?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: Food safety is of increasing global concern, and a OneHealth issue requiring attention of many disciplines. Aflatoxins are toxins produced by fungi and found in foods and feeds, and exposure causes negative health effects in humans and animals. When lactating animals consume aflatoxin B1, the metabolite (AFM1) is transferred to milk.
Methods: A cross-sectional study was designed to determine characteristics of smallholder dairy farming in urban and peri-urban areas of Kisumu and quantify AFM1 in milk. Data was collected from 97 randomly selected dairy farms on farming practices, milk production, and awareness about aflatoxins. Collected milk samples were analyzed using enzyme-linked immunosorbent assay for AFM1.
Results: Average milk produced was 13 liters per day per household and mainly used for household consumption and sold to neighbours. Farmers mainly fed cows on forage and concentrates (62.9%). Levels of AFM1 ranged from below the detection limit to 151 ppt, with a mean of 29.67 ppt; 26.4% exceeding the EU limit. Concentrate feeding was associated with higher AFM1 levels (p = 0.002); with farms feeding concentrates more likely to have levels exceeding 50 ppt (OR = 10.1).
Conclusion: In conclusion, milk produced by small holder dairy farmers in Kisumu County frequently is contaminated with AFM1, implying health risks for human and animals.
Introduction
Animal-source foods are important for food and nutrition security, but risks of transmission of biological or chemical hazards cause food safety issues and potential negative health impacts. Food and feed contamination with mycotoxins is a major public health concern especially in tropical and subtropical regions. Mycotoxins are secondary metabolites of fungi, and aflatoxins, produced by the Aspergillus species [Citation1], are probably the most studied and the most abundant class of mycotoxins. Aflatoxins are immunotoxic, carcinogenic, mutagenic and hepatotoxic [Citation2]. They can cause growth retardation in animals [Citation3] and have been associated with stunting in children [Citation4,Citation5]. The fungi grow when there are favourable conditions of moisture, warm temperatures [Citation6] and poor storage conditions [Citation7]. Toxin production can occur in almost all stages of the value chain, in the field, during processing, transportation and storage [Citation8]. Mycotoxins in food and feeds should therefore be monitored from farm-to-fork to assure safety to consumers. Aflatoxins B1, B2, G1 and G2 occur naturally in crops [Citation9]. Aflatoxin B1 (AFB1) is the most prevalent of all, and has been associated with acute aflatoxicosis that manifests as hepatotoxicity (with a case fatality rate of 25% in some outbreaks). Aflatoxin B1 is considered as a group I carcinogen for humans [Citation10].
Aflatoxin-contaminated feeds, when given to animals, can affect their health and productivity, and when present in animal-source foods such as milk, may affect the health of those consuming these products [Citation11]. Aflatoxin B1 is the main aflatoxin in contaminated feed. Aflatoxin M1 (AFM1), a metabolite of AFB1, is present in milk of animals that have been fed diet contaminated with AFB1. About 3% of dietary intake of AFB1 is excreted as AFM1 [Citation12], but this may vary with the cow productivity and other cow factors [Citation13,Citation14]. AFM1 is excreted within 12 hours of administration of contaminated feeds [Citation15]. Considering that cow’s milk is often among the first food a child is introduced to, and given that children at this early age are not immune competent, intake of milk contaminated with AFM1 may further suppress their immunity and make them more susceptible to other diseases.
Smallholder dairy farmers in Kenya face a number of challenges, including inadequate feeding. Many farmers are unable to buy commercial feeds, and often lack properly constructed feed stores as well as knowledge on safe formulation of feed rations. As a result, they rely on crop residues and cereals to feed their animals, which may have been discarded due to mold spoilage [Citation16]. Urban dairy farmers spend nine times more money on purchasing commercial feeds than their rural counterparts [Citation17] and are also at a higher risk of feeding AFB1-contaminated animal feeds [Citation18]. There is very scarce and scattered data of AFM1 in milk from small-scale dairy farms in Kisumu County, yet the county is characterized by high temperatures (over 25 °C) and high humidity (40–89%) which foster mould growth and aflatoxin contamination. A few studies of aflatoxins in Kisumu County, focusing on marketed milk, found AFM1 levels up to 130 ppt (parts per trillion) [Citation19] despite the recommend limit of 50 ppt by EU. It is important to keep aflatoxin contamination at levels as low as possible as exposure to small amounts may still be harmful to human health. The objectives of this study were to 1) determine baseline characteristics of smallholder dairy farming in urban and peri-urban areas of Kisumu and 2) quantify the levels of AFM1 in milk produced in the County.
