ABSTRACT
Background: Brucellosis is an economically important zoonotic disease with worldwide distribution, with low-income countries being more affected. The disease is endemic in India, a country that house the world’s largest cattle and buffalo population and produce the most milk in the world.
Results: Prevalence of the disease in the country is reported as low as 1% to as high as 60% by different researchers but many of the published studies that reported higher prevalence were conducted in non-randomised samples. Based on this review, overall prevalence in the country is likely 12% or less. About 20 different risk factors are reported that contribute/predispose to occurrence of bovine brucellosis. The risk factors could be classified in four groups: host factors, farmer’s factors, managemental factors, and agro-ecological factors. Various studies reported high economic burden of the diseases in dairy animals but there is dearth of comprehensive and rigorous economic studies.
Conclusions: In the absence of highly effective vaccines and because of difficulties in executing a segregation and slaughter policy of infected animals in countries like India, control of bovine brucellosis remains a challenge.
Introduction
Brucellosis is one of the most common but often neglected zoonotic diseases in the world [Citation1]. The disease occurs worldwide, except in some high-income countries [Citation2,Citation3]. In low-income countries, the disease is often underreported and there is little or no effective control, resulting in major health, economic and livelihood burdens [Citation4]. The disease is caused by bacteria of the genus Brucella. Twelve species of Brucella have been identified so far [Citation5]. Most species of Brucella can infect multiple species of animals, including humans [Citation6]. In cattle, the infection is predominantly caused by B. abortus, less frequently by B. melitensis and occasionally by B. suis [Citation7]. In sexually mature female cattle, infection localizes in the reproductive system and produces placentitis followed by abortion, causing production losses [Citation8,Citation9]. Most infected animals abort only once in their lifetime, but may remain infected their entire life [Citation6]. The disease is often asymptomatic in non-pregnant female cattle and after the first abortion. Adult male cattle may develop orchitis, and brucellosis may cause infertility in both sexes. Hygromas can occur in leg joints and are a common manifestation of brucellosis in some tropical countries [Citation3]. Bovine brucellosis can also occur in buffaloes, bison and yak and clinical manifestations in these animals are similar to those in cattle [Citation3].
India, a country with more than one billion people, is the world’s leading milk producer, contributing around 17% of the world’s total milk production [Citation10,Citation11]. India has the world’s largest dairy cattle population at around 300 million, both buffalos and cows [Citation10]. Dairy products are the main animal-source food for the large vegetarian population, and 70 million households engage in milk production [Citation11]. The cultural, religious and historical importance of cows in India, including the common ban to slaughter cattle and the free roaming, further adds a layer of complexity to disease control, and the predominance of an informal market of raw milk adds to the public health risks of people. This review therefore focuses on India, where brucellosis is common but under-researched.
Prevalence in India
To assess prevalence in a population, researchers need to consider sample size, sampling frame, and selection of serological tests. Developing an appropriate sampling strategy frame for epidemiological studies is of primary importance. This review found that many surveys in India either did not report the selection method or used non-randomised methods.
A large study conducted in 23 states of India with 30,437 cattle and buffalo samples found an overall sero-prevalence of 2% [Citation12]. The same study found 17% sero-positivity in organized farms with a history of abortion, repeat breeding and retention of placenta. However, the study used samples originally collected for sero-monitoring purposes of rinderpest over a period of 4 years, and seems to have lacked probabilistic selection. Another epidemiological study found 5% sero-prevalence in cattle and 3% in buffalo in India [Citation13]; however, this study also used the same kind of samples collected for sero-monitoring of rinderpest. A study in Karnataka (India) reported 6% prevalence by iELISA and 5% by PCR in non-randomised samples of different size of cattle and buffalo farms [Citation14].
