ABSTRACT
Vietnam is a tropical country where mosquito-borne diseases are common. This review explores the transmission of mosquito-borne flaviviruses in urban areas of Vietnam. It concludes that urban transmission has mainly been studied for Dengue virus, and so far, much less for Japanese encephalitis virus. Dengue is the most common flavivirus in Vietnam. Due to fast urbanization and favorable climatic conditions, the viral transmission concentrates mainly to large cities with high population density including Ha Noi, Nha Trang and Ho Chi Minh. Human cases of Japanese encephalitis have been controlled by an expanded immunization program. However, this virus is still circulating throughout the country, also in cities due to the pig rearing practices in urban and peri-urban areas. Zika virus is an additional major concern because it has long circulated in the Northern area and is now increasingly diagnosed in urban areas of the Central, Central Highlands and Southern regions using the same mosquito vectors as Dengue virus. There was alarge outbreak of Zika disease from 2016 to early 2017, with most infections observed in Ho Chi Minh city, the largest town in Vietnam. Other flaviviruses circulate in Vietnam but have not been investigated in terms of urban transmission.
Introduction
The Flavivirus genus comprises more than 70 different viruses belonging to Flaviviridae family, including the five well-known mosquito-borne viruses Dengue (DENV), Japanese encephalitis (JEV), Zika (ZIKV), West Nile (WNV) and Yellow fever (YFV) viruses [Citation1,Citation2]. These are all common viruses causing life-threatening infectious diseases in humans via mosquitoes. According to the World Health Organization (WHO), flaviviruses transmitted by mosquitoes cause millions of human cases and nearly a million of human deaths every year [Citation3]. With increased urbanization and significant climatic changes, it is likely that we will see increased disease transmissions in cities, and additional mosquito-borne viruses may adapt to these circumstances [Citation4]. Humans are dead-end hosts for JEV infections [Citation5,Citation6] and WNV transmission [Citation1,Citation7], but are the major amplifying hosts in the transmission cycle of DENV [Citation2] and ZIKV, and involved in the transmission cycle of YFV [Citation1,Citation8]. Therefore, it should be noted that all these flaviviruses pose a major threat to the human society.
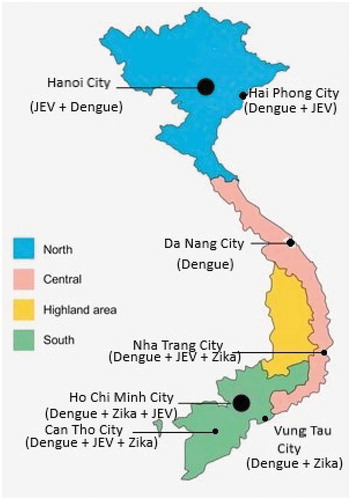
Vietnam is a tropical country in Southeast Asia where three of these mosquito-borne viruses (JEV, DENV and ZIKV) are endemic. Geographically, Vietnam can be divided into four regions including the Northern part, the Central Part, the Highlands Central part and the Southern part ().
This review focuses on the transmission of flaviviruses in urban areas of those regions, where the increasing urbanization contributes to an increased risk for mosquito-borne diseases.
Japanese encephalitis virus
Japanese encephalitis virus is prevalent in 24 Asian countries, encompassing almost half of the world’s population [Citation9–Citation11]. This flavivirus is the most common mosquito-borne pathogen causing viral encephalitis [Citation1,Citation7] and has been estimated to cause 68,000 human cases each year in global [Citation9,Citation10,Citation12]. About 75% of all Japanese encephalitis (JE) cases are observed among children in the age group 0–14 years [Citation12]. The clinical symptoms of a JEV infection are not always evident, and some cases develop only mild fever and headache. In case of severe symptoms such as high fever, headache, neck stiffness, disorientation, coma, seizures, spastic paralysis, the fatality rate can range from 20% to 30%, while 30–50% of the survivors will suffer severe neurologic or psychiatric sequelae [Citation10,Citation12].
The enzootic transmission cycle of JEV involves mosquitoes, pigs and water birds [Citation10]. Mosquitoes of the genus Culex, and in particular Culex tritaeniorhynchus, were early described as the most important vectors of JEV [Citation13]. JEV has been isolated from more than 30 species of mosquitoes since it was first isolated in the 1930s [Citation5], but mosquitoes from the Cx. vishnui subgroup, especially Cx. tritaeniorhynchus are still considered the most important vectors [Citation1,Citation4]. Cx. gelidus and Cx. fuscocephala are also recognized vectors, and the anthropophilic Cx. quinquefasciatus has been shown to be naturally infected and capable of transmitting JEV experimentally [Citation14,Citation15]. In addition, mosquitoes from other genera, such as Aedes, Armigeres, Anopheles and Mansonia have been shown to carry the virus or to be able to transmit it experimentally [Citation6,Citation14,Citation16–Citation18]. However, it is not yet known if these mosquitoes have any significant role in the epidemiology of JEV. Birds and pigs serve as reservoirs and amplifiers of the virus, whereas humans and other animals are considered accidental dead-end hosts [Citation4,Citation11]. Similar to human infections, JEV may cause fatal encephalitis in horses, whereas pigs often are only sub-clinically infected, or show reproductive symptoms, such as abortion or stillbirths [Citation19]. Many other domestic animals seroconvert without clinical symptoms, and dogs have recently been suggested as possible sentinels. Serological screening of dogs may be more representative for assessing human risks in urban environments, compared to screening of pigs [Citation20]. Emergence of JEV can be associated with both the growth of the human population and intensification and structural changes in agricultural practices. In Asia, the agricultural sector is rapidly growing. Especially the closeness to pigs and rice fields are established factors that increase the risk of contracting JE, and rice fields are favoured breeding sites for Cx. tritaeniorhynchus and other species in the Cx. vishnui subgroup [Citation21–Citation23], and also Cx. gelidus [Citation24], but this species is also known to breed in wastewaters [Citation25]. Therefore, the disease is most common in rural areas where growing rice fields and irrigation systems make up breeding grounds for vectors [Citation11]. In contrast, the urban Cx. quinquefasciatus often breeds in dirty stagnant waters, sewers and drains [Citation26]. In an outbreak of JEV in Australia; closeness of stagnant water and the density of the human population was believed to be important risk factors for infection [Citation27]. However, the epidemiology of JEV is likely to change with ongoing demographic and climate changes. The effects that the climate changes may have on disease transmission are difficult to predict since both vector and host factors will be affected [Citation28]. Although JE is generally viewed as a rural disease, JEV infection is also observed in pigs and humans living in urban environments [Citation4,Citation29–Citation31].