Materials and methods
Selection of study sites and sample size calculation
Four urban and peri-urban sub-counties; Kisumu East, Kisumu Central, Kisumu West, and Nyando were purposively selected for this study. They were selected because of high levels of urban and peri-urban agriculture, and presence of smallholder dairy farmers managing their animals through zero – grazing systems. The County Veterinary Department provided the list of sub-counties meeting the inclusion criteria. Livestock extension officers in each sub-county were then asked to provide a list of dairy farmers in their areas, which then constituted the sampling frame, from which 100 farms were randomly selected, using randomization in MS Excel. Sample size (n = 100 smallholder dairy farms) was calculated using the method suggested by Daniel [Citation20]. Replacements were made for farms that, though selected, were not available to participate in the study. Ethical review permit was obtained from the Institutional Research Ethics Committee of the International Livestock Research Institute, approval number ILRI-IREC2017–10.
Participatory appraisals and household survey
A one-day participatory meeting was held with key dairy stakeholders in Kisumu County, including a representation of farmers, livestock extension officers, Kenya Dairy Board, Directorate of Veterinary Services and milk traders. The objective of the meeting was to provide details (goals, phases of the study etc.) for the project and officially launch the project activities. Farm visits were organized immediately after the stakeholder meeting. A pre-tested questionnaire was used to capture data on household characteristics (e.g. gender, age, level of education etc.), herd characteristics (species kept, their number etc.), animal health challenges, feeding practices, milk production levels and respondents awareness about moulds and aflatoxins.
Milk sample collection
Raw bulk milk samples were collected from each study farm, except for farms where there was no milk at the time of the visit. In such cases, we requested the farmer to obtain for us a small quantity of milk from one of the cows at the time of the visit. Samples were collected by trained research assistants, to achieve consistency and to minimize the risks of microbial contamination at the time of sampling. The samples were collected in duplicate, in sterile 50 millilitre falcon tubes. The samples, while in the field, were safely kept in cooler boxes, and later transferred to freezers at the County Veterinary Department, where they were kept frozen awaiting transportation to the laboratory at International Livestock Research Institute (ILRI), for further storage and analyses. Best practices for handling of laboratory samples were observed throughout the sample handling and storage processes.
Enzyme immunoassay for aflatoxin M1 (AFM1) in milk
Milk samples were analysed using commercial ELISA kit for AFM1 (Helica Biosystems, Inc., Santa Ana, CA 92704, USA, Catalog No. 961AFLM01M-96) according to the manufacturer’s instructions. The samples were thawed prior to being analyzed and reagents brought to room temperature before use. The limit of detection of AFM1 was 2 parts per trillion (ppt). Samples with AFM1 values above the highest standard concentration (100 ppt) were diluted further and the assay repeated until the AFM1 value quantification fell below the 100 ppt aflatoxin values in the standards.
Samples and 200 μl aliquots of the standards were dispensed into appropriate wells in duplicate. The plate was covered with sealing tape to avoid evaporation while providing protection from excess light, and incubated at ambient temperature (19°-25°C) for 2 hours. The contents of the wells were discarded and washed thrice by filling with PBS-Tween 20® and using a multi-channel pipette. The wells were tapped on a layer of absorbent paper facing down to remove any residual wash buffer. A 100 μl of conjugate was added to each well, resealed and incubated for 15minutes at ambient temperatures. Washing was repeated as earlier described, after which 100 μl of enzyme substrate was added to each well and incubated for 15 minutes. The result was a colour change, from clear solution to blue. The reaction was stopped by adding 100 μl of ‘stops’ solution and the optical density of each microwell was read with a micro plate reader at 450 nm using a differential filter of 630 nm.
Data analysis
Data were entered and cleaned in Microsoft excel (MS Excel®), and analyzed using SPSS (version 22) statistical package. Log transformation of AFM1 levels was done to attain a more normal distribution. Descriptive analyses, for quantitative data, included determination of measures of central tendency, mean (± standard deviation) and median. Qualitative data were summarized using frequency tables, graphs and trends. Inferential analyses included the use of Chi square statistics to assess statistical associations, Students t-tests and ANOVA to assess significance of differences in group means, and univariable linear regression to see the association between milk yield and contamination level. All factors that could potentially affect AFM1 levels i.e. feeding of concentrates storage facilities, occurrence of mold on farm, were included in the full multivariable linear regression model. A backward (manual) approach was used to model the relationship between these factors and detection of AFM1 levels (≥50 ppt) in milk. Elimination of variables was done until only those with significant (p < 0.05) associations remained in the model.
Results
Response rate
A total of 97 farms were interviewed as part of the study corresponding to a response rate of 97% (n = 100).