Two studies using probabilistic sampling in Punjab reported an overall sero-prevalence of 21% and 18% respectively [Citation9,Citation15]. However, two other Punjabi studies (based on random sampling and non-random sampling) reported (12%) [Citation16,Citation17]. One of these studies suggested that prevalence had been increasing from the 1970s to 1990s [Citation17]. Similar prevalence was reported in Assam (14% in cattle and 10% in buffalo, based on non-random sampling), Gujarat (12%, based on random sampling), Bihar (12%, based on random sampling) and Andhra Pradesh (12%, based on non-random sampling) (Bhattacharya, Ahmed, & Rahman, 2005; Trangadia et al. 2012; Patel et al. 2014; Pandian et al. 2015). A relatively lower sero-prevalence (8%) was reported in both organized and marginalized dairy farms in Uttar Pradesh (no mention about sampling strategy) [Citation18].
Other studies [Citation9,Citation15,Citation19–Citation26] reported much higher rates of sero-positivity (20–60%) in cattle and buffalo in different parts of India but most were conducted in farms with a history of bovine brucellosis/abortion/retention of placenta or in a small number of farms selected purposively or from particular dairy belts. Therefore, these sero-positivity rates might not reflect the overall prevalence in a general population. Similarly, higher herd seroprevalence (66%) were reported than individual prevalence (26%) in samples delivered by farmers for laboratory examination in Punjab and Haryana [Citation27]
From the discussion above it seems that most studies found 12% or lower prevalence in several states in India, which may reflect the overall prevalence situation in the country, although there is likely to be differences between the different states. However, the country’s government document reported only 4 outbreaks of brucellosis in cattle and buffalo with 46 reported cases in 2016 [Citation28], indicating under-reporting.
Risk factors
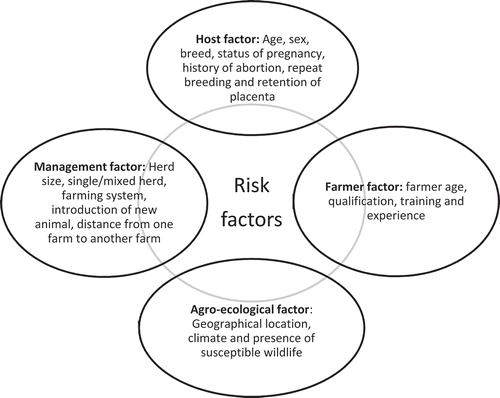
Risk factors include production systems, agro-ecological zones, husbandry practices, contact with wild animals and management factors. Reviewing literature from India and other countries, we classify these broadly into four groups ().
A significant association between Brucella infection and risk markers, such as abortion, retention of placenta and repeat breeding is reported by some researchers. Aulakh et al. [Citation15] found significant association between brucellosis and abortion and retention of placenta, but not between brucellosis and repeat breeding. Mugizi et al. [Citation29] and Asmare et al. [Citation30] found no significant association between Brucella sero-positivity and abortion and retention of placenta. In the case of B. melitensis infection in cattle, sero-prevalence was lower and abortion occurred less frequently than in the case of B. abortus infection [Citation30].
Many researchers found significant associations between species, sex, breed and age of animals with sero-positivity [Citation14,Citation27,Citation29,Citation31,Citation32]. Other risk factors reported include: lack of clean water, insufficient manure removal and cleaning, poor management of aborted materials, introduction of new animals from herds that were not free from brucellosis or of unknown status, herds kept in close confinement, and mixed herds [Citation15,Citation16,Citation33–Citation35]. Animals bred with natural mating is reported to be more seropositive for Brucella infection than animals bred with artificial insemination. The same study found that the farms that practice routine milk testing for screening Brucella infection is less likely sero-positive than those who do not follow such practice [Citation14]. Farmer’s knowledge and awareness about brucellosis significantly reduces sero-positivity of Brucella infection in animals [Citation14,Citation26,Citation27]. Inadequate floor space has also been reported as one of the risk factor of Brucella infection [Citation26]. Reports suggest that younger cows are less likely to be sero-positive than older cows [Citation36] and pregnant cows are more likely to be sero-positive than non-pregnant cows [Citation3,Citation37]. Lower sero-prevalence of brucellosis in young animals could be attributed to resistance of sexually immature cattle to infection, or to less time of risk of exposure. Increased susceptibility to clinical disease with age, could be more associated with sexual maturity due to the effects of sex hormone and placenta erythritol on the pathogenesis of brucellosis [Citation30]. However one study reported higher sero-prevalence of Brucella infection in younger calves (10%) than older animals (9%) and the study suggested that age does not have positive correlation with sero-positivity [Citation38]. Higher prevalence of Brucella infection in female than male is also reported [Citation16] ().
