ABSTRACT
Melioidosis is an infectious disease endemic in Northern Australia and South East Asia. It is associated with high degrees of morbidity and mortality. On average, around five cases are diagnosed annually in the USA. Diagnosis remains a challenge, as it mimics many other conditions, especially tuberculosis, hence its other name, the ‘great mimicker.’ The present case involves a recent traveler to the Philippines, who presented with episodic fevers and weight loss to his primary care physician. Blood cultures ordered grew Burkholderia pseudomallei. Primary care physicians should suspect melioidosis in symptomatic patients with travel history to endemic areas.
1. Case report
A 60-year-old Filipino male with poorly controlled diabetes mellitus (HbA1c:12%), hypertension, and dyslipidemia presented to the medicine clinic. He complained of episodic fevers of up to 101°F, chills, loss of appetite, muscle aches, and a 17lb weight loss over the last 3.5 weeks. Travel history revealed a recent trip to Manila, Philippines, 3 weeks prior, where the patient spent a month. During his stay, it rained for the first week, and the patient was outdoors frequently. Initial outpatient workup revealed leukocytosis of 14,100/mL. In the clinic, blood cultures were drawn, and he was given intramuscular ceftriaxone due to episodic fevers. The patient was sent home on oral ciprofloxacin. However, the patient remained febrile with persistent leukocytosis. He was admitted for intravenous antibiotics and tight glycemic control. On admission, he had low-grade fever and tachycardia. His exam was unremarkable, except for malaise. Chest X-ray, urine culture, and human immunodeficiency virus were negative. Final blood cultures grew Burkholderia pseudomallei, and it was confirmed by the Center for Disease Control and Prevention (CDC). The patient was diagnosed with melioidosis with no evident focus.
After consultation with the infectious disease physician, he was started on intravenous ceftazidime. The patient was discharged home after 48 h, as the leukocytosis resolved and he remained afebrile. He was instructed to complete 4 weeks of intravenous ceftazidime and then to start on oral Bactrim, with follow-up arranged with the infectious disease clinic. The patient was also given instructions to improve glycemic control, and his insulin regimen was adjusted. The patient was seen in the clinic by his primary care physician after 4 weeks of induction therapy. He had complete resolution of symptoms. Repeat blood cultures were negative, and he was tolerating eradication therapy with oral Bactrim.
2. Discussion
2.1. Introduction
Melioidosis is caused by a Gram-negative bacterium B. pseudomallei [Citation1,Citation2]. Typically seen in countries in Southeast Asia and in Australia, it is now an emerging infection in India, Africa, and the Middle East [Citation2,Citation3]. Exposure to contaminated soil or water through ingestion, inhalation, or skin inoculation causes infection [Citation4]. It is believed that the present patient acquired melioidosis through inhalation while being outdoors during the rainy season in an endemic area. Person-to-person transmission is extremely rare but may occur through contact with the blood or body fluids of an infected person in a laboratory setting. Most cases of melioidosis in the USA are diagnosed in travelers from endemic areas. At risk are individuals with chronically immunosuppressed states, such as poorly controlled diabetes mellitus, alcoholism, chronic renal or liver disease, and patients on chronic steroid therapy [Citation5,Citation6].
2.2. Clinical manifestations
Manifestations of melioidosis vary and can include localized to multifocal infection with or without septicemia [Citation4,Citation6]. Though the most common presentation is pneumonia with symptoms similar to pulmonary tuberculosis [Citation4], it can also present as encephalomyelitis, septic arthritis, osteomyelitis, and skin and visceral organ abscesses involving renal, splenic, prostatic, and hepatic sites, hence making this disease a ‘great mimicker’ [Citation7].
In non-endemic regions, such as the USA, diagnosis should be considered for every symptomatic patient with a history of travel to endemic regions, especially if immunocompromised. In case of immunosuppression, melioidosis may be reactivated, with reports of latency periods lasting up to 26 years, as seen in US veterans returning from Vietnam, earning it the name the ‘Vietnamese time bomb’ [Citation2,Citation4,Citation8].
2.3. Diagnosis
The gold standard for diagnosis remains positive cultures for B. pseudomallei. However, due to low sensitivity, samples should be collected from multiple sites. In all patients, blood and urine cultures and a throat swab must be obtained, even in the absence of urinary symptoms or pharyngitis. Organ-specific samples should be collected, for example sputum in pneumonia or aspirate in abscesses. Selective media such as Ashdown agar should be used to grow the cultures [Citation9].
