ABSTRACT
Invasive aspergillosis is an important cause of morbidity and mortality among immunocompromised patients. Prolonged neutropenia is the most common risk factor. It has rarely been reported to occur in non-neutropenic critically ill patients in the intensive care unit setting. Mortality rate in this group has been reported to be as high as 92%. We report a case of tracheobronchial aspergillosis in a non-neutropenic critically ill patient to highlight the fact that critically ill patients admitted in the intensive care unit can develop opportunistic infections such as invasive aspergillosis even in the absence of classic risk factors and prior history of immunosuppression. Early diagnosis and prompt initiation of antifungal therapy may improve the outcome and decrease mortality rate.
1. Introduction
Invasive aspergillosis (IA) is an important cause of morbidity and mortality among immunocompromised patients. Risk factors for IA include bone marrow or solid organ transplant, hematologic malignancies, neutropenia, human immunodeficiency virus (HIV) infection, chronic granulomatous disease (CGD) and chronic obstructive pulmonary disease (COPD) with prolonged corticosteroid use.[Citation1,Citation2] Prolonged neutropenia is the most common risk factor for IA. It has rarely been reported to occur in non-neutropenic critically ill patients in the critical care setting. Mortality rate in this group has been reported to be as high as 92%.[Citation3] In rare circumstances, tracheobronchial involvement is seen without pulmonary parenchymal disease. In such cases, bronchoscopy shows localized invasion of hyphae and may reveal pseudomembranes. We report a case of tracheobronchial aspergillosis in a non-neutropenic critically ill patient in the intensive care unit (ICU).
2. Case
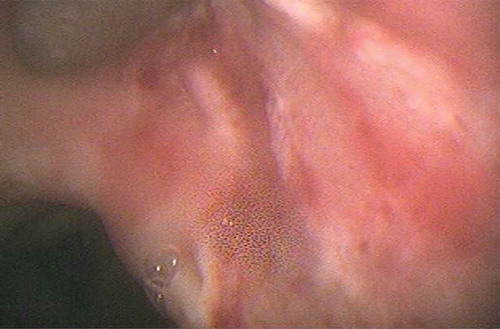
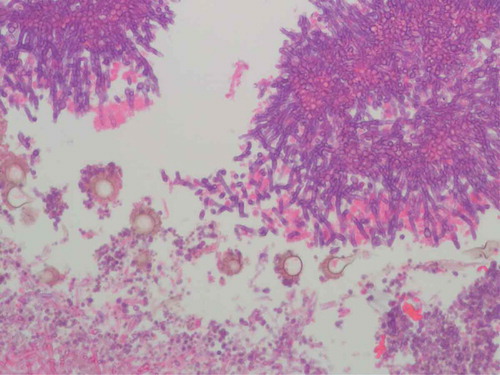
A 70-year-old Caucasian male was admitted to the ICU with acute peritonitis and septic shock secondary to perforated gastric volvulus. His only prior comorbidity was hypertension. He underwent emergent surgical repair of the gastric volvulus. Supportive care was initiated with broad spectrum antimicrobials, vasopressor support and mechanical ventilation. The clinical course was complicated by the development of acute kidney injury that required continuous renal replacement therapy. Subsequently, he developed stress-induced cardiomyopathy, atrial fibrillation and persistent ventilator dependent respiratory failure. Chest X-ray showed bibasilar atelectasis and bilateral pleural effusions. Computerized tomography of the chest showed bilateral pleural effusions and compressive atelectasis in lower lobes that necessitated placement of pleural drainage catheters. In light of the clinical deterioration, fiberoptic bronchoscopy was done with diagnostic and therapeutic intent. It revealed severe mucopurulent tracheobronchitis and a well demarcated area of increased friability with white-colored pseudomembrane involving the carina and right upper bronchus (). Cultures of bronchoalveolar lavage fluid grew Aspergillus fumigatus. Brushings from the pseudomembrane were sent for cytology that showed clusters of septate fungal hyphae with a positive potassium hydroxide (KOH) preparation (). Serum beta D glucan was positive (>500 pg/mL). Treatment with isavucoronium was initiated. The patient improved clinically and was subsequently liberated from ventilatory support. Meanwhile, we investigated for conditions that would predispose to severe Aspergillus infection. Human immunodeficiency virus serology was negative and HbA1c was within normal limits. Neutrophil count was not low. He had no history of underlying malignancy or prolonged steroid use. His absolute CD4 count was 366 cells µl–1 and CD4/CD8 ratio was 7.7. Immunoglobulin levels were within normal limits and revealed IgG level of 728 mg/dL, IgA level of 438 mg/dL, IgM level of 61 mg/dL, IgD level of 4.2 mg/dL and IgE level of 21 mg/dL. IgG subclasses 1–4 were also within normal limits.
3. Discussion
In patients without classic risk factors for IA, mortality rates are particularly high as the diagnosis is often delayed due to lack of awareness of clinicians for the risk of IA in non-neutropenic patients.