In Vietnam, JEV was isolated for the first time in 1951 [Citation32]. Until now, the incidence rate of JE in humans has diminished dramatically after introduction of an immunization program in the form of campaigns in high-risk areas in 1997. Until 2007, JE vaccination schemes covered 65% districts of Vietnam and was further scaled up nationwide in 2015 through an expanded program of vaccination [Citation32,Citation33]. Before 2003, JEV was endemic throughout the country with the annual incidence of 1000–3000 cases, mainly in rural areas [Citation11,Citation34]. However, the virus has also been circulating in cities and their vicinities for long [Citation35], where JEV has been isolated from pigs, mosquitoes and humans [Citation36].
Regarding the mosquito vector of JEV, in 2003, an entomological study was conducted in a suburban area, namely the Ha Tay province (at present part of Hanoi city since 2008). Maiko Hasegawa and co-workers found that the Culex population was predominant as compared to other mosquito species and its abundance was associated with the density of cattle [Citation37]. Another survey in both urban and rural areas of eight cities/provinces throughout Vietnam between 2006 and 2008 indicated that JEV existed in adult mosquitoes collected at different time points of the year and in different provinces in three out of four regions of Vietnam. Cx. tritaeniorhynchus collected in the Ha Tay province in May 2006 were found positive for JEV [Citation34]. Lindahl and co-workers investigated vectors of JEV in the urban environment of Can Tho city in southern Vietnam and found that vectors known to be competent transmitters of JEV were present in and around urban homes. Pigs in the close vicinity were associated with an increased number of competent vectors, in particular Cx. tritaeniorhynchus. An increased density of people in the household was related to higher numbers of Cx. quinquefasciatus [Citation38]. Cx. quinquefasciatus is a mosquito species known to prefer urban habitats and breed in stagnant waters such as sewers and other polluted waters [Citation26], explaining its abundance in the study, and this species was indicated as a potential urban vector of JEV in Can Tho city [Citation39]. Additionally, a recent study revealed that 60.4% of pigs sampled in slaughterhouses in Hanoi city were infected with JEV [Citation40]. It is possible that JE may become an increasing concern as increased urbanisation combined with a need to keep livestock (in particular pigs) creates new conditions for disease transmission in cities [Citation38].
In humans, surveillance data from the five Northern provinces of Vietnam: Thai Binh, Hai Phong, Thanh Hoa, Hai Duong, Bac Giang in 2004–2005 showed that more than half of 421 samples were positive for IgM against JEV, with 91% of these JEV infections observed in children under 15 years. Remarkably, among these five provinces, Hai Phong which is an urban city, recorded 61% of acute encephalitis syndrome cases laboratory confirmed as JEV [Citation32]. A recent serological survey of children between 1 and 10 years in Nha Trang city showed that JEV was still co-circulating with DENV, but at a lower rate. In 80 randomly selected ELISA test-positive samples; 21.3% were found seropositive for JEV; 87.5% were found positive to DENV, while 17.5% were positive to both JEV and DENV by a plaque reduction neutralization test [Citation41].
Dengue virus
DENV is one of the most important mosquito-borne viruses causing disease in humans throughout all tropical areas [Citation42,Citation43]. Every year, DENV infections result in 50–100 million cases of symptomatic illness in over 100 affected countries [Citation44]. The total number of DENV infections has been estimated to 390 million with approximately 70% of the infections occurring in Asia [Citation45]. More than half (3.97 billion people) of the world’s population is at risk of being infected with DENV [Citation46]. There are two diverse transmission cycles that maintains DENV endemic: a human cycle and a sylvatic cycle. The human cycle includes the transmission between Aedes mosquitoes and humans, where humans acts as the only known reservoir. DENV is transmitted from humans to humans through the bites of female Aedes mosquitoes. In contrast, the sylvatic cycle involves non-human primates and Aedes mosquitoes [Citation47]. There are four distinct serotypes of DENV named as DENV-1, DENV-2, DENV-3, and DENV-4 [Citation48]. Recently, another type called DENV-5 and related to the sylvatic cycle has been described [Citation49]. The serotypes 1–4 are all spread globally today. Infection with any DENV serotype can produce a spectrum of illness ranging from a mild, non-specific febrile syndrome, to classic dengue fever (DF), or more severe forms of disease, such as dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS), that can be fatal [Citation48]. The Aedes vectors prefer clean water for breeding; therefore, this virus can also be transmitted in rural areas where clean water is accessible [Citation50]. However, DF has been considered as an urban disease [Citation46] with the main vector Aedes aegypti being common in urban areas [Citation51,Citation52]. Aedes albopictus has more recently also become abundant in urban and peri-urban areas. Further geographical expansion of Aedes albopictus is expected in the near future, mainly due to urbanization, migration and a changing climate [Citation52–Citation54].
