ABSTRACT
Small ruminants are the main reservoirs for brucellosis and coxiellosis, two zoonotic diseases affecting livestock production, and posing a public health threat in India. Understanding disease prevalence and risk factors associated with small ruminant infection can help mitigate disease transmission.
We report a cross-sectional survey in the states of Assam and Odisha in Eastern India. We interviewed 244 farmers to assess knowledge, attitude and practices relevant to brucellosis and coxiellosis infection. Serum samples from 411 goats and 21 sheep were analysed using enzyme-linked immunosorbent assay and Rose-Bengal Brucella agglutination plate test. Higher Brucella and Coxiella burnetii seroprevalence were found in Odisha (22% and 11.5%, respectively) than Assam (9.8% and 1.6%, respectively), and certain districts in Odisha were at higher risk. No association was found between seropositive animals and clinical signs, a challenge when attempting to identify seropositive animals in the herd. None of the farmers interviewed were aware of brucellosis, its aetiology, clinical form, or zoonotic risk. This study acts as a first indication of the extent of these diseases among small ruminants in these Indian states, highlighting how farming practices are associated with increased risk of infection. More research is urgently needed to mitigate zoonoses transmission in this region.
Introduction
Sheep and goats help support the livelihoods of millions of poor rural households in India [Citation1]. An estimated 98% of small ruminants are owned by small, landless, and often illiterate farmers [Citation2]. Prioritising small ruminant health is challenging due to the ad hoc approach taken to goat and sheep rearing [Citation3] and the difficulty in estimating small ruminant disease costs [Citation4]. Disease prevention and control in small ruminants are further hampered by inadequate veterinary services in rural areas [Citation3,Citation4]. However, given that many zoonotic pathogens of serious animal and public health concern in India have small ruminants as reservoirs [Citation5,Citation6], it is of paramount public health importance to understand zoonoses prevalence in small ruminants.
Brucellosis is endemic in India [Citation7,Citation8] and sheep and goats are a major source of infection [Citation9,Citation10]. A sharp increase in human brucellosis in recent years has been attributed to Brucella melitensis [Citation11,Citation12], which is the species usually responsible for small ruminant brucellosis [Citation13]. To date, a limited number of small ruminant studies have been carried out in India to understand brucellosis seroprevalence [Citation1,Citation14–Citation16], but other infectious agents have been overlooked [Citation17].
One such agent is Coxiella burnetii, causing coxiellosis, or Q-fever as it is called when affecting humans, a widely distributed zoonotic disease of animal and public health concern [Citation6,Citation18,Citation19]. Coxiella burnetii has multiple hosts and its transmission is also affected by environmental factors [Citation18]. It is ranked among the top 13 global priority zoonoses [Citation20]. Small ruminants have been identified as one of its primary reservoirs [Citation18,Citation21,Citation22]. Despite its ubiquitous nature globally [Citation21], human cases are underdiagnosed and underreported in India [Citation23], and little is understood of its prevalence in Indian livestock [Citation24].
Brucellosis and coxiellosis in small ruminants are both characterised by abortion during late pregnancy, stillbirths or the delivery of weak kids, thus causing severe reproductive losses [Citation22,Citation25]. Both pathogens cause chronic infection in the uterus and mammary glands of infected goats and sheep [Citation26] and are both shed mostly in placental membranes and birth fluids, but also in milk and faeces [Citation27]. Both cause fever and chronic disease in humans [Citation1], are resistant in the environment, can spread as aerosols and may cause large-scale outbreaks due to their low infectious dose [Citation27]. Coxiellosis can also be transmitted to livestock via tick bites [Citation18,Citation22]. Control of either pathogen is challenging due to the latent nature of infection; a normal parturition often follows an abortion, making culling of infectious animals within a herd complicated [Citation11,Citation22].
In India, animal husbandry is the second largest occupation in rural areas [Citation28]. A One Health approach including transdisciplinary collaboration and participation of communities is necessary for successful interventions to mitigate human infection at the animal, environmental, human interface. Prevention of human brucellosis and Q-fever from a public health perspective is based on control of the disease in the small ruminant reservoir [Citation29]; thus, understanding how farmers interact with their sheep and goats is vital. In this study, a twofold approach examined the seroprevalence of Brucella and C. burnetii in small ruminants in the northeastern Indian states of Assam and Odisha. These states were chosen due to the dearth of reports on small ruminant zoonoses [Citation2,Citation12,Citation30]. A farmer knowledge, attitude, and practices (KAP) questionnaire was used to gain a deeper understanding into farming practices, rearing conditions, and contact between small ruminants and other species, all important risk factors identified for C. burnetii and Brucella infection [Citation2,Citation22]. Through an integrated approach, combining serology sampling with farmer interviews, this study created a deeper understanding of the epidemiology and risk factors for Brucella and Coxiella seropositivity, benefiting future intervention programmes and policy development in mitigating transmission risk in Eastern India.
