ABSTRACT
Background:
The aim of this study was to provide a descriptive account of carbapenem resistance and risk factors for mortality from invasive Acinetobacter infections in the south of Sweden.
Methods:
Blood isolates with growth of Acinetobacter species between 2010 and 2019 in Skåne county were subtyped using MALDI-TOF and subjected to susceptibility testing against clinically relevant antibiotics. Association between risk factors and 30-day mortality were analysed in univariate and multivariate logistic regression models.
Results:
There were 179 bacteraemia episodes in 176 patients included in the study. The 30-day all-cause mortality was 16%. In all, two percent of Acinetobacter strains were carbapenem resistant. Independent risk factors associated with 30-day mortality in the multivariate regression model were Acinetobacter growth in all blood cultures drawn at the day of bacteraemia onset (OR 5.0, 95% CI: 1.8 to 13.7, p= 0.002), baseline functional capacity (1–4 points, OR 2.0, 95% CI: 1.2 to 3.4, p= 0.010) and correct empiric antibiotics at time of culture (OR 3.5 95% CI: 1.0 to 11.8, p= 0.045).
Conclusion:
This study on Acinetobacter bacteraemia in South Sweden found low 30-day mortality and low carbapenem-resistance rates compared to previous international studies which may be due to a higher rate of contaminant findings.
Introduction
The bacterial genus of Acinetobacter is a diverse group comprising gram-negative, strictly aerobic coccobacilli of more than 60 different species [Citation1,Citation2]. Acinetobacter is primarily known as an opportunistic pathogen causing nosocomial infections, particularly in intensive care units (ICUs). Risk factors for infection include longer duration of hospitalization, exposure to broad-spectrum antibiotics, mechanical ventilation, venous and urinary catheterization and other impairments of natural barriers such as wounds and surgical procedures [Citation3,Citation4]. Pneumonia (commonly ventilator-associated) and venous catheter-related bacteraemia dominate the infection-type spectrum, but urinary tract infections, wound infections and endocarditis, amongst others, are also frequently reported [Citation3,Citation4]. Community-acquired Acinetobacter infections occur, albeit less frequent compared to nosocomial infections [Citation3,Citation4]. A. baumannii is the most common species to cause infection[Citation3].
The southern and eastern parts of Europe saw the highest rates of carbapenem resistance amongst invasive Acinetobacter isolates in Europe in 2019, with Greece, Croatia and Romania reporting rates of 92%, 92% and 88%, respectively[Citation5]. A. baumannii strains resistant to all available antibiotics have been reported[Citation6].
Even though Acinetobacter is a pathogen of increasing importance globally, data on Acinetobacter infections in Sweden are scarce. Contrary to other bacteria with large potential for antibiotic resistance, Acinetobacter is not under national surveillance. We aimed to investigate invasive Acinetobacter infections in our clinical setting in the south of Sweden. The study objective was to provide a detailed descriptive account of invasive Acinetobacter infections with regard to epidemiology, antibiotic resistance, clinical characteristics, treatment and outcome.
Materials and methods
Study design and data collection
We performed a population-based observational study in Skåne County in southern Sweden, with a population of 1.3 million[Citation7]. Data on blood cultures positive for Acinetobacter species (spp.) between the years of 2010–2019, including personal identification numbers, were obtained from the Department of Clinical Microbiology at Skåne University Hospital. This department handles and analyses all microbiological cultures from primary, secondary and tertiary care in Skåne. All blood cultures positive for an Acinetobacter-species during the study period were included. Exclusion criteria was complete absence of data in medical records and clinical microbiology records. Medical records were reviewed using the software Melior (Melior, Siemens Healthcare Services, Upplands Väsby, Sweden). Laboratory Registers at the Department of Clinical Microbiology, Region Skåne, were accessed through LIMS-systems ww-lab (Autonik, Nyköping, Sweden) and Bactius (Clinical Microbiology, Lund, Sweden).