Dengue was first reported in Vietnam in 1959 [Citation55] and dengue has since then become endemic across the country with the circulation of all four serotypes [Citation41,Citation56–Citation60]. Nevertheless, there is a spatial heterogeneity in DENV transmission in Vietnam and the incidence rate differs for each region and province. The Southern part of the country has a higher number of human cases as compared to other regions of the country [Citation55,Citation61]. DENV has been shown to be imported from the Southern region into the Central and Northern regions of Vietnam [Citation62]. At present, DENV is disseminating in both urban and rural Vietnam [Citation63,Citation64]. However, research findings in Vietnam still pointed out that people mainly get infected with dengue when living in the cities of Hanoi (Northern part); Nha Trang, Khanh Hoa province (Central part) and Ho Chi Minh (Southern part). Less research on urban transmission has been performed in the Central Highlands although this region is also an endemic area for dengue [Citation64].
With a warm climate and a speed of urbanization, Hanoi city has been the hotspot for dengue in the whole northern area of Vietnam and witnessed several big outbreaks during the last decade. The capital city reported 16,263 DF cases in an outbreak in 2009, which was 87% of the total number of DF cases in the whole northern region [Citation65]. The latest as well as the largest DF outbreak so far in Hanoi occurred in 2017 with 37,651 cases and 7 deaths [Citation66]. Four DENV serotypes (1–4) have been confirmed to co-exist in Hanoi [Citation59]. Previous studies also revealed that the DF cases were mainly concentrated to the central urban districts [Citation65,Citation67–Citation69]. In the central region of Vietnam, a recent comprehensive study in Nha Trang city revealed 12,655 dengue cases recorded between 2006 and 2016 [Citation41]. Here it was also reported that all four serotypes of DENV were found in hospitalized patients during a single season (2015). During the peak years of DF in this coastal city, the incidence has been highest in the central urban wards [Citation41]. The southern region of Vietnam has been known as a dengue hyperendemic area where its tropical climate constitutes favorable conditions for the breeding of the vector. Ho Chi Minh city has been identified as a critical urban center for DENV transmission, due to the high population density and intense transportations [Citation70,Citation71]. DENV3 was first discovered in this location of southern Vietnam and has then spread over wider areas [Citation72]. Similar to the capital Hanoi, Ho Chi Minh city needs to cope with overloaded hospitals during the dengue outbreaks during recent years.
Vietnam is in an intense phase of industrialization and modernization leading to a rapid increase of urbanization. This factor in combination with climatic variability have influenced the distribution and density of dengue vectors [Citation73–Citation75]. Aedes aegypti is the dominant vector of DENV in urban areas of Vietnam, mainly breeding in plastic buckets, jars or flower vases [Citation76–Citation80]. Studies in urban areas of Hanoi city and Ho Chi Minh city identified DENV PCR-positive female Ae. aegypti [Citation80,Citation81]. Ae. albopictus has also been found to breed in urban and suburban areas of Vietnam, however, with lower density as compared to Ae. aegypti, which could be connected to climatic conditions [Citation75,Citation76,Citation78,Citation80]. Additionally, although low temperature is a negative factor for an increase of DENV vector populations, both Ae. aegypti and Ae. albopictus have been shown to survive during the winter in concrete tanks with broken lids in central districts of Hanoi city [Citation82]. In conclusion, DENV transmission is frequently presented also in urban areas of Vietnam. Therefore, public health interventions are needed to investigate the circulation of this dangerous flavivirus since the current dengue vector control program is working inefficiently [Citation83].
Zika virus
ZIKV was first discovered in a rhesus monkey in Uganda, Africa in 1947 [Citation84]. In 1954, a Nigerian female was the first human case diagnosed with a ZIKV infection [Citation85]. Prior to 2007, ZIKV only circulated in Africa and in Asia [Citation86,Citation87]. However, it has since then spread to the Pacific, Europe and the Americas, with the first described ZIKV outbreak in humans on the Island of Yap – Federated States of Micronesia in 2007, where 75% of the population was infected [Citation86,Citation88–Citation90]. While receiving only minor attention until 2013, ZIKV infections have now been recognized as a global threat and many countries in the Americas and in Asia had to cope with a large-scale epidemic [Citation91]. Confronting this new situation in 2016, WHO declared ZIKV as a Public Health Emergency of International Concern [Citation89,Citation90]. Like DENV, ZIKV has two transmission cycles: a sylvatic cycle and a human cycle. The sylvatic transmission cycle involves non-human primates and Aedes mosquitoes [Citation87]. In the human transmission cycle, ZIKV is transmitted between humans by infected Aedes mosquitoes [Citation90]. In addition, ZIKV has also been isolated from other species of mosquitoes such as Culex, Anopheles and Mansonia [Citation92,Citation93]. However, different species of Aedes mosquitoes are still recognized as the main vector for human infections [Citation86,Citation87,Citation92]. Ae. aegypti is the main vector in urban settings while Ae. albopictus can act as the vector of ZIKV infection in both urban and rural settings [Citation87,Citation92,Citation94,Citation95].
During recent years, new evidence revealed novel routes of transmission for ZIKV. It has been found that transfusions and perinatal transmission can transmit the virus [Citation96,Citation97]. Additionally, sexual contacts can also spread ZIKV among humans [Citation98–Citation100], which is in line with earlier indications of JEV being potentially sexually transmitted between pigs [Citation101]. ZIKV infections usually cause milder illness with common ‘dengue-like’ symptoms such as slight fever or rash, muscle and joint pain, tiredness. The symptoms usually last from 2 to 7 days. Similarly to the other flaviviruses, the majority of the infected persons do not develop clinical symptoms [Citation86,Citation95]. The most severe clinical complication of ZIKV infection is congenital microcephaly of the fetus when mothers get infected during pregnancy [Citation89,Citation102]. A link to Guillain-Barré syndrome has also been confirmed [Citation103,Citation104].