Table 1. Factors reported to be associated with Brucella sero-positivity in the literature.
There are reports that prevalence in organized dairy farms is typically higher than in marginal herds [Citation18,Citation19,Citation22,Citation38,Citation39]. This higher prevalence in organized dairy farms may be due to higher prevalence of the disease in exotic and cross-bred animals, compared to indigenous cattle [Citation40], transmission of disease during natural mating/artificial insemination, physical contact because of close confinement and exposure to diseased animals [Citation18,Citation22]. On the contrary, a few studies reported significantly lower prevalence in organized farms than marginal herds possibly because of proper management, good sanitation and disinfection, proper disposal of placenta, better animal health awareness and management and vaccination; however, the number of these types of farms where studies were conducted were few and not representative of organized farms overall [Citation17,Citation33]. While large herds have been reported more prone to Brucella-infection, large herds may be owned by farmers who have more resources and are more knowledgeable and this may result in less disease. In this case wealth and education are confounding factors that mask the positive relation between large herd size and occurrence of brucellosis. Another study reported higher prevalence in medium size organised farms (26–100) than small or large size farms [Citation14]. This might be because of indiscriminate replacement of herd from unknown source or poor hygiene and management in medium size farm than larger size farms. In addition, significantly higher prevalence in cattle than buffalo was reported by some studies [Citation9,Citation15,Citation17,Citation33], but contrary findings are also not uncommon [Citation16,Citation22,Citation35,Citation39]. Higher herd prevalence than individual prevalence was also reported [Citation31], which is expected when multiple animals are sampled per farm.
Based on the above discussion, the risk factors are summarised at under four different categories:
Economic impact, loss and cost of bovine brucellosis
Bovine brucellosis causes huge losses to the dairy industry; however, there is a dearth of comprehensive economic studies. It is also observed that terms such as economic impact, loss, and cost of brucellosis are used by some researchers loosely and inter-changeably. Economic impact can include direct (e.g. reduced milk yield, increased mortality) and indirect (e.g. vaccination, culling) costs. Direct impacts may further be classified as visible (e.g. abortion, repeat breeding), invisible (e.g. lower fertility), additional costs (e.g. treatment, vaccination) and revenue forgone (e.g. distress selling) [Citation43]. Loss may comprise only those parameters that reduce benefits (e.g. reduced milk yield, reduced weight gain, reduced fertility, increased replacement cost, increased mortality etc.) while cost would comprise amounts spent for treatment and control (e.g. biosecurity, vaccination, movement control, disease surveillance, research etc.) of the disease [Citation4,Citation43]. Most economic estimates have not taken into consideration the loss caused by distress selling, feeding and management loss of pregnant animals in the event of abortion, person-days loss for treating animals, cost of antiseptic and detergents, cost of transportation related to treatment, cost of diagnosis etc. Most studies extrapolate the economic figures based on limited epidemiological information and assumptions developed in the given country or elsewhere. Few studies that estimate the economic impact of the disease based on rigorous epidemiological data collected from a randomly selected population. Because of lack of uniformity in approach to measurement of economic impact/cost/loss, and the fact that these are highly context specific, the estimates have also varied widely.