Respondent and household characteristics
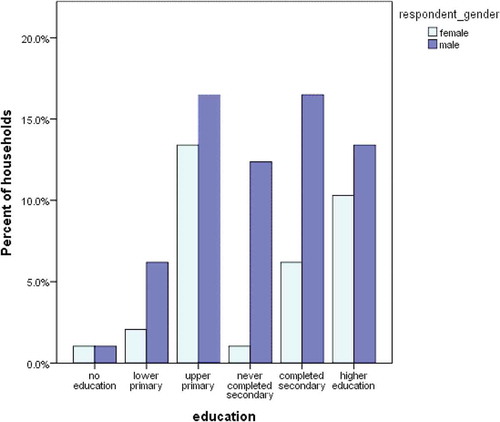
Those interviewed were aged between 20 and 83 years and most were men (66%; 64/97). Among our respondents there were more males 64/97 than females 33/97. Most (30%; 29/97) of those that had attained upper primary education were men. Women were 34% (33/97) respondents and tended to have attained less education (). Their main source of income was farming (73.7%), 27% were casually employed and 12.5% were employed on fulltime basis. Out of the interviewed, 47.9% were the head of the households, 24% were wives to the household heads, 21.9% were the farm workers, 6.3% were sons and relatives of the household. Majority 85.3% of respondents decided on what feed to buy, 72.6% acquired the feeds while 61.1% fed the cows.
Feeding and milking was most commonly done by male workers whereas the wife of the household head was most often responsible for cleaning milk utensils, transporting and selling of milk. Dairy activities performed by the different household members are summarized as () below.
Table 1. Percentage of household members tasked with different dairy tasks.
A small percentage (37.1%; 36/97) of the respondents had received training on different aspects of dairy production such as milk production (32%), hygiene (12.4%) and health (22.7%). Although proportionally more women (42.4%; 14/33) had received dairy training this proportion did not differ significantly from the proportion of women that was trained (34.4%; 22/64) (p = 0.4).
Herd composition
Of the 97 farms visited, 28.9% owned sheep, 29.9% goats, 7.2% pigs and 87.6% poultry. The average number of livestock species kept per household was: cattle (5.44 ± 6.88), sheep (2.58 ± 6.04), goats (1.34 ± 3.94), pigs (1.12 ± 5.91) and poultry (29.41 ± 69.32). Ayrshire (42.7%) and Friesian (38.2%) were the most common cattle breed type kept by the farmers. Other breeds were Jersey (10.9%) and Guernsey (8.2%) (n = 97). In terms of herd composition, adult males were kept on 14.7% farms, milking cows on 82.5%, dry cows on 53.1%, heifers on 52.6%, and calves and weaners on 75.3%. Most (90.7%; 88/97) households practiced zero grazing, 47.4% also practiced tethering and 22.8% pasture grazing. The average number of milking cows per farm was 2 and a minimum of 1.
Milk production and utilization
Most farmers (94%) milked their cows twice each day i.e. morning and evening, 4.8% milked once daily, while only 1.2% milked three times daily. The average daily milk production was 13 ± 15.83 liters per farm, with a range of between 0.25–80 liters, while milk yield per cow was 0.25–27 liters. The price at which fresh raw milk was sold at was US dollars 0.4–1.2 per liter. In most cases (73.4%; 58/97), customers visited the farms to purchase the milk ().
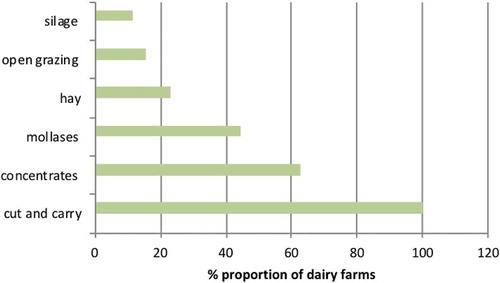
Figure 2. Feed types used by smallholder dairy farmers in selected urban and peri-urban areas of Kisumu
Milk was bought by neighbors, milk traders, hotel shops, bulking traders, and 2.75 (± sd 4.79) liters was retained for household consumption (). Milk was, in most farms (62%), sold within 30 minutes of milking, a few farms (12.8%) retained milk for more than 7 hours after milking. Milk was preserved by either boiling (40.7%); refrigeration (39.6%) or no form of treatment was done (19.7%) before being sold. Most (91.7%) families boiled their milk before consumption, 7.1% consumed it raw and 7.2% had it fermented.
Table 2. Milk produced and consumed per day in smallholder dairy farms in urban and peri-urban Kisumu, Kenya.
Most farmers (76.3%; 66/97) had not experienced any incidence of milk spoilage in their farms. Of the respondents (n = 31) who had problems with milk getting spoilt (23.7%), 17.5% (17/97) used it either to prepare fermented milk commonly known as ‘maziwa lala’, added vegetables which was consumed by the household members, or gave out to their pets. The rest (6.2%; 6/97) discarded the spoilt milk.
Dairy feeds (types, sources, storage and feeding practices)
Dairy animals were intensively managed (90.7%; 88/97). The cows were fed on forages (mostly cut and carry) and supplemented with concentrates such as dairy meal, cotton seed cake, maize germ and wheat bran. Some farmers used silage and molasses. Details of feeds are summarized in . Most (58.8%) farmers fed dairy meal. Farmers sourced their various feed either from on-farm formulations or local purchases ( and ).