In Vietnam, the attention to ZIKV has been increased during recent years. In April 2016, Vietnam declared the first Zika cases identified in two females from Nha Trang city and Ho Chi Minh city, respectively [Citation105]. However, it should be noted that ZIKV has been retrospectively detected by RT-PCR in two children living in Ho Chi Minh city and the suburban area of the Long An province of the Southern region already in 2013 [Citation106]. In addition, ZIKV infections have earlier been shown by the presence of neutralizing antibodies in two adults in North Vietnam in 1954 [Citation107]. Hence, this virus appeared in Vietnam for several decades, nevertheless, because of the poor understanding of ZIKV infection and the lack of diagnostic tests at that time, it has earlier not been given any attention. After the detection of human cases in 2016, the number of recognized ZIKV infections increased dramatically.
According to statistics summarized in , a total of 212 cases of ZIKV infection were reported in 2016. This outbreak in Vietnam occurred in more than 10 cities and provinces of the Central, Central Highlands and Southern regions, mainly in urban and semi-urban areas. A number of cases were identified in pregnant women [Citation108]; however, only one microcephaly case was found in the Dak Lak province, Central Highlands [Citation109]. Since 2017, the ZIKV transmission decreased significantly with only 24 newly recorded cases from January to April and one newly infected province, namely Lam Dong in Central Highlands [Citation64]. All of these cases were identified to be local mosquito-borne infections [Citation110].
Table 1. ZIKV infection in Vietnam in 2016.
Vietnam is one of the five countries which have been identified as sentinel markers for ZIKV transmission [Citation99]. Many visitors have been confirmed to have acquired ZIKV infection after their stay in Vietnam, particularly in Ho Chi Minh city, the biggest city in the country, which suffered the most severe outbreak. Israel and Korean travelers were suspected to have been infected with ZIKV in Vietnam in early 2016, while Zika disease was not reported at all in Vietnam in 2015 [Citation111,Citation112]. After that, two cases, a German woman and a Japanese man were hospitalized in Japan in September and November 2016, respectively, and were both diagnosed to be infected by ZIKV in Ho Chi Minh city [Citation113,Citation114]. Taiwan Centers for Disease Control recorded five ZIKV cases originating from Vietnam in 2016, and one new case was confirmed in a person who worked in Vietnam in late 2018 [Citation115]. Therefore, it seems that ZIKV is still circulating in Vietnam.
Although unsafe sexual practices and blood transfusions can be a route for ZIKV transmission, no such cases have been identified in Vietnam. The most important risk factor of ZIKV transmission is referred to the high density of the main vector, the Aedes mosquito population [Citation110]. In an entomological report from Nha Trang city in 2016, ZIKV existed in Ae. aegypti with a high prevalence of 0.24% [Citation116]. This was in accordance with an entomological finding in an urban district of Brazil where three out of one hundred and ninety-eight pools of Ae. aegypti were naturally infected with ZIKV [Citation117]. In Vietnam, the practice of storing water in tanks or containers at home is common, enabling Aedes mosquitos, the main vector of DENV and ZIKV, to expand quickly [Citation110]. In addition, the impact of climate change and urbanization plus the lack of awareness among urban inhabitants on the importance of removing the breeding grounds of mosquitoes have lead to a significant increase of the vector density [Citation33,Citation83]. In addition to encouraging people to use personal protective measures to avoid mosquito bites, new effective strategies for vector control need to be built globally [Citation118] because the current program has its limitations and is difficult to achieve [Citation119]. Hence, ZIKV is concentrated mainly in urban and semi-urban areas of the Central, Central Highlands and Southern regions of Vietnam, but there is a significant risk that this virus can re-emerge in the Northern region due to a huge human migrations [Citation110].
Other flaviviruses
Quang Binh virus is another flavivirus that was isolated from Cx. tritaeniorhynchus mosquitoes collected in the Quang Binh province, Central Vietnam, in 2002 [Citation120]. However, no human infection has yet been detected, and no circulation in mosquitoes has been shown since 2002. Knowledge of the epidemiology, particularly regarding urban transmission, of this virus in Vietnam is still limited.
Some important flaviviruses have never been detected in Vietnam. YFV shares the same mosquito vector as DENV and ZIKV (Ae. aegypti) and a similar urban cycle of viral transmission. However, interestingly, YFV has never been identified in Asia, so far only in Africa and the Americas [Citation121,Citation122].
WNV is distributed extensively in Africa, the Americas, parts of southern Europe, the Middle East and western Asia. Viral transmission to humans has mainly been associated with bites by Culex mosquitoes. Eighty percent of the infections are asymptomatic or mild febrile illness. However, 1 in 150 WNV infections results in meningitis or encephalitis, and even deaths [Citation2,Citation123]. It is not yet known why Southeast Asia has so far been spared from this flavivirus, and more research into the vector potential of the regional Culex species may be warranted.
Conclusion
This review focuses on flaviviruses in urban Vietnam, in order to bring more clarity to its transmission in cities (see in the ). In conclusion, three of the most important flaviviruses: JEV, DENV and ZIKV co-exist in urban Vietnam and pose a major risk for severe diseases in humans via mosquito bites.
Table 2. Summary points of urban transmission of mosquito-borne flavivirus in Vietnam.
The urban epidemiology of JEV has mainly been studied in three regions of Vietnam. Several potential mosquito vectors for JEV transmission have been identified in entomological surveys; found RT-PCR positive for this virus. JEV has the potential to rapidly emerge in urban communities, particularly when pig-keeping is practiced in peri-urban and urban areas of cities, as frequently occurring in Vietnam. DENV is the most widespread flavivirus in Vietnam where all DENV serotypes are circulating. They are regularly transmitted in urban and semi-urban areas yearly throughout the country and constitute a major threat; mainly in the larger cities. ZIKV was first detected in the Northern region of Vietnam in the 1950s but seems to have recently re-emerged only in urban areas of the Central, Central Highlands and Southern regions, especially in Ho Chi Minh city, where migration and international travel are contributing to risks of further spread. In conclusion, there is an urgent need for intensified research to be able to create new appropriate strategies to cope with the increasing mosquito populations during the high-risk season and to prevent human infections from the flaviviruses.