Definitions of variables
Multiple blood cultures positive for Acinetobacter during the same hospitalization were considered one single event of bacteraemia. The bacteraemia was registered as carbapenem resistant if at least one of the blood cultures with Acinetobacter growth were carbapenem resistant. Patients with repeated bacteraemia at different episodes of in-patient care could be included multiple times. Cases were classified as primary bacteraemia if no other infectious foci were found and secondary if another focus was present (ie presence of positive Acinetobacter cultures from other locations, as well as the clinical assessments of the treating physician). Bacteraemia were defined as nosocomial if the first blood cultures positive for Acinetobacter was drawn after more than 48 hours of hospitalization, otherwise the infection was defined as community acquired. The variable ‘abroad recently’ was defined as abroad stay within 30 days prior to the bacteraemia onset. Immunosuppression was defined as a daily corticosteroid dose equivalent to ≥15 mg of prednisolone, chemotherapy or other medication with significant immunosuppressive effect, such as TNF-alpha inhibitors, or innate immune deficiencies. Charlson comorbidity index was calculated according to the model developed and validated by Charlson et al [Citation8]. National Early Warning Score 2 (NEWS2) was calculated in accordance with the model developed by Royal College of Physicians[Citation9]. A modified version of the Brighton Paediatric Early Warning Score (PEWS) proposed by Solevåg et al was used for patients aged <18 years [Citation10]. Baseline functional capacity was defined according to the categories used by Dickstein et al [Citation11].
Microbiological analyses
All blood isolates drawn between 2010 and 2019 and preserved at the Department of Clinical Microbiology at Skåne University Hospital Lund with growth of Acinetobacter spp. were re-cultured. Isolates were plated on Mueller-Hinton-agars (Oxoid, Basingstoke, UK) and incubated for 18 hours. Isolates were tested for susceptibility to cefiderocol, doripenem, imipenem, meropenem, imipenem-relebactam, ciprofloxacin, levofloxacin, amikacin, gentamicin, tobramycin, colistin and trimethoprim-sulfamethoxazole by disc diffusion using antibiotic-impregnated discs (Oxoid, Basingstoke, UK for all but colistin which were from Mast Group, Bootle, UK) and E-tests (bioMérieux, Askim, Sweden and Liofilchem, Roseto degli Abruzzi, Italy). Inhibition zone diameters were interpreted using the NordicAST (Nordic Committee on Antimicrobial Susceptibility Testing) breakpoints (www.nordicast.org). For strains not preserved (n = 72), the original susceptibility testing (performed at time when the Acinetobacter strain was uncovered) was used. Acinetobacter strains were identified to species level using Matrix Assisted Laser Desorption/Ionization-Time Of Flight (MALDI-TOF) Mass Spectrometry (MS) and analysed with software MALDI Biotyper (MBT) Compass (Bruker, Billerica, Massachusetts, USA).
Statistical analyses
We used multivariable logistic regression as our main statistical method for investigating an association between putative risk factors and the outcome. We limited the number of investigated risk factor to six due to a limited sample size and few events. Variables were chosen in two steps, the first part was based on a known or suspected association with the outcome to enable a well calibrated survival model (age, comorbidities, baseline functional capacity and vital parameters). The second part was more exploratory, and variables were chosen to investigate putative associations with the outcome (all blood cultures positive and correct empiric antibiotics). We first fit univariate logistic regression models using all variables on their original scale and using complete case analysis respectively. We investigated patterns of missingness and concluded there to be a missing at random mechanism with >5% missingness in at least one variable. We performed chained multiple imputation using predictive mean matching and 10 imputations including all variables in the final model, the outcome variable, and additional data on nosocomial infection, immunosuppression and ICU admission. Non-linearity between the included variables and the outcome was tested with likelihood ratio tests using 4 knot restricted cubic splines for continuous variables and categorization for factor variables. We then fit a multivariable logistic regression model using original and imputed data and reported odds ratios, 95% confidence intervals and p-values for all variables together with measures of discrimination (area under receiver operating curve) and model fit (pseudo R2) for the full model. We checked model assumptions using variable inflation factor <4 for multicollinearity and Hosmer-Lemeshow goodness-of-fit test for calibration. All results are reported with 95% confidence intervals and p-values below 0.05 were regarded as statistically significant. Statistical analyses were performed using Stata MP version 16.1 (StataCorp, College Station, Texas, USA).