Materials and methods
Sampling design
This cross-sectional study was conducted in the Indian states of Assam and Odisha from March to December 2017, using a multistage sampling technique for household sample selection in both states. The first stage was to select three districts each from the 33 districts in Assam and 30 districts in Odisha. District selection was guided by consultations with the Animal Husbandry and Veterinary Department officials of each state who have access to goat and sheep census numbers. Accordingly, Kamrup, Bangaigaon, and Sonitpur districts were selected in Assam, and Cuttack, Kendrapara, and Mayurbhanj districts selected in Odisha. Secondly, two community development blocks (CDBs) from each district, one urban and one rural were randomly selected. Thirdly, two villages were selected randomly from each CDB. A list of households with goats and sheep in each of these selected villages was created with the support of key informants, including the local non-governmental organisations (NGO) and village headsmen. From these lists, 10 households were selected randomly, however from two villages in Assam and Odisha, 11 households per village were included. One hundred and twenty-two households were thus selected in total from each state for the study. Random selection was done using the random number function in MS Excel.
Ethics statement
Ethical permission for the study was granted by the Institutional Research Ethical Committee of the International Livestock Research Institute ILRI-IREC2017-39 as well as by the ethical board at Indian Council of Agricultural Research-National Institute of Veterinary Epidemiology and Disease Informatics (ICAR-NIVEDI).
Data collection
The farmers were contacted before the study by key informants. Informed consent was obtained from all participants included in the study and farmers were compensated for their time. A pre-tested household questionnaire with closed questions was distributed by the veterinary scientists who conducted the fieldwork at the time of blood sampling and who spoke the local language. Two male interviewers worked in the state of Odisha and five male interviewers worked in the state of Assam. The questionnaire was piloted in the area before the start of the survey, and the interviewing personnel were trained in order to have a common methodology. Knowledge on zoonoses was assessed, with only brucellosis being named since animal health extension efforts have until now only ever focused on this disease.
Serological sampling
To evaluate seroprevalence, the aim was to sample two randomly selected female small ruminants per farm, although sometimes farmers only had one animal, or only allowed sampling of one. Blood samples from 411 goats and 21 sheep were collected from the jugular vein into a sterile syringe, transferred to vacutainer tubes, allowed to clot, and stored using ice packs before being transported back to a local lab where the samples were frozen until shipment to ICAR-NIVEDI.
Coxiella burnetii
The 432 serum samples were tested for antibodies against C. burnetii inactivated phase I and II antigens using an indirect commercial ELISA test as per the manufacturer’s instructions [Citation31]. Results were expressed as a percentage of the optical density (%OD) reading of the test sample calculated as %OD = 100 * (S-N)/(P-N), where S, N, and P are the values of the sample (S) and OD of negative (N) and positive (P) controls, respectively. Samples with %OD ≥37% were considered positive.
Brucella spp.
The 432 serum samples were tested for antibodies against Brucella spp. using an indirect, multi-species, commercial enzyme-linked immunosorbent assay (ELISA) kit as per the manufacturer’s instructions [Citation32]. Results were expressed as a percentage of the optical density (%OD) as described above. Samples with %OD ≥ 30% were considered positive. According to the manufacturer, the test had 100% specificity and sensitivity for ovine sera [Citation32].
The sera were also screened using a Rose-Bengal Brucella agglutination plate test (RBPT) following the standard procedure as described by Citation33. The plates were shaken for 4 min and any agglutination that appeared within this time was recorded as a positive reaction. The results were read by experienced technical staff.
Statistical analysis
Data were entered into Microsoft Excel and analysed using ‘R’ statistical software [Citation34]. Given scarcity of sheep numbers, for the purpose of this analysis, sheep and goats were grouped together and referred to using the term ‘small ruminant’. Initial univariable analyses were conducted using Chi2 testing to identify each potential risk variable firstly for Brucella and then for Coxiella seropositivity. Mixed-effects multivariable logistic regression models were built, starting with all independent variables with a p value <0.1 in the univariable analysis, using manual backward elimination of variables, with district and village as random effects for the farm level model and farms as a random effect for the animal level model. The Goodman and Kruskal’s tau measure were used to check for correlated pairs of variables using a correlation matrix prior to the multivariable analysis. The R lme4 package [Citation35] was used to fit the mixed-effects multivariable logistic regression models as described above. The lowest Akaike’s Information Criteria (AIC) value was used as a measure of best model parsimony. Maps were created in ‘R’ with the R leaflet package [Citation36].