Panchasara et al. [Citation44] reported that economic losses caused by brucellosis was mainly due to reduction in milk production followed by cost of treatment and loss of the aborted calf. It was further stated that there was an average loss of 231 litres and 177 litres of milk (10% of total lactation yield) in Brucella positive cows and buffalo cow respectively, causing an economic loss of around USD 40. The average costs of treatment following abortion, repeat breeding and retention of placenta of dairy cattle were estimated at USD 4, USD 5 and USD 7 respectively [Citation44]: (USD values are at exchange rate of early 2018). Another study in Gujarat, India reported the highest quantified losses (46%) because of reduced milk yield followed by extended calving interval (18%), treatment cost of abortion (14%), and treatment cost of metritis/endometritis (8%) out of the total loss caused by brucellosis to peri urban dairy farms of cattle and buffalo [Citation24]. This estimation was based on simple economic calculation of losses obtained from the primary data. Similar approach was used to assess the economic loss in Sudan. The study found that on an average each sero-positive animal causes economic loss of USD 202 [Citation45].
An estimate of overall economic loss caused by brucellosis in India was based on secondary information (e.g. prevalence data, decrease in milk production, decrease in carcass weight, draught power, life expectancy, reproductive rates, market price, livestock population etc.) collected from different published literature in India and abroad. The study suggested that brucellosis caused a median loss of USD 3.4 billion to the livestock sector of which 96% was to the dairy sector alone [Citation46]. Another study in India estimated an economic loss of USD 58.8 million per year based on an active surveillance program [Citation47] for bovine brucellosis but the paper did not explain how they arrived at the figure. Another study estimated that abortion caused a loss of USD 89 per animal [Citation16] but with no mention of other losses.
Outside India, a study conducted in Brazil, found a loss of USD 2.10 per cattle. Every 1% increase or decrease in prevalence of brucellosis was expected to increase or decrease the economic burden of brucellosis by USD 0.37/head [Citation48].
Vaccination and control
In low and middle-income countries, the classical approach of vaccination, testing, quarantine, and slaughter with compensation policies is less successful or feasible. More targeted control measures may be more useful [Citation4]. Vaccination may be the most appropriate control measure, while stamping out may be too economically burdensome [Citation32]. Test and slaughter is not an easy option because of huge economic loss to the poor farmers. In India, it is even more difficult because of socio-religious factors, which makes slaughter of cattle unacceptable. Cattle slaughter is also officially banned in some parts of India [Citation13]. An action research project in Uttar Pradesh found that periodic testing of all animals and segregation of sero-positive animals (test and segregation method) in a specialized farm away from the main farm, reduced seropositive animals from 12.4% to 1.2%. This coupled with better housing, proper hygienic disposal of aborted materials and calf-hood vaccination could help in reducing prevalence of brucellosis [Citation47]. Another study in Punjab found, B. abortus S19 vaccine reduced the rate of abortion from 8% to1% in cows and from 3% to 1% in buffalo [Citation17].
Brucella abortus S19 is the most widely used and was the first effective vaccine (described in 1930) against brucellosis in bovines. The S19 vaccine induces reasonable protection against brucellosis but does not protect cattle against B. melitensis [Citation8]. The problem of positive reactions in serological screenings is partly overcome by the development of a live vaccine devoid of smooth liposaccharide (SLPS) that can help separate infected from vaccinated animals, and therefore eliminates unnecessary further test and slaughter of animal. Brucella abortus RB51 strain has proved safe and effective in the field against bovine brucellosis and exhibits negligible interference with diagnostic serology [Citation49,Citation50]. RB51 has been successfully used in USA since 1996 [Citation3] for prevention and eradication of bovine brucellosis and has been suggested to be used in India [Citation50]. Both Brucella S19 and RB51 vaccines are recommended by OIE but vaccine efficacy may be limited under heavy exposure [Citation8]. Efficient vaccination benefit from improved livestock management systems, good access to veterinary services, adequate supply of vaccines and constant refrigeration, which are often lacking in low and middle-income countries, where vaccines may need to be more thermostable and longer acting to reduce the burden of vaccinating dairy animals too frequently. Efforts therefore need to be increased on producing more effective vaccine that are best suitable in low-income country context.
