ABSTRACT
Loperamide is an over-the-counter antidiarrheal agent that is considered by many patients to be safe, but has been used as a drug of abuse due to its opioid properties. However, cardiotoxicity has been reported, prompting the FDA to release a warning regarding the arrhythmogenic potential of loperamide. We present a case of a 38-year-old female presenting with cardiac arrest thought to be secondary to abuse of the loperamide that she was using to alleviate the heroin withdrawal symptoms. Cardiac ischemia and other drug toxicities were ruled out. Loperamide induces QTc prolongation and cardiac dysrhythmias. She had recurrent ventricular arrhythmias with multiple cardiac arrests. The persistence of the cardiotoxicity for a longer duration than previously reported in the literature is unique in this clinical presentation. We also highlight the potential mechanisms for loperamide cardiotoxicity and its challenging management.
Abbreviations: ACLS: Advanced cardiac life support; GI: Gastrointestinal
1. Introduction
Loperamide is a commonly used, readily available over-the-counter anti-diarrheal agent that is widely believed to be safe and has both proven effectiveness and safety when used at FDA-approved dosages (a maximum 16 mg/d for prescription products and 8 mg/d for over-the-counter (OTC) products) [Citation1,Citation2]. Since dissemination of its opioid properties in online recreational drug use forums, poison control centers have noticed a doubling in referrals for loperamide overdose since 2014 [Citation3,Citation4]. Loperamide has peripheral mu receptor agonistic properties in the gastrointestinal tract, and accidental and deliberate overdosage has been associated with opioid-like euphoria [Citation5,Citation6]. In fact, loperamide was, at one time, a schedule V drug due to its opioid properties, and has been described as the ‘poor man’s methadone’ [Citation4]. In the absence of studies indicating any tolerance developing, even at supra-therapeutic dosages, it was removed from scheduling [Citation7]. Due to its ability to control diarrhea, the same drug forums advocated for its use to control the diarrheal symptoms of opioid withdrawal, and it has been increasingly utilized in this manner [Citation3,Citation6]. However, with increasing abuse, there has been a concomitant increase in the number of reports of cardiotoxicity, particular dysrhythmias during the post-marketing drug safety surveillance [Citation2,Citation3] These reports led to a 2016 FDA warning on potential arrhythmias from usage [Citation7]. Herein, we report prolonged cardiotoxicity in a patient abusing loperamide to manage her heroin withdrawal and review the complexities of managing this presentation.
2. Case presentation
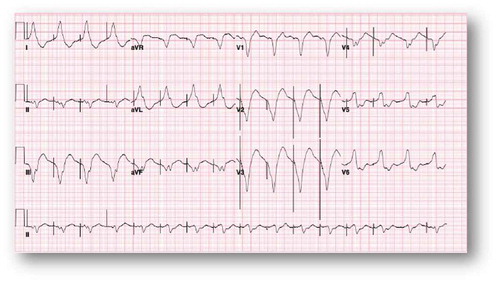
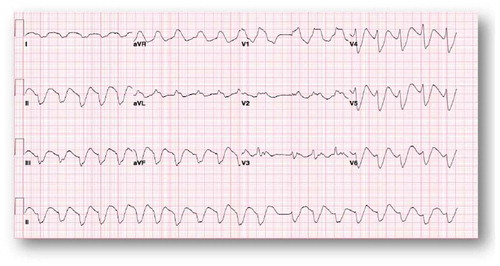
A 38-year-old female with an extensive history of poly-substance abuse presented after an outside hospital cardiac arrest that was recognized in the field to be caused by ventricular fibrillation of uncertain duration in the field. Her family reported daily use of two boxes of loperamide (144 tabs, 288 mg) daily for an indefinite period of time for the purpose of controlling symptoms of heroin withdrawal. She formerly used codeine and suboxone for opioid withdrawal symptom control but no longer had access to those treatments. Other than the polysubstance abuse, she was not known to have any known chronic medical illness and was not taking any prescription medications. There was no family history of cardiac arrhythmias or sudden cardiac death (SCD). The patient was successfully resuscitated in the field as per ACLS protocol with defibrillation, and, for airway protection, was intubated. She was initially taken to an outside facility and then emergently transferred to a tertiary care center. Hypothermia protocol was not initiated because of non-availability at the outside hospital. At the time of arrival, electrocardiogram (EKG) revealed a wide complex undetermined atrial rhythm at a rate of 58 bpm with prolonged QT and QTc intervals (). The patient had no available baseline EKG for comparison. Emergent coronary angiography indicated normal coronary arteries. Urine toxicology screen was negative. The regional poison control center was notified and recommended sodium bicarbonate and lipid infusions. Electrolytes, including potassium, magnesium, and calcium, were maintained within normal limits through intravenous supplementation.