Table 3. Feed storage conditions that were routinely monitored for by smallholder dairy farmers in urban and peri-urban Kisumu, May 2017.
Cut and carry, which was predominately used by farmers, involved harvest of Napier grass, legumes and any other local grass that could be fed to cows. This included the actual practice of cutting, collecting and transporting natural forages from different areas away from the farm homesteads.
On the question of what feed additives were used, farmers reported using routinely added phosphorous salt in the animal feeds (47.4%; 46/97). Sixty three percent (63%) of the farmers had feed storage facilities present on their farms and feed was kept on raised surfaces. Those that lacked feed stores (37%) had feed stored on the ground. While feeds were in storage, farmers routinely monitored for conditions such as temperature, ventilation, moisture, mold growth, dryness, animal and pests (). While most (78%; n = 97) of farmers said they would throw away feeds if they noticed mold growth on them, 22% said they would air and still give that to their animals.
Animal health and milk hygiene practices
About half (51.5%; 50/97) of the respondents reported experiencing mastitis on their farms. Most (90%; n = 50) rarely experienced the disease, 6% had experienced it at least every two months while 4% had it at least once a month. We asked the respondents (n = 50) to state what they did when they observed the cases on their dairy farms; 90.8% called a veterinary doctor for treatment and 4.7% managed these on their own, either using milking cream or salve (4.1%) or using warm water (4.1%) (). One percent of the farmers reported doing nothing in response to occurrence of mastitis on their farms. Milk from cows with mastitis (n = 50) was either discarded (46%; 23/50), given to calves and pets (38%; 19/50), or consumed (14%; 7/50) consumed. A few of the farmers (2%; 1/50) did not milk the cow. Assessment of mastitis was only done by 50.5% of the farmers, of which 35% did it regularly at every milking; 15.5% said they tested for the disease sometimes. Most respondents (94.9%; n = 97) carried out daily cleaning of both the milking shed and feeding areas.
Table 4. Milking hygiene practices reported by smallholder dairy farmers in urban and peri-urban Kisumu, May 2017.
Broom was used by 50.5% of farmers in daily cleaning of the milking shed and 16.3% of respondents used brooms when doing a more thorough cleaning. One percent of respondents did not clean hands before milking, 74.2% did not wash udder and teats after milking, 3.1% did not do daily cleaning of the milking shed, 32% did not do thorough cleaning of the milking shed and 2.1% did not ensure milking equipment were clean after milking.
Awareness about mold growth and aflatoxins
Fifty nine percent of the farmers had seen molds on their farms. Ingestion of moldy feeds was mostly thought to cause gastrointestinal problems ().
Figure 3. Origin of feeds used by smallholder dairy farmers in urban and peri-urban Kisumu, May 2017
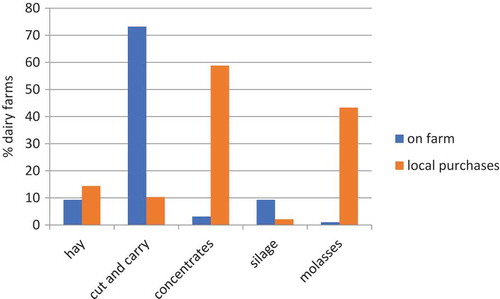
Figure 4. Effects of feeding moldy feeds to dairy cows, as reported by smallholder dairy farmers in urban and peri-urban areas of Kisumu, May 2017
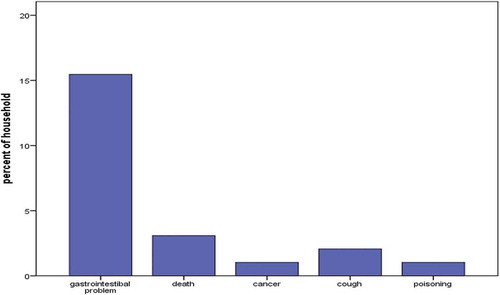
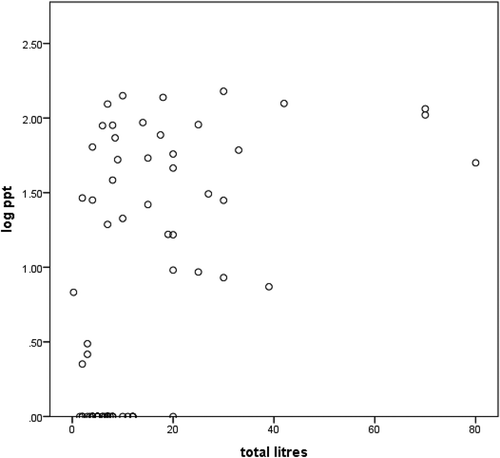
Figure 5. Relationship between aflatoxin M1 levels (logarithmic scale) and quantities of milk (in liters) produced in total on the farm
Most farmers, 61.8%, had heard of aflatoxins, with slightly more men (67.2%) than women (48.5%) having heard of it, but the difference was not significant (p = 0.07). Of the 61.8% that had heard of aflatoxins, 37.1% could correctly define what aflatoxins are i.e. as food poison (19.6%), as toxic mold (17.5%). Incorrect definitions (24.7%) included responses that classified aflatoxins as bacteria (10.3%), disease (10.3%), and those that have heard but could not define (4.1%). We asked farmers to indicate the foods they considered more likely to be contaminated with aflatoxins, 5.2% did not know; those who knew (n = 97) identified maize (46.4%) – forage (21.6%) and dairy meal (17.5%). Other foods that were thought to be contaminated with aflatoxins included cassava, millet, potatoes, milk, meat, wheat, beans and bread. In humans, aflatoxin was thought to result to gastrointestinal problems (18.3), cancer (1.9%) and even death (19.2%).