Results
In total 244 farms were visited, 122 farms per state, with one farmer questionnaire administered per farm. Of the 244 respondents of the farmer questionnaire, 133 were male and 111 were female. The average age of male farmers was 45.7 years and 39.4 years for females. A mean of five people lived in each farming household. Odisha had larger herd sizes compared to Assam; mean number of goats and sheep per farm in Odisha was 7.8 goats and 2 sheep compared to 4.7 goats and 0.2 sheep in Assam. Five farms reported to produce small ruminant milk, while most animals were kept for meat production.
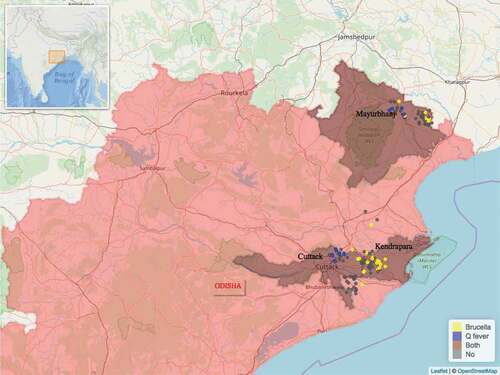
In total 432 blood samples were collected from 411 goats and 21 sheep. All 21 sheep were of local indigenous breed. Three of the 411 goats were crossbreeds, all remaining 408 goats were of local indigenous breed. Of the 432 blood samples, 43 were found to be seropositive for Brucella, 20 were seropositive for Coxiella and 1 sample was seropositive for both pathogens. Of the 244 farms visited, 53 were seropositive, i.e. a farm where one or more seropositive animals were found. The distribution of the 53 seropositive and 191 seronegative farms found can be seen on the maps in and . Farm seroprevalence for Brucella in Odisha was 22% (95% CI 15.6%-30%) and 9.8% (95% CI 5.7%-16%) in Assam. Farm seroprevalence for Coxiella in Odisha was 11.5% (95% CI 7%-18%) and 1.6% (95% CI 0.5%-6%) in Assam.
Figure 1. Distribution of the study farms within the state of Assam and the location of Assam within India (insert). Seropositive farms for Brucella spp. are in yellow and seropositive farms for Coxiella burnetti are in blue colour.
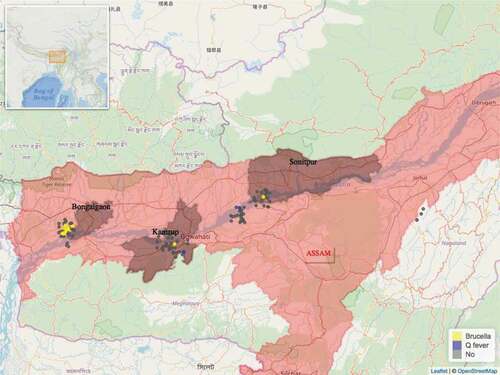
Figure 2. Distribution of the study farms within the state of Odisha and the location of Odisha within India (insert). Seropositive farms for Brucella spp. are in yellow colour and seropositive farms for Coxiella burnetti are in blue colour. Only one farm, in brown colour, had animals seropositive for both Brucella spp. and Coxiella burnetti.
Analysis of factors associated with Brucella and Coxiella infection at the farm level
Univariable analysis identified Odisha state and certain districts as having higher Brucella and Coxiella farm seroprevalence. Odisha’s district of Kendrapara was associated with high Brucella seroprevalence risk while Cuttack was associated with high Coxiella seroprevalence risk. Small ruminants kept by men had higher Brucella seroprevalence risk. Farmer age and education level were not identified as risk factors. In terms of farm hygiene, cleanliness of farms, use of disinfectant, and farmers’ hand-washing habits were not associated with seropositivity risk. Results of the univariable analysis are shown in .
Table 1. Univariable analysis showing risk factors associated with Brucella and Coxiella farm seroprevalence (with 95% confidence interval (CI)).
In Odisha, 60 farms vaccinated small ruminants against peste des petits ruminants (PPR), foot and mouth disease (FMD) and haemorrhagic septicaemia (HS), while only one farm in Assam vaccinated small ruminants. Vaccinated farms were associated with increased brucellosis seroprevalence risk. Farms where small ruminant houses were of a floor type different to earthen had higher brucellosis risk. Larger herd size and increased introduction of new animals into a herd were risk factors for Coxiella farm prevalence.