Acknowledgments
We would like to thank Dr Vu Trong Duoc from National Institute of Hygiene and Epidemiology, Vietnam for providing us with essential data for writing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Thang Nguyen-Tien
Thang Nguyen-Tien is a PhD fellow of International Livestock Research Institute and Uppsala University, following the research track of Infection since 2018. He is focusing on one health approach to deal with vector-borne and infectious diseases in his PhD project.
Åke Lundkvist
Åke Lundkvist is a Professor in Virology. He is the co-founder and head of the Zoonosis Science Center (ZSC) at Department of Medical Biochemistry and Microbiology, Uppsala University, Sweden. His research group is focused on viral zoonoses, with a specifc interest in hanta-, favi- and avian infuenza viruses. His research has included basic virology, immunology, genetics, molecular epidemiology, animal models, virus evolution, antivirals and diagnostics.
Johanna Lindahl
Johanna Lindahl is a veterinary epidemiologist working at the International Livestock Research Institute (ILRI) in Hanoi, Vietnam, and is an associate professor at Uppsala University since 2017. Johanna graduated from the Swedish University of Agricultural Sciences after doing her PhD working on Japanese encephalitis virus in Vietnam. Since her PhD she has been focusing her research on food safety, and vector-borne, zoonotic and emerging infectious diseases in developing countries.
References
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of japanese encephalitis, west nile and dengue viruses. Nat Med. 2004;10:1-10.
- Huang YJS, Higgs S, Horne KME, et al. Flavivirus-mosquito interactions. Viruses. 2014;6:4703–4730.
- WHO. Mosquito-borne diseases [Online]; 2017 [cited 2019 Mar 01]. Available from: https://www.who.int/neglected_diseases/vector_ecology/mosquito-borne-diseases/en/
- Solomon T, Dung NM, Kneen R, et al. Japanese encephalitis. J Neurol Neurosurg Psychiatry. 2000;68(4):405–10.
- van Den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35.
- Endy TP, Nisalak AN. Japanese encephalitis virus: ecology and epidemiology. In Japanese encephalitis and West Nile viruses 2002 (pp. 11–48). Springer, Berlin, Heidelberg.
- Go YY, Balasuriya UBR, Lee CK. Zoonotic encephalitides caused by arboviruses: transmission and epidemiology of alphaviruses and flaviviruses. Clin Exp Vaccine Res. 2014;3(1):58.
- Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85(2):328–345.
- Wang H, Liang G. Epidemiology of Japanese encephalitis: past, present, and future prospects. Ther Clin Risk Manag. 2015;11:435–448.
- WHO. JE factsheet [Online]; 2015 [cited 2019 Mar 01]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/japanese-encephalitis
- Erlanger TE, Weiss S, Keiser J, et al. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15(1):1–7.
- Campbell GL, Hills SL, Fischer M, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011;44:337–344.
- Buescher EL, Scherer WF. Ecologic studies of Japanese encephalitis virus in Japan. Am J Trop Med Hyg. 1959;8(6):719–722.
- Rosen L. The natural history of Japanese encephalitis virus. Annu Rev Microbiol. 2002;40(1):395–414.
- Lindahl J. Japanese encephalitis virus in pigs and vectors in the mekong delta with special reference to urban farming. Department of Clinical Sciences, Swedish University of Agricultural Sciences. 2012.
- Thenmozhi V, Rajendran R, Ayanar K, et al. Long-term study of Japanese encephalitis virus infection in anopheles subpictus in Cuddalore district, Tamil Nadu, South India. Trop Med Int Heal. 2006;11(3):288–293.
- Liu H, Lu H-J, Liu Z-J, et al. Japanese encephalitis virus in mosquitoes and swine in yunnan province, China 2009–2010. Vector-Borne Zoonotic Dis. 2012;13(1):41–49.
- Chen W-J, Dong C-F, Chiou L-Y, et al. Potential role of armigeres subalbatus (diptera: culicidae) in the transmission of Japanese encephalitis virus in the absence of rice culture on Liu-Chiu Islet, Taiwan. J Med Entomol. 2009;37(1):108–113.
- Burns KF. Congenital Japanese B encephalitis infection of swine. Exp Biol Med. 1950;75(2):621–625.
- Shimoda H, TAMARU S, MORIMOTO M, et al. Experimental infection of Japanese encephalitis virus in dogs. J Vet Med Sci. 2011;73(9):1241–1242.
- Liu W, GIBBONS RV, KARI K, et al. Risk factors for Japanese encephalitis: a case-control study. Epidemiol Infect. 2010;138(9):1292–1297.
- Impoinvil DE, Solomon T, Schluter WW, et al. The spatial heterogeneity between Japanese encephalitis incidence distribution and environmental variables in Nepal. PLoS One. 2011;6(7):e22192.
- Keiser J, Maltese MF, Erlanger TE, et al. Effect of irrigated rice agriculture on Japanese encephalitis, including challenges and opportunities for integrated vector management. Acta Trop. 2005;95(1):40–57.
- Abu Hassan A, Dieng H, Satho T, et al. Breeding patterns of the JE vector culex gelidus and its insect predators in rice cultivation areas of northern peninsular Malaysia. Trop Biomed. 2010;27(3):404–416.
- Whelan P, Hayes G, Tucker G, et al. The detection of exotic mosquitoes in the Northern Territory of Australia the detection of exotic mosquitoes in the Northern. Arbovirus Res Aust. 2001;8:395–404.
- Epstein PR. West Nile virus and the climate. J Urban Health. 2001;78(2):367–371.