Critically ill patients in the ICU are prone to developing nosocomial infections but these patients may also be at a higher risk to develop opportunistic infections which are more commonly seen in immunocompromised patients. During acute severe illnesses such as sepsis, trauma and multi organ dysfunction syndrome (MODS), there is a biphasic immunological response consisting of an initial hyperinflammatory phase followed by an anti-inflammatory phase. This anti-inflammatory response leads to a state termed compensatory anti-inflammatory response syndrome (CARS) or immunoparalysis. Critically ill patients in the ICU may exhibit this immune alteration, termed ‘immunoparalysis’.[Citation4–Citation6] It is characterized by macrophage and monocyte deactivation, reduced monocyte HLA-DR antigen expression and altered cellular response that allows development of opportunistic infections including invasive fungal infections in patients without classic predisposing risk factors.[Citation5] Since alveolar macrophages form the first line of defense against inhaled Aspergillus spores, macrophage deactivation and loss of antigen presenting capacity allows development of invasive aspergillosis. Other factors that have unfavorable influence on the immune function during critical illness include acute hyperglycemia, corticosteroid use that affects distribution and function of neutrophils, and use of broad-spectrum antibiotics affecting distribution of normal flora.[Citation4] lists the risk factors for IA in non-neutropenic patients during critical illness as described in the literature. Critical illness itself can cause unintended immunomodulation and therapies that are common in the ICU setting also have undesired and unintended effects on immune system such as proinflammatory effect of catecholamines and anti-inflammatory effects of insulin, narcotics and furosemide.[Citation6]
Table 1. Risk factors for IA in non-neutropenic patients during critical illness.
Diagnosis of IA can be challenging as patients typically have nonspecific signs and symptoms as well as radiological findings. Isolation of Aspergillus species from respiratory tract samples may be misleading as the fungus is ubiquitous and may colonize airways without causing infection.[Citation5] The gold standard for the diagnosis of invasive disease remains histopathological examination, which requires invasive procedures that are sometimes not feasible in critically ill patients.
Isavuconazonium is a pro-drug of isavuconazole, a triazole antifungal drug. It has been approved by the Food and Drug Administration (FDA) for IA and was recently (January 2015) recommended by the FDA Anti-infective Drugs Advisory Committee for the treatment of IA in adults. Its safety and efficacy was compared to voriconazole in a phase 3, multicenter, double-blind, controlled non-inferiority trial (SECURE) in treating patients with IA. Isavuconazonium was found to have similar efficacy as voriconazole;[Citation15] however, IV formulations of isavuconazonium do not contain cyclodextrin which has the potential to cause nephrotoxicity and this is an advantage over voriconazole.[Citation16]
4. Conclusion
The above case highlights the fact that critically ill patients admitted in the ICU can develop opportunistic infections such as invasive aspergillosis even in the absence of classic risk factors and prior history of immunosuppression. Hence, IA should be included in the differential diagnosis of non-immunocompromised patients with pneumonia or sepsis resistant to broad-spectrum antimicrobials. Physicians should be aware of immunoparalysis syndrome, which can lead to opportunistic infections in patients with no history of immunosuppression prior to their ICU admission. A high index of suspicion is required in patients without well-established risk factors for IA as early diagnosis and prompt initiation of antifungal therapy may improve the outcome and decrease mortality rate.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Turc J, Lamblin A, Vitry T, et al. Clinical diagnosis of invasive pulmonary aspergillosis in a non-neutropenic critically ill patient. Resp Care. 2014;59(9):137–45.
- Falcone M, Concia E, Iori I, et al. Identification and management of invasive mycoses in internal medicine: a road-map for physicians. Intern Emerg Med. 2014;9:501–511.
- Nataloni S, Gabbanelli V, Rossi R, et al. Successful early voriconazole treatment of Aspergillus infection in two non immunocompromised patients in intensive care unit. Minerva Anestesiol. 2007;73:371–375.
- Trof RJ, Beishuizen A, Debets-Ossenkopp YJ, et al. Management of invasive pulmonary aspergillosis in non-neutropenic critically ill patients. Intensive Care Med. 2007;33:1694–1703.
- Hartemink KJ, Paul MA, Spijkstra JJ, et al. Immunoparalysis as a cause for invasive aspergillosis? Intensive Care Med. 2003;29:2068–2071.
- Frazier WJ, Hall MW. Immunoparalysis and adverse outcomes from critical illness. Pediatr Clin North Am. 2008;55(3):647–xi.
- Kosmidis C, Denning D. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70:270–277.
- Kaiser P, Thurnheer R, Krause M, et al. Invasive aspergillosis in non-neutropenic patients. Eur J Intern Med. 2009;20:e131–e133.
- Rello J, Esandi ME, Mariscal D, et al. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: report of eight cases and review. Clin Infect Dis. 1998;26:1473–1475.
- Garcia RJ, Troya P, Edwards C. Invasive aspergillosis with central nervous system dissemination in a presumably immunocompetent, non-neutropenic patient: case report and review. South Med J. 2006;99(6):607–610.
- Sahlen AO, Survarna SK, Wilkie ME. A case of invasive pulmonary aspergillosis in renal failure. Nephrol Dial Transplant. 2004;19:2687.
- Marr KA. Epidemiology and clinical manifestations of invasive aspergillosis. In: UpToDate, Post TW, editor. UpToDate. Waltham (MA). 2015. Available from: http://www.uptodate.com/contents/epidemiology-and-clinical-manifestations-of-invasive-aspergillosis
- ter Maaten JC, Golding RP, Strack van Schijndel RJ, et al. Disseminated aspergillosis after near-drowning. Neth J Med. 1995;47:21–24.
- Leroy P, Smismans A, Seute T. Invasive pulmonary and central nervous system aspergillosis after near-drowning of a child: case report and review of the literature. Pediatrics. 2006;118:e509–e513.
- Alexander J. Introductory remarks: overview of NDA 207,500 and 207,501 Isavuconazonium. Anti-Infective Drugs Advisory Committee Meeting; 2015. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM431845.pdf
- Falci DR, Pasqualotto AC. Profile of isavuconazole and its potential in the treatment of severe invasive fungal infections. Infect Drug Resist. 2013;6:163–174.