Ethics
The study received ethical approval. This committee waived the need for written consent from the patients of the study due to the retrospective nature of the study and the fact that the patients are unidentifiable. The study was performed in accordance with the Declaration of Helsinki.
Results
The yearly incidence of Acinetobacter bacteraemia did not increase over the study period
Between 2010 and 2019, there were 233 blood cultures positive for Acinetobacter species in Skåne. When comparing multiple positive blood cultures against hospitalization episodes, 189 cases of bacteraemia were identified. Of these, 10 cases were excluded from the study due to absence of data. Thus, 179 cases of bacteraemia in 176 patients were included in the study; three patients had two episodes of bacteraemia each.
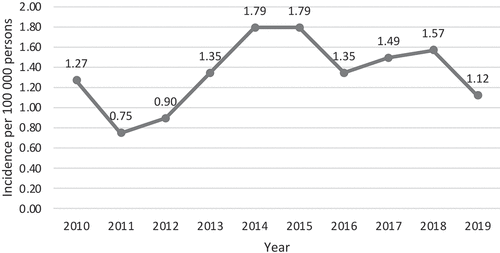
The yearly incidence of Acinetobacter bacteraemia in Skåne remained fairly constant during the study period, ranging between 0.75 and 1.79 cases per 100,000 citizens and year (mean and median 1.3). Yearly incidence of cases 2010–2019 are displayed in .
Baseline characteristics of the study population
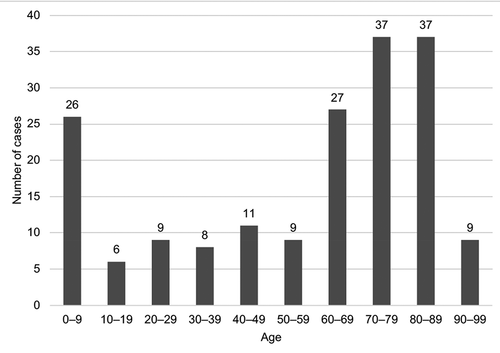
Out of the 179 included cases of bacteraemia, 92 (51.4%) occurred in male patients, and the median age was 67 years (range 0–94). The median Charlson comorbidity index score was four (range 0–15), and 44 patients (25%) were considered immunosuppressed. The median length of hospitalization was 10 days (range 0–320), and 26 patients (15%) were admitted to the intensive care unit (ICU). The baseline characteristics of the study population are presented in and age distribution of patients is displayed in .
Table 1. Patient characteristics.
The majority of the episodes of bacteraemia were community-acquired
Out of the 179 episodes of bacteraemia included in this study, 139 (78%) were community-acquired (). Of the 40 nosocomial cases, the median time to bacteraemia was 15 days (range 2–136). Out of the 26 patients treated in the ICU, 16 (62%) had onset of bacteraemia before ICU admission, while six (23%) had onset during the ICU stay and four (15%) after ICU discharge. Ten patients (6%) had been abroad recently prior to hospitalization. At the time of bacteraemia onset, 47 patients (26%) were treated with antibiotics.
Table 2. Bacteraemia and treatment characteristics.