- Hanna JN, Ritchie SA, Phillips DA, et al. An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med J Aust. 1996;165(5):256–260.
- Githeko AK, Lindsay SW, Confalonieri UE, et al. Climate change and vector-borne diseases: a regional analysis. Bull World Health Organ. 2000;78(9):1136–1147.
- Tseng HF, Tan HF, Chang CK, et al. Seroepidemiology study of Japanese encephalitis neutralizing antibodies in southern Taiwan: A comparative study between urban city and country townships. Am J Infect Control. 2003;31(7):435–440.
- Cappelle J, Duong V, Pring L, et al. Intensive Circulation of Japanese encephalitis virus in peri-urban sentinel pigs near Phnom Penh, Cambodia. PLoS Negl Trop Dis. 2016;10(12):e0005149.
- Bi P, Zhang Y, Parton KA. Weather variables and Japanese encephalitis in the metropolitan area of Jinan city, China. J Infect. 2007;55(6):551–556.
- Yen NT, Duffy MR, Hong NM, et al. Surveillance for Japanese encephalitis in Vietnam, 1998–2007. Am J Trop Med Hyg. 2010;83(4):816–819.
- Ministry of Health Vietnam. Joint annual health review 2015: strengthening primary health care at the grassroots toward universal health coverage. Med Publ House. 2016;211.
- Kuwata R, Tsuda Y, Sawabe K, et al. Surveillance of Japanese encephalitis virus infection in mosquitoes in Vietnam from 2006 to 2008. Am J Trop Med Hyg. 2013;88(4):681–688.
- Thoa NT, Vien NT, Mai TT, et al. Japanese encephalitis vectors: isolation of virus from culicine mosquitoes in the Saigon area. Southeast Asian J Trop Med Public Health. 1974;5(3):408–412.
- Do, QH, Vu, TQH, Huynh, TKL, et al. Current situation of Japanese encephalitis in the South of Vietnam, 1976–1992. Trop Med. 1995;36(4):202–214.
- Hasegawa M, Tuno N, Nguyen TY, et al. Influence of the distribution of host species on adult abundance of Japanese encephalitis vectors - culex vishnui subgroup and culex gelidus - in a rice-cultivating village in Northern Vietnam. Am J Trop Med Hyg. 2008;78(1):159–168.
- Lindahl J, Chirico J, Boqvist S, et al. Occurrence of Japanese encephalitis virus mosquito vectors in relation to urban pig holdings. Am J Trop Med Hyg. 2012;87(6):1076–1082.
- Lindahl JF, Ståhl K, Chirico J, et al. Circulation of Japanese encephalitis virus in pigs and mosquito vectors within can Tho City, Vietnam. PLoS Negl Trop Dis. 2013;7(4).
- Ruget A-S, Beck C, Gabassi A, et al. Japanese encephalitis circulation pattern in swine of northern Vietnam and consequences for swine’s vaccination recommendations. Transbound Emerg Dis. 2018;65(6):1485–1492.
- Le Quyen D, Thanh Le N, Van Anh CT, et al. Epidemiological, serological, and virological features of dengue in Nha Trang City, Vietnam. Am J Trop Med Hyg. 2018;98(2):402–409.
- WHO. Global strategy for dengue prevention and control 2012–2020. World Heal Organiszation. 2012;43.
- Cucunawangsih, Lugito NPH. Trends of dengue disease epidemiology. Virol Res Treat. 2017;8:1178122X17695836.
- WHO. Dengue [Online]; 2014 [cited 2017 Jan 30]. Available from: http://www.wpro.who.int/mediacentre/factsheets/fs_09032012_Dengue/en/
- Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013 Apr;496(7446):504–507.
- Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760.
- Vasilakis N, Cardosa J, Hanley KA, et al. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol. 2011 Jul;9(7):532–541.
- WHO. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: WHO Library. 2009:10–12.
- Mustafa MS, Rasotgi V, Jain S, et al. Discovery of fifth serotype of dengue virus (denv-5): a new public health dilemma in dengue control. Med J Armed Forces India. 2015;71(1):67–70.
- Guo C, Kim TK, Pinto AFM, et al. Global epidemiology of dengue outbreaks in 1990–2015: a systematic review and meta-analysis. Front Cell Infect Microbiol. 2017;7:317.
- Zahouli JBZ, Koudou BG, Müller P, et al. Urbanization is a main driver for the larval ecology of aedes mosquitoes in arbovirus-endemic settings in south-eastern côte d’Ivoire. PLoS Negl Trop Dis. 2017;11(7):e0005751.
- Jansen CC, Beebe NW. The dengue vector aedes aegypti: what comes next. Microbes Infect. 2010;12(4):272–279.
- Li Y, Kamara F, Zhou G, et al. Urbanization increases aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl Trop Dis. 2014;8(11):e3301.
- Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;4:e646.
- Vietnam WHO. Dengue factsheet [Online]; 2016 [cited 2017 Jan 30]. Available from: http://www.wpro.who.int/vietnam/topics/dengue/factsheet/en/
- Thai KTD, Phuong HL, Thanh Nga TT, et al. Clinical, epidemiological and virological features of dengue virus infections in vietnamese patients presenting to primary care facilities with acute undifferentiated fever. J Infect. 2010;60(3):229–237.
- Ha DQ, Huong VTQ, Loan HTK, et al. Virological and serological surveillance of dengue epidemics in 19 provinces in Southern Viet Nam during 2001. Dengue Bull. 2003;27:46–51.
- Tuan LV, Van NT, Nga PT, et al. Seasonal distribution of dengue fever in the central highlands region, Vietnam (2010–2015). Am J Epidemiol Infect Dis. 2017;5(1):8–13.