No farm reported to have purchased sick or weak animals over the previous 12 months, only two farms in Assam reported an animal becoming sick after purchase, this was not significantly associated with any seroprevalence risk. Two farms reported culling of animals. No farmer knew of or used quarantine methods. No farmer possessed knowledge of brucellosis, its aetiology, clinical form, or zoonotic risk.
No association was found between placenta disposal on farms and seroprevalence or abortion cases. Of the 31 farms that reported cases of small ruminants aborting, 54.8% (95% CI 36.0%-72.7%) throw the placenta into an open field, 29.0% (95% CI 14.2%-48.0%) throw it in an open drain and 16.1% (95% CI 5.5%-33.7%) bury it. Most farmers, 86% (95% CI 82%-91%), lived adjacent to their small ruminant house, no association between farm seropositivity and proximity of small ruminant shed to residential homes was found.
Higher numbers of farms in Assam allowed small ruminants to mix with other animal sources of Brucella spp. and C. burnetii: poultry (119 farms in Assam, 9 farms in Odisha), cattle (121 farms in Assam, 50 in Odisha), dogs (117 farms in Assam, 2 in Odisha) cats (109 farms in Assam, 0 in Odisha) and pigs (23 farms in Assam, 0 in Odisha). In the univariable analysis poultry, dogs, and cats mixing with small ruminants was associated with lower Brucella and C. burnetii farm seroprevalence. No association was found between small ruminants mixing with cattle or pigs and farm seroprevalence. In Assam, 108 farms reported veterinary visits over the previous 12 months compared to 3 farms in Odisha. Veterinary visits to farms were associated with lower Coxiella seropositivity risk ().
Results of the multivariable analyses modelling for combined Brucella and Coxiella seropositivity at farm level are shown in . For both Brucella and Coxiella seroprevalence at the farm level, the most parsimonious model showed that state and mixing with poultry were significantly associated. If mixing with poultry was removed from this model, the AIC value increased with estimates for state-changing, thus highlighting it as a confounding variable. Farms in Odisha had far higher odds ratio (OR) of being seropositive for either pathogen than farms in Assam (OR = 17.4, 95% CI 1.5%-190%).
Table 2. Multivariable analyses of risk factors for combined seropositivity of Coxiella and Brucella.
Analysis of factors associated with Brucella and Coxiella infection at animal level
Results of the univariable analysis at the animal level are presented in . Out of 433 sampled small ruminants, 64 were seropositive for either or both bacteria. In Odisha 32 (14%, 95% CI 10–19%) were positive for Brucella spp. and 12 (6%, 95% CI 3.5%-10) in Assam, while 19 small ruminants were seropositive for C. burnetii in Odisha (8%, 95% CI 5–12%) and 2 (1%, 95% CI 0.3–4%) in Assam. Only one sheep in Odisha was seropositive for both pathogens (0.43%, 95% CI 0.022–2.4%). The ELISA kit for Brucella spp. yielded 43 positives results, higher than the RBPT method which gave one positive result. Few differences were found between risk factors already identified at farm level with those found at individual animal level; small ruminant shed floor type was no longer seen as a risk factor for Brucella seroprevalence and small ruminants grazing extensively full time were found to be associated with increased risk of Coxiella seroprevalence. History of abortions, number of pregnancies, age, and species were not associated with Coxiella seroprevalence. Older animals and sheep, more than goats, were identified as risk factors for Brucella seropositivity. No association was found between seropositive small ruminants and the presentation of clinical signs; abortion, mastitis, vaginal discharge, or lameness.
Table 3. Univariable analysis showing risk factors associated with Brucella and Coxiella seropositivity at animal level. Results shown as seropositivity (95% confidence interval (CI)) or mean (standard deviation (SD)).
In the multivariable model at animal level, the most parsimonious model for Brucella showed that district, gender, and mixing with poultry were significantly associated with seropositive animals (). Small ruminants living in Odisha’s Kendrapara district had 5.9 (95% CI 1%-34%) higher odds or being Brucella seropositive compared to Mayurbhanj or Cuttack district. Small ruminants mixing with poultry had 3.3 (95% CI 0.7–15) times higher odds of being at risk compared to those coming from herds who did not mix with poultry. Due to the low number of Coxiella seropositive small ruminants, the multivariable mixed model did not converge, so results are not shown here.
Table 4. Multivariable model for risk factors for Brucella seropositivity at animal level.
