ABSTRACT
Clinical copper deficiency is now more frequently recognized. Hematologically, it can present as anemia (microcytic, normocytic, or macrocytic) and neutropenia. Thrombocytopenia is relatively rare. Neurologically, it can manifest as myelopathy and peripheral neuropathy simulating subacute combined degeneration. Bone marrow findings can mimic myelodysplasia resulting in occasional inappropriate referral for bone marrow transplantation. Other conditions with similar presentations include infections, drug toxicity, autoimmunity, B12 deficiency, folate deficiency, myelodysplastic syndrome, aplastic anemia, and lymphoma with bone marrow involvement. Hematological, but not neurological, manifestations respond promptly to copper replacement, making early diagnosis essential for good outcome. Common risk factors for copper deficiency are foregut surgery, dietary deficiency, enteropathies with malabsorption, and prolonged intravenous nutrition (total parenteral nutrition). We present a unique case of copper deficiency, with no apparent known risk factors.
KEYWORDS:
1. Case report
A 72-year-old female with known phlebotomy-dependent hemochromatosis, pernicious anemia, hypertension, hypothyroidism, and bipolar disorder presented with progressive generalized weakness, lower extremity numbness, tingling, abnormal balance, and a 26-lb weight loss over several months. Her family history was significant for colon cancer in paternal grandfather, and pancreatic and breast cancer in her sisters. She lived alone, smoked cigarettes, but denied alcohol consumption. Her medications included acetaminophen, aspirin, carbamazepine, cyanocobalamin, darbepoetin alfa, fluticasone nasal spray, levothyroxine, lisinopril, magnesium hydroxide, meclizine, metoprolol tartrate, pantoprazole, fluoxetine, quetiapine, and multivitamins. Physical examination revealed pallor, lower extremity hyporeflexia, and sensory ataxia.
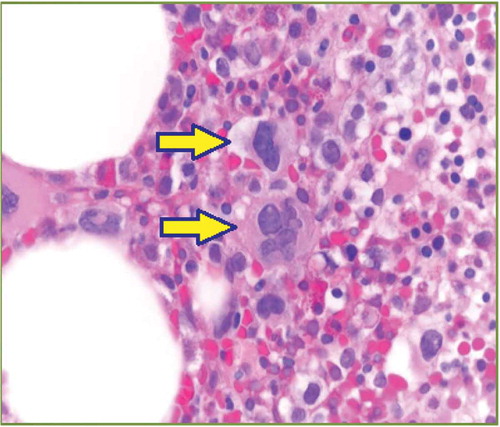
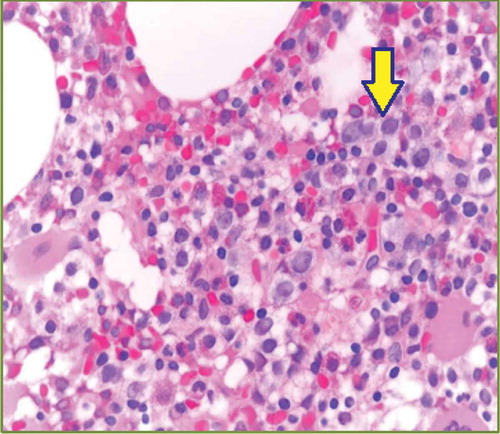
Laboratory values checked at the time of presentation are displayed in . Her complete metabolic panel was essentially unremarkable. Bone marrow biopsy showed myelodysplasia with no megaloblasts, with normal iron stains ( and ). Esophagogastroduodenoscopy and colonoscopy showed normal findings.
Table 1. Laboratory values in parenthesis represent reference range.
Figure 2. Dyspoietic changes in myeloid cells (center and top right): hypogranularity and megaloblastoid changes (solid arrow).
Her copper level was undetectable at <5 (reference range 70–125 µg/dL). She was given intravenous followed by oral copper supplementation. Within 4–6 weeks of copper replacement, her macrocytic anemia and leukopenia resolved but neurological manifestation persisted. shows her laboratory values after copper supplementation. Her neurological symptoms started to alleviate in 5–6 months after copper supplementation but did not resolve completely. On subsequent outpatient visits over a year, her weight remained stable and she reported only mild generalized weakness.
Table 2. Laboratory values after copper supplementation.
Table 3. Common causes of copper deficiency.
2. Discussion
Copper, a trace element, is heavily involved in cell oxidation and signaling systems. Etiology of anemia in copper deficiency is complex and multifactorial. Ceruloplasmin, a major copper carrying protein in the blood, oxidizes ferrous iron to ferric form which allows iron to be transported in the circulation and bind to transferrin. Another important element in copper–iron interaction is Hephaestine, which is a copper-dependent ferroxidase. This is a transport protein involved in iron absorption from enterocytes [Citation1], which can explain the development of microcytic hypochromic anemia in some patients. Macrocytosis, neutropenia, and neurological manifestations are related to the role of copper as an important cofactor in enzymatic processes involved in cell division and protein synthesis. Copper acts as a cofactor in several enzymes that have a critical role in the structure and function of the central nervous system. These enzymes include cytochrome-c-oxidase in the mitochondrial electron transport chain and oxidative phosphorylation, superoxide dismutase for oxidative protection, crosslinking of collagen and elastin by lysyl oxidase, dopamine beta-hydroxylase for catecholamine biosynthesis, and peptidylglycine alpha-amidating monooxygenase for peptide neurotransmitters and hormones processing [Citation2].