- M. H. P. Ly, Moi ML, Vu TB et al., Dengue virus infection-enhancement activity in neutralizing antibodies of healthy adults before dengue season as determined by using FcγR-expressing cells. BMC Infect Dis. 2018;18(1):31.
- Kim Lien PT, Yatawara L, Nagataki M, et al. Surveillance of dengue and chikungunya infection in Dong Thap, Vietnam: A 13-month study. Asian Pac J Trop Med. 2016;9(1):39–43.
- Lee HS, Smouse SL, Tau NP, et al. Seasonal patterns of dengue fever and associated climate factors in 4 provinces in Vietnam from 1994 to 2013. BMC Infect Dis. 2017 Dec;17(1):218.
- Rabaa MA, Simmons CP, Fox A, et al. Dengue virus in Sub-tropical Northern and Central Viet Nam: population immunity and climate shape patterns of viral invasion and maintenance. PLoS Negl Trop Dis. 2013;7(12):e2581.
- Schmidt W-P, Suzuki M, Dinh Thiem V, et al. Population density, water supply, and the risk of dengue fever in vietnam: Cohort study and spatial analysis. PLOS Med. 2011;8(8):e1001082.
- Duoc VT. Situations of dengue and Zika virus diseases - control activities in Vietnam. National Institute of Hygiene and Epidemiology, Vietnam. 2017.
- An DTM, Rocklöv J. Epidemiology of dengue fever in hanoi from 2002 to 2010 and its meteorological determinants. Glob Health Action. 2014;7(1):23074.
- Hanoi Preventive Medicine Center. Report on communicable disease control in Hanoi in 2017. Communicable disease control Department, Hanoi Preventive Medicine Center, Hanoi; 2017.
- Cuong HQ, Gros -P-P, Tam M, et al. Quantifying the emergence of dengue in Hanoi, Vietnam: 1998–2009. PLoS Negl Trop Dis. 2011;5(9):e1322.
- Duong TN, Cam NN, Duoc VT. Epidemiological situation of Dengue hemorrhagic fever in Ha Noi, 2006–2011. Vietnam J Prev Med. 2013;23(6):58.
- Hanh DTK, Binh NTT, Anh NTL, Cam NN. Epidemiological characteristics of Dengue fever in Hanoi from 2004 to 2014. Vietnam J Prev Med. 2016;26(2):25.
- Rabaa MA, Hang VTT, Wills B, et al. Phylogeography of recently emerged DENV-2 in Southern Viet Nam. PLoS Negl Trop Dis. 2010;4(7).
- Raghwani J, Rambaut A, Holmes EC, et al. Endemic dengue associated with the co-circulation of multiple viral lineages and localized density-dependent transmission. PLoS Pathog. 2011;7(6):e1002064.
- Ha DQ, Tien NTK, Huong VTQ, et al. Dengue epidemic in Southern Vietnam, 1998. Emerg Infect Dis. 2000;6(4):422–425.
- Thai KTD, Cazelles B, Nguyen NV, et al. Dengue dynamics in binh thuan province, southern vietnam: periodicity, synchronicity and climate variability. PLoS Negl Trop Dis. 2010;4(7):e747.
- Pham HV, Doan HTM, Phan TTT, et al. Ecological factors associated with dengue fever in a central highlands province, Vietnam. BMC Infect Dis. 2011 11.
- Higa Y, Thi Yen N, Kawada H, et al. Geographic distribution of aedes aegypti and aedes albopictus collected from used tires in Vietnam. J Am Mosq Control Assoc. 2010;26(1):1–9.
- Phong TV, Nam VS. Key breeding sites of dengue vectors in Hanoi, Vietnam, 1994–1997. Dengue Bull. 1999;23:67–72.
- Huber K, Le Loan L, Hoang TH, et al. Aedes aegypti in South Vietnam: ecology, genetic structure, vectorial competence and resistance to insecticides. Southeast Asian J Trop Med Public Health. 2003;34(1):81–86.
- Tsuzuki A, Duoc VT, Higa Y, et al. High potential risk of dengue transmission during the hot-dry season in Nha Trang City, Vietnam. Acta Trop. 2009;111(3):325–329.
- Tsuzuki A, Vu TD, Higa Y, et al. Effect of peridomestic environments on repeated infestation by preadult Aedes aegypti in urban premises in Nha Trang City, Vietnam. Am J Trop Med Hyg. 2009;81(4):645–650.
- Kim Lien PT, Duoc VT, Gavotte L, et al. Role of aedes aegypti and aedes albopictus during the 2011 dengue fever epidemics in Hanoi, Vietnam. Asian Pac J Trop Med. 2015;8(7):543–548.
- Anders KL, Nga LH, Thuy NTV, et al. Households as foci for dengue transmission in highly urban Vietnam. PLoS Negl Trop Dis. 2015;9(2):e0003528.
- Tsunoda T, Cuong TC, Dong TD, et al. Winter refuge for aedes aegypti and ae. albopictus mosquitoes in Hanoi during winter. PLoS One. 2014;9(4):e95606.
- Nguyen-Tien T, Probandari A, Ahmad RA., et al. Barriers to engaging communities in a dengue vector control program: an implementation research in an urban area in Hanoi City, Vietnam. Am J Trop Med Hyg. 2019;100(4):964–973.
- Dick GWA, Kitchen SF, Haddow AJ. Zika virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–520.
- Macnamara, MacNamara FN. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48(2):139–145.
- Hamel R, Liégeois F, Wichit S, et al. Zika virus: epidemiology, clinical features and host-virus interactions. Microbes Infect. 2016;18(7–8):441–449.
- Weaver SC, Costa F, Garcia-Blanco MA, et al. Zika virus: history, emergence, biology, and prospects for control. Antiviral Res. 2016;130:69–80.
- Duffy MR, Chen T-H, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543.
- Kindhauser MK, Allen T, Frank V, et al. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ. 2016;94(9):675–686C.