Aflatoxin M1 detection
A total of 72 milk samples were collected, 62.5% had LOD levels of ≤19 ppt, 11% had levels of 20–49 ppt, and 26.4% were above 50 ppt. The lowest levels of AFM1 were below the limit of detection, while the highest level was 151 ppt.
Assessing the effect of exposure factors on levels of AFM1
Univariate analyses revealed significant relationships (p < 0.05) between some of the variables in the study and AFM1 levels (>50 ppt). Dairy farmers giving concentrates to their cows were more likely to have high AFM1 levels (above 50 ppt) in milk than those not giving these to their animals (p = 0.002). The risk of elevated aflatoxin was ten-fold higher in these farmers OR = 10.06. Despite some farmers having knowledge on aflatoxins, there was no significant statistical difference in AFM1 levels between those who were considered aware of aflatoxins and those who were not (p = 0.109). Also Pearson χ2 statistics revealed no significant association between AFM1 status i.e. less than 50 ppt and ≥50 ppt, and the ability of the respondents to correctly define aflatoxins i.e. ‘correct definition’ and ‘incorrect definition’ (χ2 = 12.97, p > 0.05). There was also absence of any significant association between presence or absence of storage facilities and occurrence of AFM1 in the sampled milk (χ2 = 0.67, p > 0.05). No significant association was observed between gender of dairy farmer and AFM1 levels (χ2 = 1.701, p > 0.05).
We observed a correlation between AFM1 levels and quantities of milk produced (p < 0.001), with higher AFM1 levels being reported in the high milk producing farms ().
Multivariable analysis () showed that feeding of concentrates and total liters produced was associated with significantly increased levels of AFM1 (p < 0.05). Farmers that gave concentrates had a mean production of 16.69 ± 17.96 while those that did not feed concentrates had a mean of 5.55 ± 5.94. High milk production could be attributed to feeding of concentrates which was statistically significant (p = 0.001). High log ppt values (0.98 ± 0.88) of AFM1 levels were observed in milk from farmers that gave concentrates compared to those that did not (0.33 ± 0.59), this was statistically significant (p = 0.000).
Table 5. Multivariable analysis of feeding concentrates and total milk produced.
Discussion
Food safety is an increasing cause of concern, and the safety of animal-source foods is a OneHealth issue, requiring attention of multiple disciplines and ministries. This study assessed the dairy production to obtain insights into aflatoxins in milk in Kisumu County. Most milk was sold locally, mainly to neighbours and for income. However, milk was also consumed by the households, and children below 5 years of age consumed, on average, a half a litre of milk per day, which is similar to results reported before [Citation21]. The rest of the family consumed milk mainly in the form of tea; a few took milk either as lala (fermented milk) or yoghurt.
Women mostly ensured milking equipment were clean; this was also found by [Citation21]. In our study the farmers observed practices that could increase risk of exposure to mycotoxins. The presence of moulds on feeds did not motivate destruction to some farmers. They aired the mouldy feed and later mixed it with good feed and gave to the animals, while others reported discarding the feed as manure. Moreover, farmers often over-report practices they regard as desirable so this may be an over-estimation (Bronsvoort et al attached). Most farmers ensured proper daily cleaning of the cow shed removing feed leftovers which can contribute to mould growth and replacing with fresh ones.
Small-scale dairy farms often have low productivity, which could be attributed to poor management and disease. Mastitis, particularly the subclinical type, is one of the most persistent and widely spread disease conditions of importance to milk hygiene and quality among dairy cattle worldwide [Citation22]. Mastitis influences total milk output and as milk may contain high loads of bacteria, mastitis may modify milk composition and its technological usability. Subclinical mastitis is a prevalent disease in smallholder dairy herds in Kenya [Citation22,Citation23], our study was not exceptional given that 51.5% of the farms had reportedly experienced the disease at some point on their farms. Mastitis may also increase the secretion of AFM1 into milk [Citation24]. Understanding the prevalence of mastitis in dairy farming is essential as it contributes to bacterial loads in milk this leads to loss of milk as it is spoilt and may produce disease in the consumers. Lack of adequate dairy infrastructure and limited knowledge of milk hygiene contribute to higher incidence of milk borne pathogens. Milk-borne diseases cause serious and life threatening health risks to individuals with compromised immune system, the pregnant women, newborns and the elderly [Citation25].