Discussion
This study is the first of its kind to look at the spatial distribution and risk factors for both Brucella and Coxiella seroprevalence in small ruminants in Assam and Odisha in Eastern India. In Assam high Coxiella seroprevalence has already been reported in cattle and identified as a public health risk [Citation37], but no reports on coxiellosis in small ruminants exist [Citation30]. In Odisha, Coxiella seroprevalence in goats has been reported at 10.6% [Citation2], higher than the 8% (95% CI 5–12%) we reported. For Brucella spp., our study found a seroprevalence of 14% in Odisha (95% CI 10–19%), and 6% in Assam (95% CI 4–10%), higher than observations previously made of 5% seroprevalence among sheep and goats in Odisha [Citation15], and a 2% seroprevalence recorded in goats in Assam [Citation38]. Previous studies have however shown particularly high prevalence (70% herd prevalence) in cows in peri-urban Guwahati, the capital of Assam [Citation39].
Our study found 43 seropositive animals for Brucella spp. using the ELISA method compared to one positive animal found using RBPT. This result supports findings from previous Indian studies that report a higher diagnostic sensitivity of the ELISA compared to RBPT method [Citation40–Citation42]. The RBPT method serves an indirect function, however. Standard RBPT favours B. abortus with B. melitensis-infected small ruminants showing negative results [Citation43]. Therefore, one positive result using RBPT compared to 43 positives using ELISA indicates that the small ruminants sampled are likely to be infected with B. melitensis rather than B. abortus, an important public health finding given that B. melitensis is more pathogenic to humans [Citation9,Citation12].
If brucellosis serology testing alone had been carried out in this study and coxiellosis neglected, our combined farm seroprevalence would have decreased from 15% to 10%, a total of 21 infected small ruminants (19 in Odisha and 2 in Assam) would have been undetected. These 21 animals highlight the benefits of extending beyond single serology screening approaches, gaining greater insights into zoonoses prevalence.
To understand risk factors associated with brucellosis and coxiellosis infection, serology alone can be misleading; a significant proportion of animals that shed C. burnetii or Brucella spp. are not seropositive, furthermore animals can be seropositive and not shed the pathogen [Citation21,Citation22,Citation27]. In a developing world context, studies which have focused solely on serology have failed to give sufficient insight into sustainable control options [Citation44]. Therefore in this study, a farmer questionnaire was used to deepen our understanding of animal-human-environmental contact patterns, necessary information to reduce zoonotic transmission risks [Citation45].
In terms of knowledge about brucellosis, transmission pathways, or control measures, none of the 244 farm respondents possessed any information, this concurs with other research showing a strong lack of knowledge among Indian livestock keepers on zoonotic disease and highlights the urgent need for intervention [Citation6,Citation46]. Our study shows the proximity within which small ruminants and their keepers live, 86% of respondents in this study live adjacent to their small ruminant house. Increased brucellosis and coxiellosis transmission to humans and other animals occurs through exposure to placenta membranes and birth fluids from infected small ruminants [Citation22,Citation29,Citation47], and so we investigated methods of placenta disposal on farms. We found more than half of the farmers throw the placenta from aborted small ruminants into an open field, or in an open drain, and only 16% bury it. While no association was found between placenta disposal and seropositive farms or farms reporting abortions, inappropriate disposal of hazardous farm waste material must be discouraged to reduce environmental contamination and intra- and inter-species transmission.
No farmer practiced quarantine in the study. Increased herd numbers, increased introduction of new small ruminants into herds, and full-time extensively grazed small ruminants were associated with higher Coxiella seroprevalence risk, all highlighting the opportunistic, infectious nature of this pathogen when herd density increases [Citation18]. Increased farmer knowledge is needed regarding the implementation of biosecurity measures. In addition, the risks and cost-effectiveness of vaccination interventions, where the mixing of herds increases Brucella transmission [Citation48] and close animal-human contact occurs [Citation49], require further investigation given our findings of increased Brucella seroprevalence among vaccinated small ruminants.
Inapparent or ‘silent’ clinical signs of brucellosis and coxiellosis in small ruminants complicates their clinical diagnosis [Citation21,Citation29]. We found no association between Brucella or Coxiella seropositive small ruminants with clinical signs; abortion, mastitis, lameness, or vaginal discharge. Farms that reported abortion did not report infertility and only two farms reported to have culled animals, suggesting a poor perception level among farmers regarding small ruminant production parameters. The increased risk of Brucella seroprevalence in older small ruminants, as seen in our study, could help identify animals for culling. However, engaging with farmers for future disease control plans will be challenging given their current lack of zoonoses knowledge and the lack of tangible disease manifestation in their herds.