- WHO Zika virus [Online]; 2018 [cited 2018 Aug 25]. Available from: http://www.who.int/en/news-room/fact-sheets/detail/zika-virus
- Duong V, Dussart P, Buchy P. Zika virus in Asia. Inter J Infect Dis. 2017;54:121–128.
- Vasilakis N, Weaver SC. Flavivirus transmission focusing on Zika. Curr Opin Virol. 2017;22:30–35.
- Boyer S, Calvez E, Chouin-Carneiro T, et al. An overview of mosquito vectors of Zika virus. Microbes Infect. 2018;20(11–12):646–660.
- Grard G, Caron M, Mombo IM, et al. Zika virus in Gabon (Central Africa) - 2007: a new threat from aedes albopictus? PLoS Negl Trop Dis. 2014;8(2):e2681.
- Ioos S, Mallet HP, Leparc Goffart I, et al. Current Zika virus epidemiology and recent epidemics. Med Mal Infectieuses. 2014;44(7):302–307.
- Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Eurosurveillance. 2014;19(14):20761.
- Besnard M, Lastère S, Teissier A, et al. Evidence of perinatal transmission of zika virus, French Polynesia, december 2013 and february 2014. Eurosurveillance. 2014;19(13):20751.
- Hastings AK, Fikrig E. Zika virus and sexual transmission: a new route of transmission for mosquito-borne flaviviruses. Yale J Biol Med. 2017;90(2):325–330.
- Wilder-Smith A, Chang CR, Leong WY. Zika in travellers 1947–2017: a systematic review. J Travel Med. 2018;25(1).
- Moreira J, Peixoto TM, Siqueira AM, et al. Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect. 2017;23(5):296–305.
- Maes D, Nauwynck H, Rijsselaere T, et al. Diseases in swine transmitted by artificial insemination: An overview. Theriogenology. 2008;70:1337–1345.
- Zanluca C, Nunes C. Zika virus e an overview. Microbes Infect. 2016;18(5):295–301.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet. 2016;387(10027):1531–1539.
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barré syndrome case report, French Polynesia, December 2013. Eurosurveillance. 2014;19(9):20720.
- WHO. Zika virus infection – Viet Nam [Online]; 2016 [cited 2018 Aug 25]. Available from: http://www.who.int/csr/don/12-april-2016-zika-viet-nam/en/
- Quyen NTH, Kien DTH, Rabaa M, et al. Chikungunya and zika virus cases detected against a backdrop of endemic dengue transmission in Vietnam. Am J Trop Med Hyg. 2017;97(1):146–150.
- Pond WL. Arthropod-borne virus antibodies in sera from residents of South-East Asia. Trans R Soc Trop Med Hyg. 1963;57(5):364-371.
- Bui TC, Huynh XV, Nguyen H, et al. Zika virus outbreaks and treatment in pregnant women in Ho Chi Minh City, Vietnam. Int J Gynaecol Obstet. 2018;141:390–391.
- Moi ML, Koen A, Fix A, et al. Zika virus infection and microcephaly in Vietnam. Lancet Infect Dis. 2017;17(8):805–806.
- Chu DT, Ngoc VTN, Tao Y. Zika virus infection in Vietnam: current epidemic, strain origin, spreading risk, and perspective. Eur J Clin Microbiol Infect Dis. 2017;36(11):2041–2042.
- Meltzer E, Lustig Y, Leshem E, et al. Zika virus disease in traveler returning from Vietnam to Israel. Emerg Infect Dis. 2016;22(8):1521–1522.
- Jeong YE, Cha GW, Cho JE, et al. Viral and serological kinetics in Zika virus-infected patients in South Korea. Virol J. 2017;14(1).
- Hashimoto T, Wang N, Wilksch JJ, et al. Importation of Zika virus from Vietnam to Japan, November 2016. Emerg Infect Dis. 2017;23(7):1222–1225.
- Katanami Y, Kutsuna S, Taniguchi S, et al. Detection of Zika virus in a traveller from Vietnam to Japan. J Travel Med. 2017;24(5).
- Taiwan Centers for Disease Control. Taiwan CDC confirms 1 new imported Zika case [Online]; 2018 [cited 2019 Mar 01]. Available from: https://www.cdc.gov.tw/En/Bulletin/Detail/xZ_NvpydeyFPi-_sxxK_pQ?typeid=158
- Dengue Elimination project. Detection of 56 Aedes mosquitos get infected with zika virus in Nha Trang city [Online]; 2016 [cited 2019 Mar 01]. Available from: http://www.eliminatedengue.com/library/publication/document/viet-nam/20161024_ttbc_56_ca_the_muoi_zika_nt.pdf
- Ferreira-De-Brito A, Ribeiro IP, Miranda RMD, et al. First detection of natural infection of aedes aegypti with Zika virus in Brazil and throughout South America. Mem Inst Oswaldo Cruz. 2016;111(10):655–658.
- Baud D, Gubler DJ, Schaub B, et al. An update on Zika virus infection. Lancet. 2017;390(10107):2099–2109.
- Reiner RC, Achee N, Barrera R, et al. Quantifying the epidemiological impact of vector control on dengue. PLoS Negl Trop Dis. 2016;10(5):e0004588.
- Hou, Crabtree, Wu H, et al. Isolation and characterization of a new mosquito flavivirus, Quang Binh virus, from Vietnam. Arch Virol. 2009;154(5):857–860.
- Leta S, Beyene TJ, De Clercq EM, et al. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int J Infect Dis. 2018;67:25–35.
- Donadeu M, Lightowlers MW, Fahrion AS, et al. Vaccines and vaccination against yellow fever. Wkly Epidemiol Rec. 2013;III(47):445–452.
- WHO. West Nile virus [Online]. 2017 [cited 2019 Mar 01]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/west-nile-virus