study reported similar results as the present study, where bacteriophages was reported as a factor resulting in reduced Salmonella CFU/g caecal content and reduced Salmonella colonization in broiler chicks after 5 days of treatment [Citation26]. Bacteriophages have also been reported to reduce the viability of S. typhimurium in chicken cecum for up to 12 hours after inoculation [Citation25].
Thus, bacteriophage treatment has a significant effect in reducing colonization of both S. enteritidis and S. typhimurium in cecum of broiler chickens as previously suggested by Atterbury et al. [Citation28].
In conclusion, the increasing resistance of Salmonella strains to the most used antibiotics in broiler farms is considered an important problem leading to high economic losses to the poultry industry. Antibiotic treatments does not only kill pathogenic bacteria but also affect the normal micro flora, potentially leading to secondary infections. Hence, novel bacteriophage treatments, as shown in this study, show great promise for the treatment of bacterial infections in the poultry industry. Phage therapy has reduced side effects compared to traditional antibiotic treatments due to the specificity of phages. Our results suggest that bacteriophage treatment is efficacious in reducing S. typhimurium and S. enteritidis colonization in cecum of broiler chicks within a short period after oral administration of the prepared bacteriophage. Treatments with five successive doses was highly effective and cleared the Salmonella infection in new born chicks, therefore it could be recommended to administer bacteriophages orally with five consecutive doses to reduce the Salmonella load in poultry farms. Further studies with a wider scope is recommended to investigate the implementation of bacteriophage administration programs in poultry farms as a routine treatment, phage typing and the specificity of the prepared phage(s) of S. typimurium will be tested on S. enteritidis and vice versa.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Nehal M. Nabil
Nehal Nabil Graduated in 2006 from mansoura universety with PHD in bacteriology, mycology and immunology in 2015; and the quality manager of reference lab for vetrinary quality control on poultry production, Animal health research institute, gamasa, Dakahlia, Egypt.
Maram M. Tawakol
Maram Tawakol graduated in 2004 from mansoura university with PHD in bacteriology, immunology and mycology in 2014. She is the head of Biotechnology unit at reference lab for veterinary quality control on poultry production animal health research institute, gamasa, dakahlia, Egypt
Heba M. Hassan
Heba Hassan did her PHD in bacteriology mycology and immunology in 2014. In parallel with her work as microbiologist, she is depty of residue analysis unit and the head of the biosafety officer at the reference laboratory for veterinary quality control on poultry production ,Animal Health Research Institute, Giza ,Egypt.
References
- Berchieri A Jr., Murphy CK, Marston K, et al. Observations on the persistence and vertical transmission of Salmonella enterica serovars pullorum and gallinarum in chickens: effect of bacterial and host genetic background. Avian Pathol. 2001 Jun;30(3):1–8. PubMed PMID: 19184904; eng.
- Özkalp B. Isolation and identification of Salmonellas from different samples, Salmonella Dr. Barakat S M Mahmoud, INTECHOPEN. 2012. Available from: https://www.intechopen.com/books/salmonella-a-dangerous-foodborne-pathogen/isolation-and-identification-of-salmonellas-from-different-samples
- Shivaprasad HL. Fowl typhoid and pullorum disease. Rev Sci Tech. 2000 Aug;19(2):405–424. PubMed PMID: 10935271; eng.
- Vikram A, Jayaprakasha GK, Jesudhasan PR, et al. Obacunone represses Salmonella pathogenicity islands 1 and 2 in an envZ-dependent fashion. Appl Environ Microbiol. 2012 Oct;78(19):7012–7022. PubMed PMID: 22843534; PubMed Central PMCID: PMCPMC3457478. eng.
- Knodler LA, Celli J, Finlay BB. Pathogenic trickery: deception of host cell processes. Nat Rev Mol Cell Biol. 2001 Aug;2(8):578–588. PubMed PMID: 11483991; eng.
- Golkar Z, Bagasra O, Pace DG. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J Infect Dev Ctries. 2014 Feb 13;8(2):129–136. PubMed PMID: 24518621; eng.
- Agada GOA, Abdullahi IO, Aminu M, et al. Prevalence and antibiotic resistance profile of Salmonella isolates from commercial poultry and poultry farm-handlers in Jos, Plateau State, Nigeria. Br Microbiol Res J. 2014;4(4):462–479.
- Hendrix RW. Bacteriophage genomics. Curr Opin Microbiol. 2003 Oct;6(5):506–511. PubMed PMID: 14572544; eng.
- Connerton PL, Connerton IF. Microbial treatments to reduce pathogens in poultry meat. In: Mead GC, editor. Food safety control in the poultry industry. Woodhead Publishing; 2005. p. 414–432. Available from: https://www.elsevier.com/books/food-safety-control-in-the-poultry-industry/mead/978-1-85573-954-3.