Conclusion
It can be concluded that bovine brucellosis is widely prevalent in India. More systematic, large scale, randomised studies are required to accurately assess prevalence and actual economic loss/cost of brucellosis. In middle and low-income countries control of brucellosis will remain as a major hurdle because of underreporting, inadequate funding for control or eradication programme, lack of 100% effective vaccine and difficulties in segregating and slaughtering affected animals. Control of brucellosis in India is even more difficult because of large movement of animals for different reasons, and high socio-religious sentiments towards rearing of dairy animals that does not allow slaughtering of animal. Therefore, in India’s context strengthening disease reporting system, including diagnostic capabilities and surveillance in livestock, wildlife and human; vaccination, improved cleanliness and hygiene, better management, adoption of quarantine measure, restriction in movement of animals, adoption of more artificial insemination and customised health education programme on brucellosis may be emphasised.
Acknowledgments
The work with the review was supported by the CGIAR research programme Livestock and Fish and CGIAR fund.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Ram Pratim Deka
Ram Pratim Deka is a PhD student under the Department of Clinical Sciences at Swedish University of Agricultural Sciences, Sweden. He is also working under the Animal & Human Health Programme of the International Livestock Research Institute where his research focus mainly on food borne and zoonotic diseases.
Ulf Magnusson
Ulf Magnusson is working as Professor under the Department of Clinical Sciences at Swedish University of Agricultural Sciences, Sweden. He has vast experience of guiding students on conducting studies on brucellosis and other zoonotic and reproductive diseases.
Delia Grace
Delia Grace is jointly leading the Animal and Human Health Programme of the International Livestock Research Institute (ILRI) in the globe. As a Principal Scientist she has extensive of leading several projects related to food borne and zoonotic diseases.
Johanna Lindahl
Johanna Lindahl is working as a Senior Scientist under the Animal and Human Health Programme of the International Livestock Research Institute (ILRI). She is leading a few related to food borne and zoonotic diseases in South Asia in general and India in particular. She is also teaching at SLU and Uppsala University, Sweden.
References
- WHO. The control of neglected zoonotic diseases: a route to poverty alleviation. Geneva: WHO; 2005.
- Pappas G, Papadimitriou P, Akritidis N, et al. The new global map of human brucellosis. Lancet Infect Dis. 2006 Feb;6(2):91–7.
- OIE. Bovine brucellosis, OIE terrestrial manual. 2009.
- McDermott JJ, Grace D, Zinsstag J. Economics of brucellosis impact and control in low-income countries. Sci Tech Rev off Int Des Epizoot. 2013;32(1):249–261.
- Rajala EL. Brucella in Tajikistan -zoonotic risks of urbanized livestock in a low-income country [Dr. thesis]. Sweden: SLU; 2016.
- Godfroid J, Nielsen K, Saegerman C. Diagnosis of brucellosis in livestock and wildlife. Croat Med J. 2010;51:296–305.
- OIE. Infection with Brucella abortus, Brucella melitensis and Brucella suis. Rome: OIE (World Organisation for Animal Health); 2016.
- WHO FAO OIE and M. J. Corbel. Brucellosis in humans and animals. Geneva: WHO press; 2006.
- Ul-Islam MR, Gupta MP, Filia G, et al. Sero-epidemiology of brucellosis in organized cattle and buffaloes in Punjab (India). Adv Anim Vet Sci. 2013;1(3S):5–8.
- FAOSTAT. Milk total production in India. 2015.
- Douphrate DI, Hagevoort GR, Nonnenmann MW, et al. The dairy industry: a brief description of production practices, trends, and farm characteristics around the world. J Agromedicine. 2013 Jan;18(3):187–197.
- Isloor S, Renukaradhya GJ, Rajasekhar M. A serological survey of bovine brucellosis in India. Rev Sci Tech - off Int Des Épizooties. 1998 Dec;17(3):781–785.