The study found that milk from the urban and peri-urban dairy system of Kisumu is contaminated with AFM1, but most (73.1%; 53/72) of the milk samples collected were below the EU regulation level of 50 ppt [Citation26] and only 26.9% were above the EU limit. Presence of AFM1 in milk was associated with farms feeding their cattle mainly on concentrates made of ingredients such as maize germ, dairy meal, cotton and sunflower seed cake, which are all prone to contamination of AFB1 [Citation27], although it was not in the scope of this project to test feeds. However, the concentrations of AFM1 from this study were higher than an earlier study of raw milk marketed in Kisumu which found a maximum of 130 ppt [Citation19]. Levels of AFM1 in raw milk reported in our study were lower compared to a study in peri-urban Ethiopia that showed 91.8% of milk samples exceeded the maximum level set by EU regulations [Citation28]. This study also had fewer samples above 50 ppt than that reported from surveys conducted in Nairobi and other parts of Kenya [Citation29,Citation30]. This difference can be attributed to different sources of feed, different on-farm feeding practices, animal feed handling and storage conditions and perhaps also the sampling time. High milk yield was closely correlated with high levels of AFM1; this can be attributed to more feeding of contaminated feeds, mainly concentrates [Citation13]. also found that carry-over of AFM1 from AFB1 appears to increase linearly with milk yield. Our study revealed that higher producing farms had higher levels of AFM1 in milk. One likely explanation for this is that high producing cows are more likely to feed more concentrates, and we could also show an association between feeding concentrates and having AFM1 levels above 50 ppt, which is supporting this explanation. However, it is also possible that cows that are more high yielding excrete more of the AFM1 in milk [Citation13]. Therefore, higher producing farms should come up with mitigation methods of reducing aflatoxins exposure in their farms as they also distribute a lot of their milk to consumers.
Most respondents were aware of the harmful effects of aflatoxins, but had different ideas on the effects caused with majority focusing on gastrointestinal disorders. Diseases mentioned were diarrhea, bloating and loss of appetite, or even death. Most farmers (94.8%) had knowledge of some of the commodities that are contaminated by aflatoxins. It is, however, evident that all lacked knowledge on milk contamination by AFM1. Women often have less education, which may lead to poor food handling [Citation31]; in our study where fewer women had received training, and fewer had heard of aflatoxins. It is vital that women be empowered with the knowledge about the occurrence of AFB1 contamination in feeds as well as AFM1 in milk because they are accountable for family nutrition and are better placed to lessen the dangers posed by AFM1 in milk than their men counterparts [Citation18]. In our study, the majority of farmers appreciated that feeding their dairy cows with moldy feed could be fatal and some associated molds to cause cancer. Diseases reported by the farmers to be caused by molds included stomach ache, diarrhea and cough. A larger percentage generalized that ‘it causes a disease’. In another study mainly gastrointestinal disorders were mentioned by respondents, including diarrhea, vomiting, bloating and loss of appetite [Citation21].
The AFM1 levels found in our study imply that children are exposed (as they were reported to consume part of the milk), which could lead to negative health consequences. In Kisumu County 18% of children are stunted, having low height for the age [Citation32]. Exposure to AFM1 has been associated with poor growth in neonates and lower height for age score in children [Citation4,Citation33].
Globally, an increasingly higher percentage of dairy cattle are kept in intensive farming system and are fed on commercially acquired feeds which, in developing countries, often are highly contaminated with aflatoxins [Citation34]. About 80% of Kenya’s total milk production is still produced on small-scale farms [Citation35]. Due to this high demand, smallholder dairy farmers feed their dairy cows on concentrates that are often from uncertified agrovet vendors and highly contaminated with aflatoxins [Citation29]. The economic impact of feed contamination with mycotoxins includes productivity reduction and organ damage [Citation36]. Animal feeds, including hay and straw, might be contaminated during preharvest or drying stages [Citation37].
Milk supplies proteins, energy and essential micronutrients. Consumption of even small amounts can significantly increase nutritional security. It is therefore important that milk consumption is continuously encouraged, and that the milk is made as safe as possible. All age groups in Kenya widely consume milk. Therefore presence of contaminants like aflatoxin may pose serious health hazards for consumers especially children. Results of this study will help understand the provision of milk in the city and its safety standards for consumers. This will also inform intervention strategies in reducing aflatoxins in milk, which will reduce adverse effects of AFB1 in feeds to the animals’ hence increasing productivity of the cows. High productivity combined with acceptable safety standards will bring a balance between food safety and food security.