Figure 1. Wide complex undetermined atrial rhythm at rate of 58 bpm with prolonged QT 666 msec and QTc of 653 msec.
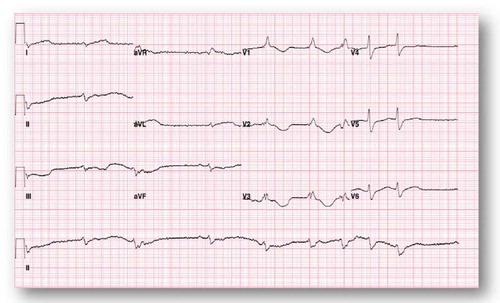
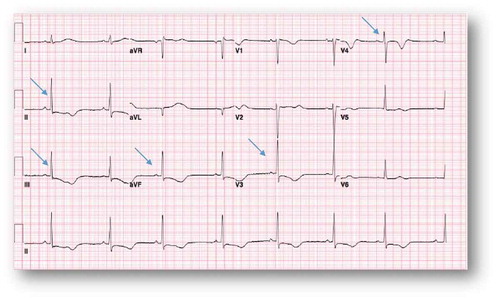
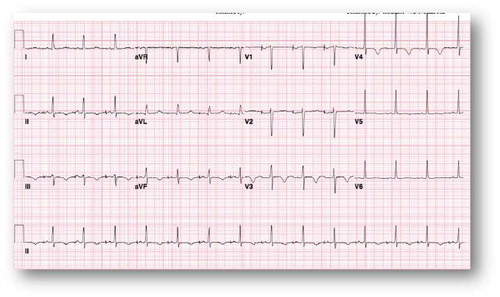
Due to bradycardia, the patient was started on an isoproterenol drip. The following day, her telemetry alarmed for a very wide QRS; EKG showed wide complex tachycardia () that culminated with a ventricular cardiac arrest. She was successfully resuscitated as per ACLS protocol with amiodarone push and lidocaine infusion. Six hours later, she developed sustained polymorphic ventricular tachycardia without loss of pulse that responded to magnesium supplementation. A temporary transvenous pacer was inserted with overdrive strategy. She was reliably paced as shown in the EKG (). Shortly after that, however, she developed a third ventricular fibrillation cardiac arrest that was successfully resuscitated as per ACLS protocol with multiple shocks (>10 times). Higher electrolytes levels were targeted with a potassium goal of 4.5 mmol/L and magnesium goal of 2–2.5 mg/dL. It was expected that the loperamide side effect would last for 24–48 hours. On Day 3, she was extubated without any residual neurologic deficit. She remained bradycardic when the pacemaker was turned down (EKG, ). Isoproterenol was weaned. On Day 5, she developed another episode of ventricular fibrillation that responded to four shocks of 200 Joules. Isoproterenol infusion was restarted transiently due to bradycardia. She was discharged to the medical floor on Day 6. However, on Day 11, she had multiple episodes of Torsade de Pointes (TdP), that were self-limiting and without symptoms. She was readmitted to the ICU due to these episodes, and temporizing measures were restarted (lidocaine and isoproterenol) with positive results. The patient threatened to leave the ICU against medical advice, so AICD/DDD ICD was placed to prevent ventricular fibrillation cardiac arrest given her recurring TdP. Repeat EKG () showed progressive shortening of the QTc and QT intervals. The patient changed her mind about leaving the ICU after the AICD placement and was discharged after medical stabilization two days later. She followed as outpatient with the EP team. She denied any symptoms or any further use of loperamide. Office EKG showed normal sinus rhythm at a rate of 85 bpm with normal QTc at 437 msec. Device interrogation demonstrated normal sensing and pacing pacemaker. She was enrolled in remote device monitoring every three months and has had no detected device shocks.