- Renukaradhya G, Isloor S, Rajasekhar M. Epidemiology, zoonotic aspects, vaccination and control/eradication of brucellosis in India. Vet Microbiol. 2002 Dec;90(1–4):183–195.
- Shome R, Shankaranarayan B, Krithiga N, et al. Bovine brucellosis in organised farms of India-an assessment of diagnostic assays and risk factors. Adv Anim Vet Sci. 2014;2(10):557–564.
- Aulakh HK, Patil PK, Sharma S, et al. A study on the epidemiology of bovine brucellosis in Punjab (India) using milk-ELISA. Acta Vet Brno. 2008;77(3):393–399.
- Dhand NK, Gumber S, Singh BB, et al. A study on the epidemiology of brucellosis in Punjab (India) using survey toolbox. Rev Sci Tech. 2005 Dec;24(3):879–885.
- Gill J, Kaur S, Joshi D, et al. Epidemiological studies on brucellosis in farm animals in Punjab state of India and its public health significance. In Proceedings of the 9th International Symposium on Veterinary Epidemiology and Economics; Colorado, USA; 2000.
- Kumar N, Pal BC, Yadav SK, et al. Prevalence of bovine brucellosis in Uttar Pradesh, India. J Vet Public Heal. 2009;7(2):129–131.
- Chand P, Sharma AK. Situation of brucellosis in bovines at organized cattle farms belonging to three different states. J Immunol Immunopathol. 2004;6(2):11–15.
- Dalvi GW, Ingle VC, Kalorey DR, et al. Seroprevalence of brucellosis in bovines in association with history of abortion and repeat breeding. Vets Commun. 2007;1:36–37.
- Londhe SP, Bannalikar AS, Dighe VD. Serodetection of bovine brucellosis by RBPT and AB-ELISA. Anim Sci Report. 2011;5(2):69–73.
- Jagapur R, Rathore R, Karthik K, et al. Seroprevalence studies of bovine brucellosis using indirect-enzyme-linked immunosorbent assay (i-ELISA) at organized and unorganized farms in three. Vet World. 2013; 6(8):550–553.
- Neha WA, Verma AK, Jain U, et al. Brucellosis in organized dairy farm: an investigation. Asian J Anim Sci. 2014 Jan;8(1):29–33.
- Patel MD, Tyagi KK, Fulsoundar AK, et al. Monetary lossesdue to bovine brucellosis in peri-urban areas of Gujarat, India. Indo-American J Agric Vet Sci. 2014;2(3):33–40.
- Shome R, Filia G, Padmashree BS, et al. Evaluation of lateral flow assay as a field test for investigation of brucellosis outbreak in an organized buffalo farm : A pilot study. Vet World. 2015;8(4):492–496.
- Pathak AD, Dubal ZB, Karunakaran M, et al. Apparent seroprevalence, isolation and identification of risk factors for brucellosis among dairy cattle in Goa, India. Comp Immunol Microbiol Infect Dis. 2016;47:1–6.
- Chand P, Chhabra R. Herd and individual animal prevalence of bovine brucellosis with associated risk factors on dairy farms in Haryana and Punjab in India. Trop Anim Health Prod. 2013 Aug;45(6):1313–1319.
- G. of I. DAHDF. Annual Report 2016–17. 2017.
- Mugizi DR, Boqvist S, Nasinyama GW, et al. Prevalence of and factors associated with Brucella sero-positivity in cattle in urban and peri-urban Gulu and Soroti towns of Uganda. J Vet Med Sci. 2015 May;77(5):557–564.
- Asmare K, Sibhat B, Molla W, et al. The status of bovine brucellosis in Ethiopia with special emphasis on exotic and cross bred cattle in dairy and breeding farms. Acta Trop. 2013 Jun;126(3):186–192.
- Patel MD, Patel PR, Prajapati MG, et al. Prevalence and risk factor’s analysis of bovine brucellosis in peri-urban areas under intensive system of production in Gujarat, India. Vet World. 2014;7(7):509–516.