Conclusion
The results of this study show that AFM1 is present in the milk in Kisumu, and the toxin of the chemical indicates that it could be a public health concern. Therefore, there is a need for monitoring of AFM1 in the raw milk and milk as well as identification of interventions to reduce the effects of aflatoxins in the feed and that are feasible to the farmers. It is foremost important to prevent toxin production in feed, as well as to create effective detoxification processes to reduce prevalence of AFM1. Milk being the first food for a child, appropriate measures should be instituted to reduce exposure to aflatoxin M1, especially in young children who are highly vulnerable.
Acknowledgments
This study was a part of the FoodAfrica Programme which is mainly financed by the Ministry for Foreign Affairs of Finland contract no. 29891501 and the CGIAR Research Program on Agriculture for Nutrition and Health.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Gladys Anyango
Gladys Anyango is a Graduate Fellow at ILRI and a graduate student at the Department of Public Health and Community Development, Maseno University. Her background is in Microbiology and her research interests are in food safety and epidemiology.
Florence Mutua
Florence Mutua is a veterinary epidemiologist lecturing at Nairobi University and on a year’s sabbatical with ILRI based in Tanzania. She has published widely and is currently researching aflatoxins in Kenya and informal dairy in Tanzania.
Irene Kagera
Irene Kagera is a Graduate Fellow at ILRI and a graduate student at the Department of Food Science and Technology, Jomo Kenyatta University of Agriculture and Technology. Her background is in Human Nutrition and her research interests are in food safety and public health.
Pauline Andang`O
Pauline Andang`o Department of Nutrition and Health, Maseno University. Pauline does research in Micronutrient Nutrition and has mainly focused on iron.
Delia Grace
Delia Grace is an epidemiologist and leads the Health Program at ILRI and the Flagship on Food Safety in the CGIAR research program on agriculture and health. She has been a lead researcher in food safety in informal markets for several decades. She has led or contributed to evidence syntheses and investment advice for World Bank, DFID, USAID, ACIAR, BMGF, FAO, OIE, WHO, AU-IBAR, OECD and others.
Johanna F. Lindahl
Johanna F. Lindahl is a joint appointee between ILRI, Swedish University of Agriculture and Uppsala University. She has a background in veterinary medicine and ten years’ experience in academia and research. She leads aflatoxin work in ILRI’s Health Program and recently edited a widely-cited special edition on aflatoxins in maize and dairy in east Africa.
References
- Guchi E. Implication of aflatoxin contamination in agricultural products. Am J Food Nutr. 2015;3(1):12–10.
- Lizárraga-Paulín EG, Moreno-Martínez E, Miranda-Castro SP. Aflatoxins and their impact on human and animal health: an emerging problem. In: R.G.Guevara-Gonzalez (Ed.). Aflatoxins-biochemistry and molecular biology. InTech; 2011. ISBN:InTech. doi:10.5772/26196.Page256 Mexico
- Massey TE, Stewart RK, Daniels JM, et al. Biochemical and molecular aspects of mammalian susceptibility to aflatoxin B1 carcinogenicity. Pro Soc Exp Biol Med. 1995;208(3):213–227.
- Kiarie G, Dominguez-Salas P, Kang’ethe S, et al. Aflatoxin exposure among young children in urban low-income areas of Nairobi and association with child growth. Afr J Food Agric Nutr Dev. 2016;16(3):10967–10990.
- Khlangwiset P, Shephard GS, Wu F. Aflatoxins and growth impairment: a review. Crit Rev Toxicol. 2011;41(9):740–755.
- Kana JR, Gnonlonfin BGJ, Harvey J, et al. Assessment of aflatoxin contamination of maize, peanut meal and poultry feed mixtures from different agroecological zones in Cameroon. Toxins (Basel). 2013;5(5):884–894.
- IARC. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. Lyon, France: World Health Organization; 2002.
- Alvarado AM, Zamora-Sanabria R, Granados-Chinchilla F. A focus on aflatoxins in feedstuffs: levels of contamination, prevalence, control strategies, and impacts on animal health. In Lukman Abdulra'UF, editor. Aflatoxin-control, analysis, detection and health risks. London: InTech; 2017.
- Strosnider H, Azziz-Baumgartner E, Banziger M, et al. Workgroup report: public health strategies for reducing aflatoxin exposure in developing countries. Environ Health Perspect. 2006;114(12):1898.
- Min W-K, Kweon D-H, Park K, et al. Characterisation of monoclonal antibody against aflatoxin B1 produced in hybridoma 2C12 and its single-chain variable fragment expressed in recombinant Escherichia coli. Food Chem. 2011;126(3):1316–1323.
- FAO. Impact of animal nutrition on animal welfare – Expert Consultation; 2011 Sep 26−30; Food and Agricultural Organization, Rome, Italy; 2012.
- Hoogenboom L, Tulliez J, Gautier J-P, et al. Absorption, distribution and excretion of aflatoxin-derived ammoniation products in lactating cows. Food Addit Contam. 2001;18(1):47–58.