The role of poultry in disseminating brucellosis to man and other animals is well reported [Citation50–Citation52], with dogs and cats also acting as mechanical disseminators of brucellosis and coxiellosis [Citation22,Citation25]. The apparent protective factor of small ruminants mixing with poultry, dogs, and cats associated with lower seroprevalence found in our univariable analysis is misleading. Mixing with these species is highly correlated with the state of Assam, where infections were less common. Increased seroprevalence risk when small ruminants are in contact with poultry did come out in the multivariable model. The ecology of farms in Assam and Odisha differs greatly, future epidemiological investigations should compare similar farming systems to truly understand the risks associated multi-species mixing on farms. Sheep and goats can infect cattle with B. melitensis [Citation7,Citation53], and given the public health implications of this, brucellosis transmission risks posed by small ruminants to large ruminant’s merits further investigation. To mitigate risk at the animal-human-environmental interface, control measures for all livestock species with shared pastures are recommended [Citation29].
In India, field veterinarians have been reported to lack knowledge on zoonoses transmission risks [Citation54]. Our study showed farms which had not received veterinary visits were associated with a higher seroprevalence risk. However, like the mixing with other species variables, a disproportionate number, 103, farms in Assam where seroprevalence is lower, compared to 3 farms in Odisha, where seroprevalence is higher, received veterinary visits suggestive of a strong correlation with state. A limitation to the questionnaire design was that the motive for the veterinary visit to farms was not recorded, this would have been of interest in furthering our understanding of animal health priorities among small ruminant keepers as well as gaining a better insight into the role of veterinarians in small ruminant medicine.
Future studies may yield more insight into risk factors if more farming systems in similar settings were compared. Nevertheless, our study contributes to the current dearth of literature on coxiellosis and brucellosis in small ruminants in India. It identifies the state of Odisha and certain districts within Odisha as higher risk for both Brucella and Coxeilla farm seropositivity. Human health services in these areas must be made aware of such prevalence in the small ruminant reservoir and the potential human health risk. The study strongly identifies small ruminant farmers as target groups for urgent zoonosis educational intervention, with special emphasis on male farmers given how gender was associated with higher Brucella seroprevalence risk. The study also highlights the need for increased understanding on veterinary involvement in zoonotic disease mitigation. The inherent farm, animal and biosecurity-related factors identified as risks for seroprevalence in this study will need to be addressed if the complex landscape of interacting agents contributing to disease emergence [Citation55], in this case brucellosis and coxiellosis, is to be understood. Further epidemiological investigation, to fully understand the transmission pathways on farms of both pathogens, is urgently needed if zoonotic risk in Eastern India is to be mitigated.
Acknowledgments
This project was supported by the ICAR-ILRI collaboration funds and the CGIAR Research Program Agriculture for Nutrition and Health. The authors would like to thank the donors to the CGIAR system for their support. We would like to acknowledge all farmers for their participation as well as the field data collection teams, and Dr HR Rahman for his support to the project. We are thankful to the Director and scientists, ICAR-NIVEDI, Bangalore for their support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Eithne Leahy
Eithne Leahy is a veterinary clinician and research graduate fellow at the International Livestock Research Institute. Her work endeavours to understand the pathways of disease transmission at the human-animal-enviromental interface using a One Health approach.
References
- Kanani A , Dabhi S , Patel Y , et al. Seroprevalence of brucellosis in small ruminants in organized and unorganized sectors of Gujarat state, India. Vet World. 2018;11(8):1030–9.
- Sahu R , Kale SB , Vergis J , et al. Apparent prevalence and risk factors associated with occurrence of Coxiella burnetii infection in goats and humans in Chhattisgarh and Odisha, India. Comp Immunol Microbiol Infect Dis. 2018;60:46–51.
- Kumar S , Rama Rao CA , Kareemulla K , et al. Role of goats in Livelihood security of rural poor in the less favoured environments. Indian J Agric Econ. 2010;65(4):760–781.
- Kumar S , Vihan VS , Deoghare PR. Economic implication of diseases in goats in India with reference to implementation of a health plan calendar. Small Ruminant Res. 2003;47(2):159–164.
- Rajkumar K , Bhattacharya A , David S , et al. Socio-demographic study on extent of knowledge, awareness, attitude, and risks of zoonotic diseases among livestock owners in Puducherry region. Vet World. 2016;9(9):1018–1024.
- Singh BB , Kaur R , Gill GS , et al. Knowledge, attitude and practices relating to zoonotic diseases among livestock farmers in Punjab, India. Acta Trop. 2019;189:15–21.
- Mantur B , Amarnath S . Brucellosis in India—a review. J Biosci. 2008;33(4):539–547.
- Singh BB , Khatkar MS , Aulakh RS , et al. Estimation of the health and economic burden of human brucellosis in India. Prev Vet Med. 2018;154:148–155.
- Renukaradhya GJ , Isloor S , Rajasekhar M . Epidemiology, zoonotic aspects, vaccination and control/eradication of brucellosis in India. Vet Microbiol. 2002;90(1):183–195.