- Makita K, Fèvre EM, Waiswa C, et al. Herd prevalence of bovine brucellosis and analysis of risk factors in cattle in urban and peri-urban areas of the Kampala economic zone, Uganda. BMC Vet Res. 2011;7(1):1–8.
- Rahman H, Bhattacharya DK, Ahmed K. Studies on sero prevalence of bovine brucellosis by different tests. J Vet Public Heal. 2005;3(2):131–133.
- Calistri P, Iannetti S, Atzeni M, et al. Risk factors for the persistence of bovine brucellosis in Sicily from 2008 to 2010. Prev Vet Med. 2013;110(3–4):329–334.
- Soomro AH, Kamboh AA, Rind R, et al. A study on prevalence and risk factors of Brucellosis in cattle and buffaloes in district Hyderabad, Pakistan. J Anim Heal Prod. 2014;2(3):33–37.
- Lindahl E, Sattorov N, Boqvist S, et al. Seropositivity and risk factors for Brucella in dairy cows in urban and peri-urban small-scale farming in Tajikistan. Trop Anim Health Prod. 2014 Mar;46(3):563–569.
- Amin K, Rahman M, Kabir S. Serological epidemiology of brucellosis in cattle of Mymensingh districts of Bangladesh. J Anim Vet Adv. 2004;3(11):773–775.
- Kumar A, Gupta VK, Verma AK, et al. Seroprevalence and risk factors associated with bovine brucellosis in Westerna Uttar Pradesh, India. Indian J Anim Sci. 2016;86(2):131–135.
- Trangadia B, Rana SK, Mukherjee F, et al. Prevalence of brucellosis and infectious bovine rhinotracheitis in organized dairy farms in India. Trop Anim Health Prod. 2010;42(2):203–207.
- Pandian S, Ray P, Chandran P, et al. Seroprevalence of Brucella abortus and Leptospira hardjo in cattle. Vet World. 2015;8(2):217–220.
- Shome R, Padmashree B, Krithiga N, et al. Bovine Brucellosis in organized farms of India—an assessment of diagnostic assays and risk factors. Adv Anim Vet Sci. 2014;2(10):557–564.
- Coelho AM, Coelho AC, Roboredo M, et al. A case-control study of risk factors for brucellosis seropositivity in Portuguese small ruminants herds. Prev Vet Med. 2007 Dec;82(3–4):291–301.
- Oseguera Montiel D, Bruce M, Frankena K, et al. Financial analysis of brucellosis control for small-scale goat farming in the Bajío region, Mexico. Prev Vet Med. 2014 Dec;118(4):247–259.
- Panchasara H. Economic implications of brucellosis in bovine. Indian J F Vet. 2012;8(1):19–21.
- Angara TE, Ismail AAA, Ibrahim AM, et al. Assessment of the economic losses due to Bovine Brucellosis in Khartoum State, Sudan. Int J Tech Res Appl. 2016;4(2):2320–8163.
- Singh BB, Dhand NK, Gill JPS. Economic losses occurring due to brucellosis in Indian livestock populations. Prev Vet Med. 2015;119(3–4):211–215.
- Kollannur J, Rathore R, Chauhan R. Epidemiology and economics of brucellosis in animals and its zoonotic significance. ISAH Tartu Est. 2007; I(83): 466–468.
- Santos RL, Martins TM, Borges ÁM, et al. Economic losses due to bovine brucellosis in Brazil. Pesqui Vet Bras. 2013;33(6):759–764.
- Schurig GG, Sriranganathan N, Corbel MJ. Brucellosis vaccines: past, present and future. Vet Microbiol. 2002;90(1–4):479–496.
- Singh R, Basera SS, Tewari K, et al. Safety and immunogenicity of Brucella abortus strain RB51 vaccine in cross bred cattle calves in India. Indian J Exp Biol. 2012 Mar;50(3):239–242.