Neurologically, copper deficiency can manifest as myelopathy and peripheral neuropathy simulating subacute combined degeneration [Citation3,Citation4]. Unlike Wilson disease, in which excessive copper deposition in tissues leads to cardiac dysfunction, liver cirrhosis, and pancreatic dysfunction, copper deficiency has not been reported to affect these organs.
An overview of copper metabolism in humans is highlighted in . The highest content of copper is found in the liver. The total amount of copper in an adult human body is approximately 50–120 mg. The liver is the fundamental organ involved in copper hemostasis. The recommended dietary allowance (RDA) for copper is 900 mcg/day in adults. Copper is predominantly absorbed in the stomach and duodenum. P-type ATPase (ATP7A) is an intestinal copper transporting protein which is deficient in Menkes disease. The latter is an x-linked genetic disorder caused by an inactivating mutation of the ATP7A gene. It is characterized by sever copper deficiency, progressive neurological decline, and early childhood death.
Figure 3. Metabolism of copper [Citation5]. The numbers represent average amount in healthy adults. Copper is largely absorbed in stomach and proximal small intestine. It is transported via portal vein to liver for ceruloplasmin biosynthesis, which carries copper to peripheral tissues. Roughly 50% copper is excreted in bile. *Menkes P-type ATPase: a transmembrane protein, which regulates copper absorption from intestine into the blood. ǂWilson P-type ATPase: copper transporting protein, involved in copper excretion into bile and plasma.
![Figure 3. Metabolism of copper [Citation5]. The numbers represent average amount in healthy adults. Copper is largely absorbed in stomach and proximal small intestine. It is transported via portal vein to liver for ceruloplasmin biosynthesis, which carries copper to peripheral tissues. Roughly 50% copper is excreted in bile. *Menkes P-type ATPase: a transmembrane protein, which regulates copper absorption from intestine into the blood. ǂWilson P-type ATPase: copper transporting protein, involved in copper excretion into bile and plasma.](/cms/asset/15775908-2683-4c62-8fc0-c4d9401d98dc/zjch_a_1351289_f0003_b.gif)
Causes of copper deficiency are listed in . The most common cause of acquired copper deficiency is malabsorption due to bariatric surgery [Citation6–Citation8]. Copper is largely absorbed in the stomach and proximal small intestine [Citation9]. Myelopathy due to copper deficiency can mimic vitamin B12 deficiency [Citation10]. Symptoms can take up to a decade to appear after the initial surgery [Citation11]. Both zinc and copper are absorbed in the stomach and proximal duodenum. Excess zinc increases the production of metallothionein, a heavy metal binding protein, by enterocytes. Copper binds with high affinity to metallothionein within the enterocytes leading to its excretion through the intestinal tract [Citation12]. Myeloneuropathy due to copper deficiency has been reported following treatment of Wilson disease with chelation and zinc [Citation13–Citation15].
Other causes of copper deficiency include enteropathies associated with malabsorption such as celiac disease and inflammatory bowel disease [Citation16]. Copper deficiency can occur in prolonged total parenteral nutrition with inadequate copper supplementation, with hematological abnormalities responding promptly to copper replacement [Citation17–Citation20]. Estimated copper requirement in patients receiving total parenteral nutrition is approximately 0.3 mg/day in the adult [Citation17].
Dietary deficiency is rare but it is possible. In our patient, rapid increase in serum copper level () after oral supplementation suggest dietary deficiency the most likely cause. Weight loss over several months also points towards poor eating habits. Copper deficiency has not been reported in hemochromatosis, which is an autosomal recessive disease characterized by increased intestinal iron absorption. Excessive dietary iron ingestion caused sever copper deficiency in mice [Citation21].
3. Conclusion/learning points
Gastrectomy and gastric bypass surgery are common causes of acquired copper deficiency. Due to the high prevalence of obesity in the USA, the number of bariatric procedures is increasing. It can take several years before the body copper stores are depleted [Citation10]. Therefore, for patients with hematological abnormalities (anemia, neutropenia, myelodysplasia) and especially neurological deficits resembling vitamin B12 deficiency after gastric surgery, deficiency of copper should be considered in the differential diagnosis [Citation22].
Copper deficiency may be mistaken for vitamin B12 deficiency or myelodysplasia. Hematological manifestations almost universally respond to copper supplementation, whereas neurological improvement is often reported as slow, incomplete, or even absent [Citation23], making prompt diagnosis and treatment essential for successful outcomes.