- Singh B , Bardhan D , Verma MR , et al. Estimation of economic losses due to peste de petits ruminants in small ruminants in India. Vet World. 2014;7(4):194.
- Smits HL , Kadri SM . Brucellosis in India: A deceptive infectious disease. Indian J Med Res. 2005;122(5):375–384.
- Sonekar CP , Kale S , Bhoyar S , et al. Brucellosis in migratory sheep flock from Maharashtra, India. Trop Anim Health Prod. 2018;50(1):91–96.
- Azam S , Rao SB , Jakka P , et al. Genetic characterization and comparative genome analysis of Brucella melitensis Isolates from India. Int J Genomics. 2016;2016:3034756. 10.1155/2016/3034756.
- Beena V , Pawaiya RVS , Gururaj K , et al. Molecular etiopathology of naturally occurring reproductive diseases in female goats. Vet World. 2017;10(8):964–972.
- Shome R , Triveni K , Padmashree B , et al. Spatial distribution of brucellosis in small ruminants of India using indigenously developed ELISA kit. J Pure Appl Microbiol. 2015;9(3):2285–2292.
- Singh SV , Singh N , Singh MP , et al. Occurrence of abortions and seroprevalence of brucellosis in goats and sheep. Small Ruminant Res. 1994;14(2):161–165.
- Keshavamurthy R , Singh B , Kalambhe DG , et al. Prevalence of Coxiella burnetii in cattle and buffalo populations in Punjab, India. Prev Vet Med. 2019;1(166):16–20.
- Marrie TJ , Raoult D . Coxiella burnetii (Q Fever). In: Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, edited by John E. Bennett, Raphael Dolin and Martin J. Blaser. Vol. 2. Elsevier; 2014. p. 2208–2216.
- OIE WOFAH . Q-fever; 2019 [cited 2019 Mar ]. http://www.oie.int/animal-health-in-the-world/animal-diseases/q-fever/
- Grace D,MF , Ochungo P,KR , Jones K , et al.; F., A. I. a. O. . Mapping of poverty and likely zoonoses hotspots. Project 4. Report to the UK department for international development. Nairobi, Kenya, UK Department for International Development; 2012. Accessed: Mar 2019. https://cgspace.cgiar.org/handle/10568/21161
- Brom RVD , Engelen EV , Roest HIJ , et al. Coxiella burnetii infections in sheep or goats: an opinionated review. Vet Microbiol. 2015;181(1/2):119–129.
- Porter SR , Czaplicki G , Mainil J , et al. Q Fever: current state of knowledge and perspectives of research of a neglected zoonosis. Int J Microbiol. 2011;2011(2011):1–22.
- Stephen S , Sangeetha B , Antony P . Seroprevalence of coxiellosis (Q fever) in sheep & goat in Puducherry & neighbouring Tamil Nadu. Indian J Med Res. 2014;140(6):785.
- Pradeep J , Stephen S , Pooja P , et al. Coxiellosis in domestic livestock of Puducherry and Tamil Nadu: detection of Coxiella burnetii DNA by polymerase chain reaction in slaughtered ruminants. Vet World. 2017;10(6):667.
- Coelho AC , Diez JG , Coelho AM . Risk factors for brucella spp. In domestic and wild animals. Updates on brucellosis; 2015 [cited 2019 Mar ]. https://www.intechopen.com/books/updates-on-brucellosis/risk-factors-for-brucella-spp-in-domestic-and-wild-animals
- Vaidya VM , Malik SVS , Bhilegaonkar KN , et al. Prevalence of Q fever in domestic animals with reproductive disorders. Comp Immunol Microbiol Infect Dis. 2010;33(4):307–321.
- Burns RJL , Barry AE , Douangngeun B , et al. Serosurveillance of Coxiellosis (Q-fever) and Brucellosis in goats in selected provinces of Lao People’s Democratic Republic. PLoS Negl Trop Dis. 2018;12(4):e0006411.
- Pathak AD , Dubal ZB , Doijad S , et al. Human brucellosis among pyrexia of unknown origin cases and occupationally exposed individuals in Goa Region, India. Emerg Health Threats J. 2014;7(1):23846.
- Ganter M . Zoonotic risks from small ruminants. Vet Microbiol. 2015;181(1–2):53–65.
- Dhaka P , Malik SS , Yadav JP , et al. Seroscreening of lactating cattle for coxiellosis by trans-PCR and commercial ELISA in Kerala, India. J Exp Biol. 2017;5:3.