- Iqbal A, Hasni S, Rahman S, et al. Preparation and evaluation of bacteriophage lysate specific for Salmonella typhimurium. Int J Curr Microbiol Appl Sci. 2016;5(1):828–835.
- Sulakvelidze A, Alavidze Z, Morris JG Jr. Bacteriophage therapy. Antimicrob Agents Chemother. 2001 Mar;45(3):649–659. PubMed PMID: 11181338; PubMed Central PMCID: PMCPMC90351. eng.
- Singleton P. Bacteria in biology, biotechnology and medicine. John Wiley and Sons Ltd; 1999. p. 4. Available from: https://www.wiley.com/en-us/Bacteria+in+Biology%2C+Biotechnology+and+Medicine%2C+6th+Edition-p-9780470090275.
- Akhtar M, Viazis S, Diez-Gonzalez F. Isolation, identification and characterization of lytic, wide host range bacteriophages from waste effluents against Salmonella enterica serovars. Food Control. 2014 Apr 01;38: 67–74.
- 6579-1 I. Microbiology of the food chain – horizontal method for the detection, enumeration and serotyping of Salmonella – part 1: detection of Salmonella spp. 1st ed. 2017. Available from: https://www.iso.org/standard/56712.html .
- Kauffmann F. Serological diagnosis of salmonella-species. In: Kauffmann, F. Kauffmann-White-Schema. 1972. pp. 126. Available from: https://www.cabdirect.org/cabdirect/abstract/19732702666.
- Grimont PAD, François-Xavier W. Antigenic formulae of the Salmonella serovars. 9th ed. Paris: WHO Collaborating Centre for Reference and Research on Salmonella; 2007.
- Rahaman MT, Rahman M, Rahman MB, et al. Poultry Salmonella specific bacteriophage isolation and characterization. Bangladesh J Vet Med. 2014;12(2):107–114.
- Andreatti Filho RL, Higgins JP, Higgins SE, et al. Ability of bacteriophages isolated from different sources to reduce Salmonella enterica serovar enteritidis in vitro and in vivo. Poult Sci. 2007 Sep;86(9):1904–1909. PubMed PMID: 17704377; eng.
- Daum LT, Barnes WJ, McAvin JC, Neidert MS, Cooper LAHuff WB, Lohman kl. Real-time Pcr Detection Of Salmonella in Suspect Foods from a Gastroenteritis Outbreak in Kerr County, Texas. J Clin Microbiol. 2002;40(8):3050-3052.
- Abd El-Ghany W, El-Shafii S, Hatem M. A survey on Salmonella species isolated from chicken flocks in Egypt. Asian J Anim Vet Adv. 2012;7(6):489–501.
- Jafari R, Ghorbanpour M, Jaideri A. An investigation into Salmonella infection status in backyard chickens in Iran. Int J Poult Sci. 2007;6(3):227–229.
- Boonmar S, Bangtrakulnonth A, Pornrunangwong S, et al. Salmonella in broiler chickens in Thailand with special reference to contamination of retail meat with Salmonella enteritidis. J Vet Med Sci. 1998 Nov;60(11):1233–1236. PubMed PMID: 9853305; eng.
- Mohamed LN, Samaha HA, Draz AA, et al. Salmonellae among birds and human beings. Alex J Vet Sci. 1999;5(1):147–154.
- Bao H, Zhang H, Wang R. Isolation and characterization of bacteriophages of Salmonella enterica serovar pullorum. Poult Sci. 2011 Oct;90(10):2370–2377. PubMed PMID: 21934022; eng.
- Berchieri A Jr., Lovell MA, Barrow PA. The activity in the chicken alimentary tract of bacteriophages lytic for Salmonella typhimurium. Res Microbiol. 1991 Jun;142(5):541–549. PubMed PMID: 1947426; eng.
- Fiorentin L, Vieira ND, Barioni W Jr. Oral treatment with bacteriophages reduces the concentration of Salmonella enteritidis PT4 in caecal contents of broilers. Avian Pathol. 2005 Jun;34(3):258–263. PubMed PMID: 16191711; eng.
- Ahn J, Lee H-Y, Biswas D. Assessment of bacteriophage-induced inflammatory mediators in Salmonella-infected chicken macrophage HD11 cells. J Poult Sci. 2015;52(3):238–243.
- Atterbury RJ, Van Bergen MAP, Ortiz F, et al. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl Environ Microbiol. 2007 May 25 [Received Jan 9; Accepted May 16];73(14):4543–4549. PubMed PMID: PMC1932804.