A written consent was obtained from the patient.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Huff JD, Keung Y-K, Thakuri M. Copper deficiency causes reversible myelodysplasia. Am J Hematol. 2007;82:625–630.
- Kumar N, Ahlskog JE, Klein CJ, et al. Imaging features of copper deficiency myelopathy: a study of 25 cases. Neuroradiology. 2006;48(2):78.
- Kumar N Jr, Gross JB, Ahlskog JE. Copper deficiency myelopathy produces a clinical picture like subacute combined degeneration. Neurology. 2004 Jul 13;63:33–39.
- Mercer JF1, Llanos RM. Molecular and cellular aspects of copper transport in developing mammals. J Nutr. 2003 May;133(5Suppl 1):1481S–4S.
- Kodama H, Fujisawa C, Bhadhprasit W. Inherited Copper Transport Disorders: biochemical Mechanisms, Diagnosis, and Treatment. Curr Drug Metab. 2012;13:237–250.
- Prodan CI, Bottomley SS, Vincent AS, et al. Copper deficiency after gastric surgery: a reason for caution. Am J Med Sci. 2009;337(4):256–258.
- Kumar N. Neurologic complications of bariatric surgery. Continuum (Minneap Minn). 2014 Jun;20(3):580–597. Neurology of Systemic Disease).
- Kumar N1, McEvoy KM, Ahlskog JE. Myelopathy due to copper deficiency following gastrointestinal surgery. Arch Neurol. 2003 Dec;60(12):1782–1785. DOI:10.1001/archneur.60.12.1782
- Wapnir RA1. Copper absorption and bioavailability. Am J Clin Nutr. 1998 May;67(5Suppl):1054S–1060S. PMID:9587151.
- Kumar N1, Ahlskog JE, Gross JB Jr. Acquired hypocupremia after gastric surgery. Clin Gastroenterol Hepatol. 2004 Dec;2(12):1074–1079.
- Juhasz-Pocsine K1, Rudnicki SA, Archer RL, et al. Neurologic complications of gastric bypass surgery for morbid obesity. Neurology. 2007 May 22;68(21):1843–1850. DOI:10.1212/01.wnl.0000262768.40174.33
- Rowin J, Lewisaff SL. Copper deficiency myeloneuropathy and pancytopenia secondary to overuse of zinc supplementation. J Neurol Neurosurg Psychiatry. 2005;76:750–751.
- da Silva-Júnior FP1, Machado AA, Lucato LT, et al. Copper deficiency myeloneuropathy in a patient with Wilson disease. Neurology. 2011 May 10;76(19):1673–1674. DOI:10.1212/WNL.0b013e318219fac8
- Horvath J, Beris P, Giostra E, et al. Zinc-induced copper deficiency in Wilson disease. J Neurol Neurosurg Psychiatry. 2010 Dec;81(12):1410–1411. Epub 2010 Oct 4. DOI:10.1136/jnnp.2009.188896
- Foubert-Samier A1, Kazadi A, Rouanet M, et al. Axonal sensory motor neuropathy in copper-deficient Wilson’s disease. Muscle Nerve. 2009 Aug;40(2):294–296. DOI:10.1002/mus.21425
- Halfdanarson TR1, Kumar N, Hogan WJ, et al. Copper deficiency in celiac disease. J Clin Gastroenterol. 2009 Feb;43(2):162–164. DOI:10.1097/MCG.0b013e3181354294
- Shike M1. Copper in parenteral nutrition. Gastroenterology. 2009 Nov;137(5Suppl):S13–S17. DOI:10.1053/j.gastro.2009.08.017
- Fuhrman MP, Herrmann V, Masidonski P, et al. Pancytopenia after removal of copper from total parenteral nutrition. JPEN J Parenter Enteral Nutr. 2000;24(6):361–366.
- Imataki O1, Ohnishi H, Kitanaka A, et al. Pancytopenia complicated with peripheral neuropathy due to copper deficiency: clinical diagnostic review. Intern Med. 47(23): 2063–2065. Epub 2008 Dec 1. 2008.
- Angotti LB1, Post GR, Robinson NS, et al. Pancytopenia with myelodysplasia due to copper deficiency. Pediatr Blood Cancer. 2008 Nov;51(5):693–695. DOI:10.1002/pbc.21661
- Ha JH, Doguer C, Collins JF. Consumption of a high-iron diet disrupts homeostatic regulation of intestinal copper absorption in adolescent mice. Am J Physiol Gastrointest Liver Physiol. 2017 Jun;15. ajpgi.00169. DOI:10.1152/ajpgi.00169.2017
- Mingyi C, Krishnamurthy A, Mohamed AR, et al. Hematological disorders following gastric bypass surgery: emerging concepts of the interplay between nutritional deficiency and inflammation. Biomed Res Int. 2013;2013:205467. Epub 2013 Jul 25. DOI:10.1155/2013/205467
- Chhetri SK1, Mills RJ2, Shaunak S2, et al. Copper deficiency. BMJ. 2014 Jun 17; 348:g3691. DOI:10.1136/bmj.g3691