Few strains were carbapenem-resistant
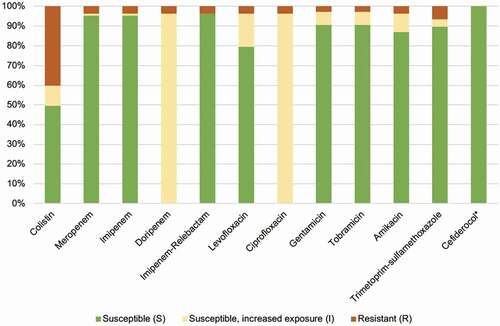
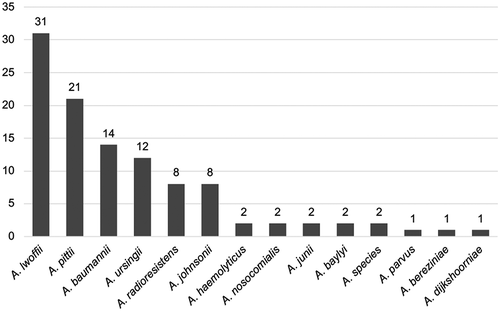
Only five Acinetobacter strains out of 169 tested (3%) were carbapenem-resistant, of which four were found in patients hospitalized abroad immediately prior to the Acinetobacter bacteraemia. The result of the susceptibility testing is displayed in . Additional bacterial species other than Acinetobacter were found in 81 (46%) of the bacteraemia episodes. Positive Acinetobacter cultures from locations other than blood were reported in 20 (12%) patients. Primary Acinetobacter bacteraemia, ie with no other foci of infection identified, was seen in 82 (46%) of cases while 97 (54%) were classified as secondary bacteraemia. In all, 107 isolates were preserved at the Department of Clinical Microbiology and available for MALDI-TOF analysis. A. lwoffii was most frequently identified (n = 31, 29%) followed by A. pittii (n= 21, 20%) and A. baumannii (n = 14, 13%). Other than these, 11 different Acinetobacter species were identified ().
The 30-day survival rate was high
As presented in , patients received empirical antibiotic treatment at bacteraemia onset in 164 of 179 cases (92%). However, only 37 patients (21%) received empirical antibiotics effective against Acinetobacter. In total, 133 patients (76%) received antibiotics with proper antibacterial activity against Acinetobacter during the course of bacteraemia. The median treatment duration was 10 days (range 1–28) for those effectively treated, either empirically or after adjusting treatment according to the antibiogram. Out of the total 179 cases, 33 patients (18%) received source control treatment, including catheter removal, nephrostomy and wound revision. In 53 cases of bacteraemia (30%), effective antimicrobial treatment targeting Acinetobacter were discontinued or not at all initiated, either due to poor prognosis of the patients (unethical to motivate further antibiotic treatment) or the Acinetobacter found being considered a contamination.
For all 179 episodes of bacteraemia, all-cause mortality rates were 16% (n = 28), 20% (n = 36) and 30% (n = 54) at 30-days, 90-days and at 1-year, respectively.
Analyses of putative risk factors for 30-day mortality
In an initial univariate complete case analysis, both Charlson comorbidity index, baseline functional capacity, age, all blood cultures positive for Acinetobacter and national/pediatric early warning score (NEWS2/PEWS) were significantly associated with 30-day mortality (Table S1). In the full multivariate model including six variables and multiple imputation for missing data (n = 176), all blood cultures positive had the strongest association with 30-day mortality (OR 5.0, 95% CI: 1.8 to 13.7, p= 0.002), baseline functional capacity the second strongest (1–4 points, OR 2.0, 95% CI: 1.2 to 3.4, p= 0.010) and finally, correct empiric antibiotics (when the culture was obtained) was also associated with an increased risk for death (OR 3.5 95% CI: 1.0 to 11.8, p= 0.045). The final full model showed good discriminatory abilities with an area under the receiver operating curve (AUROC) of 0.86 (95% CI: 0.79 to 0.93) but quite low model fit with an R2 of 23.2%. A complete case (CC) analysis (n = 148) shifted the effect estimates for blood culture positivity and baseline functional capacity somewhat, but the main difference was that correct empiric antibiotics and Charlson comorbidity index switched places for which variable had a p value below 0.05. The result of the multivariate regression analysis is detailed in .
Table 3. Multivariable logistic regression model with 30-day all-cause mortality as the outcome variable.
Discussion
The aim of this observational study was to report a detailed account of the epidemiology and outcome of Acinetobacter bacteraemia over 10 years in South Sweden. We found a low 30-day all-cause mortality of 16%, a high rate of community-acquired infections and only 3% of the Acinetobacter strains were carbapenem-resistant.