Figure 2. Wide complex tachycardia at a rate of 121 bpm that is consistent with ventricular tachycardia.
3. Discussion
There is a growing demand amongst people who are opioid-dependent for drugs to control withdrawal symptoms. As an inexpensive, over-the-counter option, loperamide has been adapted for this purpose [Citation6]. Loperamide exhibits predominantly peripheral activity, low bioavailability, and poor blood-brain barrier penetration at the FDA-approved doses. When ingested in massive doses (40–100 times the usual dose), it can cross the blood-brain barrier and exert CNS activity similar to that of other opioids [Citation3]. The patient in our vignette was a daily, long-term loperamide-abuser. The exact mechanism of the cardiotoxicity is not entirely understood. Loperamide has been reported to cause QTc prolongation and recalcitrant ventricular arrhythmias with symptoms ranging from palpitations to sudden cardiac death (SCD). At therapeutic doses, loperamide has a poor oral bioavailability of only 10–20%; it is metabolized by the cytochrome P450 (3A4 and 2C8) system with biliary excretion, resulting in minimal systemic absorption [Citation4]. Of note, other medications with effects on the 3A4 system have been linked to ventricular arrhythmias, though causation has not been proven [Citation8].
Loperamide then acts on peripheral mu receptors in the myenteric plexus of the gastrointestinal tract and has been postulated to have verapamil-like effects [Citation9]. It demonstrates structural similarities with methadone and terfenadine by having multiple phenyl rings. Therefore, it can be assumed that its QTc prolongation effect through a similar mechanism [Citation10]. Prolonged QTc interval at supra-therapeutic doses can be explained by the blockade of the cardiac human ether-a-go-go (hERG) potassium channel. These channels are major regulators of the rapid delayed rectifier current (IKr) which conducts potassium (K+) out of the cardiac myocytes [Citation11,Citation12]. Defective function of the channels affects ventricular repolarization and leads to a prolonged relative refractory period of the cardiac myocytes [Citation11]. Cells become vulnerable to extrasystole with potential TdP and ventricular arrhythmia that is manifested by prolonged QT and QTc intervals on the surface EKG. Another hypothesis suggests that congenital Long QT syndrome may be unmasked by the loperamide’s actions on the hERG channel, causing the ventricular arrhythmias [Citation13].
Managing the electrical effects of loperamide at supratherapeutic dosages is a clinical challenge. The loperamide resulted in a wide QRS morphology and prolonged QT secondary to its potassium channel blockade effect with subsequent ventricular instability. Both of the verapamil-like effects on the sinoatrial node and the prolonged QT itself result in bradycardia, which increases the risk of TdP, a phenomenon called bradycardia-induced tachyarrhythmia [Citation13]. On the basis of studies indicating successful treatment of loperamide abuse-induced ventricular arrhythmias, isoproterenol, a beta-adrenergic agonist, was used to counter the nodal blockade and to keep the heart rate more than 100 beats per minute. An overdrive pacemaker was used to achieve the same strategy. Lidocaine was added to stabilize the ventricular membrane [Citation14,Citation15]. After the initial usage during a VF arrest, amiodarone use was avoided because of the risk of QT prolongation. Time is the only important factor when it comes to the resolution of the loperamide cardiotoxicity. Only supportive management can be offered in addition to the temporizing measures, e.g., lidocaine, as noted.
The 2008 ACC/AHA/HRS Guidelines for Device-based Therapy stress the importance of excluding any reversible causes for VF before ICD therapy [Citation16]. Although the underlying etiology for the TdP and the ventricular arrhythmia (loperamide abuse) in our patient was reversible, the patient threatened to leave against medical advice and received an AICD PPM that was felt to be necessary for secondary prevention of SCD.