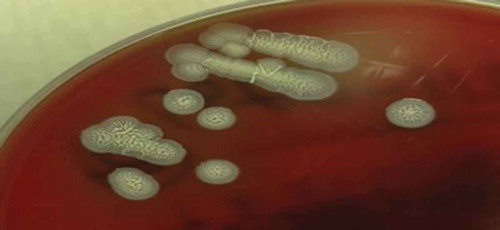
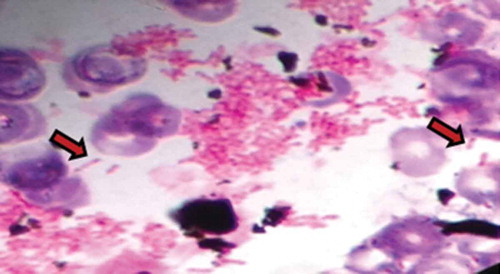
In the present patient, light microscopy of blood culture revealed bipolar staining with ‘safety pin’–like appearance () and wrinkled appearance of colonies on Ashdown agar growth medium (), which are both typical morphologies of B. pseudomallei [Citation9].
Figure 1. Light microscopy showing characteristic bipolar staining with ‘safety pin’–like appearance.
Serologic tests, such as indirect hemagglutination assay, have been widely used. These tests aid diagnosis in travelers returning to the USA from endemic regions. However, they are not useful for diagnosis in the native population [Citation4,Citation9].
2.4. Prevention and treatment
Patients who are travelling to endemic areas, especially if immunocompromised, must practice adequate hygiene measures and use safe water for drinking and cooking to decrease the risk of exposure by ingestion [Citation4,Citation5]. Travelers should stay indoors during periods of heavy wind or rain to decrease the risk of inhalation [Citation4,Citation10]. They should be instructed to use personal protective equipment such as waterproof boots and gloves. Any skin lacerations, burns, or abrasions that are contaminated with soil or surface water must be thoroughly cleaned to decrease the risk of skin inoculation. It is vital to warn laboratory personnel of suspected melioidosis, as special precautions are needed to prevent accidental exposure [Citation9].
Treatment includes two phases: the ‘induction phase’ with intravenous ceftazidime or a carbapenem (either mcMeropenem or imipenem), and the ‘eradication phase’ with oral antibiotics such as trimethoprim-sulfamethoxazole [Citation11]. Alternative eradication therapies include doxycycline and co-amoxiclav. It is vital to note that both therapies have a higher rate of relapse when compared to trimethoprim-sulfamethoxazole [Citation12]. The induction phase over 2 weeks and the eradication phase over 3 months has been shown to decrease the chances of relapse [Citation4,Citation6].
2.5. Conclusion
Primary care physicians should be vigilant of melioidosis as a differential diagnosis in any patient presenting with sepsis who has travelled to endemic areas, especially if the patient is immunocompromised. A wide array of clinical features makes melioidosis a ‘great mimicker’ [Citation7]. A detailed travel history and a high index of suspicion in this population are needed to make a timely diagnosis and treatment decision to decrease morbidity and mortality. In addition, patients who are planning to travel to endemic areas must be educated about prevention.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–1044.
- Currie BJ, Ward L, Cheng AC, et al. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4(11):e900.
- Dance DA. Melioidosis: the tip of the iceberg? Clin Microbiol Rev. 1991;4:52–60.
- Currie BJ. Burkholderia pseudomallei and Burkholderia mallei: melioidosis and Glanders. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. PA: Elsevier Saunders; 2015. p. 2541–2551.
- Stewart T, Engelthaler DM, Blaney DD, et al. Epidemiology and investigation of melioidosis, Southern Arizona. Emerg Infect Dis. 2011;17:1286–1288.
- Centers for Disease Control and Prevention (CDC). Imported melioidosis–South Florida, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:873.
- Yee KC, Lee MK, Chua CT, et al. Melioidosis, the great mimicker: a report of 10 cases from Malaysia. J Trop Med Hyg. 1988;91:249–254.
- Chodimella U, Hoppes WL, Whalen S, et al. Septicemia and suppuration in a Vietnam veteran. Hosp Pract. 1997;32:219–221.
- Hoffmaster A, AuCoin D, Baccam P, et al. Melioidosis diagnostic workshop, 2013. Emerg Infect Dis. 2015;21(2). DOI:10.3201/eid2102.141045.
- Currie BJ, Jacups SP. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis. 2003;9:1538–1542.
- White NJ, Dance DA, Chaowagul W, et al. Halving of ortality of severe melioidosis by ceftazidime. Lancet. 1989;23(2):697–701.
- Lipsitz R, Garges S, Aurigemma R, et al. Workshop on treatment of and postexposure prophylaxis for Burkholderia pseudomallei and B. mallei Infection, 2010. Emerg Infect Dis. 2012 Dec;18(12):e2. DOI:10.3201/eid1812.120638.