The incidence of invasive Acinetobacter in our study remained relatively stable throughout the 10 years studied. This is encouraging given the increasingly problematic role of Acinetobacter globally and the continuous increase in international travel seen during the last decade [Citation12,Citation13]. Our paper indicates that invasive Acinetobacter infections can be kept at a constant level with the use of proper surveillance, infection prevention and control and restrictive carbapenem usage.
The 30-day all-cause mortality of 16% found in this study is low compared to rates of 24–84% reported in previous studies [Citation14–17]. The reason for the low mortality rate could have several explanations. It could be related to the low rate of carbapenem-resistance (3%) found in this study, compared to the carbapenem-resistance rate of 32% of invasive Acinetobacter isolates in Europe 2018 [Citation5]. Multiple other studies have linked carbapenem resistance (or multi-drug resistance) to 30-day mortality [Citation3,Citation16]. In this study, only five carbapenem resistant strains were found, why such a comparison could not be performed. In Sweden, the usage of carbapenems in hospital settings is restricted and effective infection prevention measures are since long established to prevent nosocomial transmission of resistant bacteria. Furthermore, mortality could be related to Acinetobacter species. We found a predominance of A. lwoffii and A. pittii, and only a minority of strains were A. baumannii. Previous studies found appropriate antibiotic treatment associated with survival in infections caused by A. baumannii, but not by the less virulent A. nosocomialis [Citation18,Citation19].
The low mortality also raises the question whether Acinetobacter, in some cases, might constitute contamination of blood cultures. A mere 21% of patients received empiric antibiotic treatment effective against Acinetobacter, and 26% of patients never received adequate antibiotics during the course of bacteraemia. In spite of this, and in spite of other studies recognizing early administration of active antibiotics as a particularly important factor determining clinical outcome in Acinetobacter infections, the survival rate was high [Citation3,Citation15,Citation16,Citation20]. In fact, the multivariate logistic regression model found a positive association between empirical effective treatment and 30-day mortality. Previous studies support the hypothesis that Acinetobacter might contaminate blood cultures [Citation21–23]. In our multivariable survival model, Acinetobacter growth in all blood cultures drawn at the day of bacteraemia onset had the strongest association with 30-day mortality, indicating that several positive blood cultures may be a marker of actual infection. Furthermore, almost half (46%) of the patients in this study had additional species of bacteria in their blood cultures beside Acinetobacter, which could indicate contamination. Many clinicians refrained from antibiotic treatment on the suspicion of contamination rather than infection. Acinetobacter being a contamination could also explain the low rates of ICU-treated patients, as well as the high rate of community acquired infection (78%); other studies primarily regard Acinetobacter as a nosocomial pathogen [Citation24]. However, if the additional microbes also found in the blood culture were for instance other gram-negative rods or Staphylococcus aureus, this would not indicate contamination but rather a mixed infection. To what extent Acinetobacter bacteraemia constitute a contamination should preferably be studied in future prospective studies.
A strength of this study is the long inclusion time, the population-based nature and few exclusion criteria with few excluded patients and contemporary statistical methods. Limitations include the retrospective nature of the study, resulting in a risk for introducing selection bias. We used putative risk factors in our regression analyses believed to be most clinically relevant. This introduces the risk that other important variables associated with 30-day mortality were overseen. The sample size in our study is relatively small which limits the statistical power of the study. Therefore, regression modelling results should be interpreted with caution, including the association between empirical antibiotic treatment and mortality. Furthermore, since many of the standardized variables were collected from unstandardized written medical record, there is a risk of information bias. In addition, some variables have rather large proportions of missing data, although this was handled using multiple imputation. Furthermore, susceptibility testing and characterization to subspecies could only be performed in 107 of 179 strains, as the remaining were lost over time. In addition, we used Etest for testing colistin susceptibility, rather than the broth microdilution method recommended by EUCAST. This could have rendered a falsely high rate of isolates exhibiting colistin resistance and susceptibility, increased exposure.