The reported half-life of loperamide is 9–13 hours with typical dosages, but there are rare reports of half-lives extending to 40.9 hours with 16 mg dosages in healthy volunteers [Citation17,Citation18]. Given the standard half-life expectation, the poison control center suggested that it may last as long as 4–5 days before cardiac stabilization. Our patient, however, remained symptomatic for 11 days’ post-ingestion with recurrent TdP and ventricular arrhythmias. This may be explained by the slow release of the medication from her stunned GI tract, but cannot be proven without motility studies, which were not possible in her critically ill state.
Given the opioid epidemic in the USA, the increasing recognition of the abuse potential and the easy availability of loperamide, an extensive history of over-the-counter medications is a must at the time of admission in order to suspect and diagnose loperamide cardiotoxicity. Early consultation with a poison control center is a necessary adjunct to providing the safest, most effective care.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ericsson CD, Johnson PC. Safety and efficacy of loperamide. Am J Med. 1990;88:S10–S14.
- Swank KA, Wu E, Kortepeter C, et al. Adverse event detection using the FDA post-marketing drug safety surveillance system: cardiotoxicity associated with loperamide abuse and misuse. J Am Pharmacists Assoc. 2017 Mar–Apr;57(2S):S63–S67.
- Lasoff DR, Koh CH, Corbett B, et al. Loperamide trends in abuse and misuse over 13 years: 2002–2015. Pharmacotherapy. 2017;37(2):249–253.
- Stanciu CN, Gnanasegaram SA. Loperamide, the “Poor Man’s Methadone:” a brief review. J Psychoactive Drugs. 2017;49(1):18–21.
- Baker DE. Loperamide: a pharmacological review. Rev Gastroenterol Disord. 2006;7:S11–S18.
- Daniulaityte R, Carlson R, Falck R, et al. “I just wanted to tell you that loperamide WILL WORK”: a web-based study of the extra-medical use of loperamide. Drug Alcohol Depend. 2013;130:241–244.
- FDA warns about serious heart problems with high doses of the antidiarrheal medicine loperamide (Imodium), including from abuse and misuse. Safety Announcement. [ cited 2016 Jul 6].
- Hasnain M, Vieweg WVR, Howland RH, et al. Quetiapine, QTc interval prolongation, and torsades de pointes: a review of case reports. Ther Adv Psychopharmacol. 2014 Jun;4(3):130–138.
- Reynolds IJ, Gould RJ, Snyder SH. Loperamide: blockade of calcium channels as a mechanism for antidiarrheal effects. J Pharmacol Exp Ther. 1984 Dec;231(3):628–632.
- Upadhyay A, Bodar V, Malekzadegan M, et al. Loperamide induced life threatening ventricular arrhythmia. Case Rep Cardiol. 2016;2016:5040176.
- Pearlstein RA, Vaz RJ, Kang J, et al. Characterization of HERG potassium channel inhibition using CoMSiA 3D QSAR and homology modeling approaches. Bioorg Med Chem Lett. 2003 May 19;13(10):1829–1835.
- Mukarram O, Hindi Y, Catalasan G, et al. Loperamide induced Torsades de Pointes: a case report and review of the literature. Case Rep Med. 2016;2016:4061980.
- Namboodiri N. Bradycarda-induced Torsades de Pointes – an arrhythmia less understood. Indian Pacing Electrophysiol J. 2010;10(10):435–438.
- Marraffa JM, Holland MG, Sullivan RW, et al. Cardiac conduction disturbance after loperamide abuse. Clini Toxicol. 2014 Nov;52(9):952–957.
- Enakpene EO, Riaz IB, Shirazi FM, et al. The long QT teaser: loperamide abuse. Am J Med. 2015;128(10):1083–1086.
- Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;129:94–125.
- Doser K, Meyer B, Nitsche V, et al. Bioequivalence evaluation of two different oral formulations of loperamide (Diarex Lactab vs Imodium capsules). Int J Clin Pharmacol Ther. 1995;33:431–436.
- Yu JH, Kim HJ, Lee S, et al. LC-MS determination and bioavailability study of loperamide hydrochloride after oral administration of loperamide capsule in human volunteers. J Pharm Biomed Anal. 2004;36:421–427.