- Bio-X Diagnostics B . BIO K 298, Monoscreen AbELISA Coxiella burnetii/indirect; 2019 [cited 2019 Jan ]. https://www.biox.com/en/bio-k-298-monoscreen-abelisa-coxiella-burnetii-indirect-monowell-p-246/
- SVANOVA . SVANOVIR® Brucella-Ab I-ELISA; 2019 [cited 2019 Jan ]. https://www.svanova.com/content/dam/internet/ah/svanova/dk_EN/documents/bovine/Brucella-I-Infosheet_V3.pdf
- Alton GG . Laboratory techniques in brucellosis. 2nd ed. Geneva: World Health Organization; 1975.
- R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. Available from: http://www.R-project.org/
- Bates D , Mächler M , Bolker B , et al. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48.
- Cheng J , Karambelkar B , Xie Y . Leaflet: create interactive Web Maps with the JavaScript ‘Leaflet’ library. R package version 2.0.2; 2018 [cited 2019 Mar ]. https://CRAN.R-project.org/package=leaflet
- Shome R , Deka R , Milesh L , et al. Coxiella seroprevalence and risk factors in large ruminants in Bihar and Assam, India. Acta Trop. 2019;194:41–46.
- Gogoi S , Hussain P , Sarma P , et al. Prevalence of brucellosis in goat in Assam, India. Int J Chem Stud. 2017;5(4):1176–1179.
- Lindahl J , Gill J , Hazarika R , et al. Risk Factors for Seroprevalence in Peri-Urban Dairy Farms in Five Indian Cities. Trop Med Infect Dis. 2019;4(2):70.
- Reddy DA , Kumari G , Rajagunalan S , et al. Seroprevalence of caprine brucellosis in Karnataka. Vet World. 2014;7(3):182.
- Sadhu D , Panchasara H , Chauhan H , et al. Seroprevalence and comparison of different serological tests for brucellosis detection in small ruminants. Vet World. 2015;8(5):561–566.
- Sharma HK , Kotwal SK , Singh DK , et al. Sero-prevalence of sheep brucellosis in Jammu (Report). J Pure Appl Microbiol. 2016;10(3):2277.
- Ferreira AC , Cardoso R , Travassos Dias I , et al. Evaluation of a modified Rose-Bengal test and an indirect Enzyme-Linked-Immunosorbent-Assay for the diagnosis of Brucella melitensis infection in sheep. Vet Res. 2003;34(3):297–305.
- Godfroid J , Al Dahouk S , Pappas G , et al. A “ One Health” surveillance and control of brucellosis in developing countries: moving away from improvisation. Comp Immunol Microbiol Infect Dis. 2013;36(3):241–248.
- Klous G , Huss A , Heederik DJJ , et al. Human–livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Health. 2016;2(C):65–76.
- Chattopadhyay UK , Rashid M , Sur SK . Knowledge, attitude and practices about zoonoses with reference to Campylobacteriosis in a rural area of West Bengal. Indian J Public Health. 2006;50(3):187.
- Mori M , Roest HJ . Farming, Q fever and public health: agricultural practices and beyond. Arch Public Health. 2018;76(1). urn: 0778-7367. DOI:10.1186/s13690-017-0248-y
- Deka R , Magnusson U , Grace D , et al. Bovine brucellosis: prevalence, risk factors, economic cost and control options with particular reference to India- a review. Infect Ecol Epidemiol. 2018;8(1):1–7.
- Roth F , Zinsstag J , Orkhon D , et al. Human health benefits from livestock vaccination for brucellosis: case study. Bull World Health Organ. 2003;81(12):867.
- Gugong VT , Maurice NA , Ngbede EO , et al. Serological evidence of brucellosis in local chickens in Kaduna state, Nigeria. J Anim Vet Adv. 2012;11(3):418–420.
- Miller R , Sweeney S , Slootmaker C , et al. Cross-species transmission potential between wild pigs, livestock, poultry, wildlife, and humans: implications for disease risk management in North America. Sci Rep. 2017;7(1):7821.
- Onunkwo JI , Njoga EO , Njoga UJ , et al. Brucella seropositivity in chicken and risk factors for Brucella infection at the animal-human interface in Anambra State, Nigeria. Int J One Health. 2018;4:28–34.
- Memish Z . Brucellosis control in Saudi Arabia: prospects and challenges. J Chemother. 2001;13(Suppl 1):11.
- Mangalgi S , Sajjan A , Mohite S , et al. Brucellosis in occupationally exposed groups. J Clin Diagn Res. 2016;10(4):DC24–7.
- Lambin EF , Tran A , Vanwambeke SO , et al. Pathogenic landscapes: interactions between land, people, disease vectors, and their animal hosts. Int J Health Geogr. 2010;9(54):54.