Conclusions
In this retrospective cohort study of a decade of Acinetobacter bacteraemia in the south of Sweden, we found a high degree of community-acquired infection, a low rate of carbapenem resistance and low 30-day mortality, compared to previous international studies. This may reflect a high rate of microbiological contamination rather than clinical infection.
Supplemental Material
Download MS Word (18.6 KB)Disclosure statement
No competing interests to declare.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):1–9.
- Nemec A. Classification and nomenclature of species in the genus Acinetobacter. https://apps.szu.cz/anemec/Classification.pdf
- Wong D, Nielsen TB, Bonomo RA, et al. Clinical and pathophysiological overview of acinetobacter infections: a century of challenges. Clin Microbiol Rev. 2017;30(1):409–447.
- Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358(12):1271–1281.
- European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe 2018. Stockholm. 2019.
- Nichols L. Death from pan-resistant superbug. Autops Case Rep. 2019;9(3):e2019106.
- Befolkning i Skåne. 2020. Region Skåne. Cited 2020 Apr 08. Available from: https://utveckling.skane.se/digitala-rapporter/huga/befolkning/
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Royal College of Physicians. National Early Warning Score (NEWS) 2: standardising the assessment of acute-illness severity in the NHS. Updated report of a working party. London. 2017.
- Solevag AL, Eggen EH, Schroder J, et al. Use of a modified pediatric early warning score in a department of pediatric and adolescent medicine. PLoS One. 2013;8(8):e72534.
- Dickstein Y, Lellouche J, Ben Dalak Amar M, et al. Treatment outcomes of colistin- and carbapenem-resistant acinetobacter baumannii infections: an exploratory subgroup analysis of a randomized clinical trial. Clin Infect Dis. 2019;69(5):769–776.
- 2017 Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics (World Health Organization)
- International tourism, number of arrivals. 2020. The World Bank. Cited 2020 Apr 23. Available from. https://data.worldbank.org/indicator/st.int.arvl?end=2018&start=2009
- Poulikakos P, Tansarli GS, Falagas ME. Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant Acinetobacter infections: a systematic review. Eur J Clin Microbiol Infect Dis. 2014;33(10):1675–1685.
- Du X, Xu X, Yao J, et al. Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: a systematic review and meta-analysis. Am J Infect Control. 2019;47(9):1140–1145.
- Lee Y, Lee YT, Wang YC, et al. Risk of mortality of catheter-related bloodstream infections caused by acinetobacter species: is early removal of the catheters associated with a better survival outcome? J Intensive Care Med. 2018;33(6):361–369.
- Holmbom M, Moller V, Nilsson LE, et al. Low incidence of antibiotic-resistant bacteria in south-east Sweden: an epidemiologic study on 9268 cases of bloodstream infection. PLoS One. 2020;15(3):e0230501.
- Lee YT, Kuo SC, Yang SP, et al. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin Infect Dis. 2012;55(2):209–215.
- Kuo SC, Lee YT, Yang SP, et al. Evaluation of the effect of appropriate antimicrobial therapy on mortality associated with Acinetobacter nosocomialis bacteraemia. Clin Microbiol Infect. 2013;19(7):634–639.
- Lemos EV, de La Hoz FP, Einarson TR, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 2014;20(5):416–423.
- Berlau J, Aucken H, Malnick H, et al. Distribution of Acinetobacter species on skin of healthy humans. Eur J Clin Microbiol Infect Dis. 1999;18(3):179–183.
- Patil JR, Chopade BA. Distribution and in vitro antimicrobial susceptibility of Acinetobacter species on the skin of healthy humans. Natl Med J India. 2001;14(4):204–208.
- Dijkshoorn L, van Aken E, Shunburne L, et al. Prevalence of Acinetobacter baumannii and other Acinetobacter spp. in faecal samples from non-hospitalised individuals. Clin Microbiol Infect. 2005;11(4):329–332.
- Antunes LC, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014;71(3):292–301.