- Britzi M, Friedman S, Miron J, et al. Carry-over of aflatoxin B1 to aflatoxin M1 in high yielding Israeli cows in mid-and late-lactation. Toxins (Basel). 2013;5(1):173–183.
- Masoero F, Gallo A, Moschini M, et al. Carryover of aflatoxin from feed to milk in dairy cows with low or high somatic cell counts. Animal. 2007;1(9):1344–1350.
- Battacone G, Nudda A, Cannas A, et al. Excretion of aflatoxin M1 in milk of dairy ewes treated with different doses of aflatoxin B1. J Dairy Sci. 2003;86(8):2667–2675.
- Lukuyu B, Franzel S, Ongadi P, et al. Livestock feed resources: current production and management practices in central and northern rift valley provinces of Kenya. Livestock Res Rural Dev. 2011;23(5):112.
- Thorpe W, Muriuki H, Omore A, et al. Dairy development in Kenya: the past, the present and the future. Paper presented at the Annual Symposium of the Animal Production Society of Kenya (APSK). Theme: Challenges to Animal Production in this Millenium, KARI Headquaters, Nairobi March 22-23 2000. . Nairobi, (Kenya): ILRI.
- Kang’ethe E, Lang’a K. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centers in Kenya. Afr Health Sci. 2009;9(4).218–226
- Obade MI, Andang’o P, Obonyo C, et al. Exposure of children 4 to 6 months of age to aflatoxin in Kisumu County, Kenya. Afr J Food Agric Nutr Dev. 2015;15(2):9949–9963.
- Metcalfe C. Biostatistics: a foundation for analysis in the health sciences. 7th edn. Wayne W. Daniel, Wiley, 1999. No. of. pages: xiv+ 755+ appendices. Price:£ 28.95. ISBN 0‐471‐16386‐4. Stat Med. 2001;20(2): 324–326.
- Kiama T, Lindahl J, Sirma A, et al. Kenya dairy farmer perception of moulds and mycotoxins and implications for exposure to aflatoxins: a gendered analysis. Afr J Food Agric Nutr Dev. 2016;16(3):11106–11125.
- Ogola H, Shitandi A, Nanua J. Effect of mastitis on raw milk compositional quality. J Vet Sci. 2007;8(3):237–242.
- Shitandi A, Kihumbu G. Assessment of the California mastitis test usage in smallholder dairy herds and risk of violative antimicrobial residues. J Vet Sci. 2004;5(1):5–9.
- Fink-Gremmels J. Mycotoxins in cattle feeds and carry-over to dairy milk: a review. Food Addit Contam. 2008;25(2):172–180.
- Pal M. Public health hazards due to consumption of raw milk. Ethiopian Herald. 2012:10.
- van Egmond HP, Schothorst RC, Jonker MA. Regulations relating to mycotoxins in food. Anal Bioanal Chem. 2007;389(1):147–157.
- Makau CM, Matofari JW, Muliro PS, et al. Aflatoxin B1 and deoxynivalenol contamination of dairy feeds and presence of aflatoxin M1 contamination in milk from smallholder dairy systems in Nakuru, Kenya. Int J Food Contam. 2016;3(1):6.
- Gizachew D, Szonyi B, Tegegne A, et al. Aflatoxin contamination of milk and dairy feeds in the greater Addis Ababa milk shed, Ethiopia. Food Control. 2016;59:773–779.
- Senerwa D, Sirma A, Mtimet N, et al. Prevalence of aflatoxin in feeds and cow milk from five counties in Kenya. Afr J Food Agric Nutr Dev. 2016;16(3):11004–11021.
- Kirino Y, Makita K, Grace D, et al. Survey of informal milk retailers in Nairobi, Kenya and prevalence of aflatoxin M1 in marketed milk. Afr J Food Agric Nutr Dev. 2016;16(3):11022–11038.
- Okoth SA, Ohingo M. Dietary aflatoxin exposure and impaired growth in young children from Kisumu District, Kenya: cross sectional study. Afr J Health Sci. 2004;11(1–2):43–54.
- Kenya National Bureau of Statistics. Kenya demographic and health survey 2008–09. Calverton (MD); 2010.
- Haschek WM, Rousseaux CG, Wallig MA, et al. Haschek and Rousseaux’s handbook of toxicologic pathology. San Diego: Academic Press; 2013.
- Unnevehr L, Grace D. Aflatoxins: finding solutions for improved food safety. Intl Food Policy Res Inst. 2013;20.
- Ettema F. Dairy development in Kenya. In: Kenya dairy sector. 2013. p. 1–5.
- Upadhaya SD, Park M, Ha J-K. Mycotoxins and their biotransformation in the rumen: a review. Asian-Australas J Anim Sci. 2010;23(9):1250–1260.
- Bhat R, Rai RV, Karim AA. Mycotoxins in food and feed: present status and future concerns. Compr Rev Food Sci Food Saf. 2010;9(1):57–81.