ABSTRACT
We report a case of a 25-year-old obese, currently smoking, female diagnosed with EBV infectious mononucleosis. The patient complained of sudden onset abdominal pain with progressively increasing intensity in the left upper quadrant. Abdominal CT scan showed a wedge infarct of the spleen. We present this rare case that EBV may cause splenic infarct in young adults.
1. Introduction
The most common presentation of infectious mononucleosis (IM) is fever, sore throat, lymphadenopathy, and abdominal pain. The course of the disease is often benign with spontaneous resolution of symptoms after a few days. However, in certain cases IM may present with complications involving multiple organ systems like the liver, lungs, and spleen.
Table 1. Pattern of test results in patients with acute EBV infection versus past infection.
The abdominal symptoms of IM can range from an acute localized upper-left quadrant pain to a more diffuse non-localizing pain spread across the upper abdomen resulting in a broad differential. Splenic infarct and rupture are rare complications of IM. The signs and symptoms may be very subtle and a high index of suspicion has to be present in any patient presenting with IM, such as left upper-quadrant pain, to identify patients with splenic infarct. We present a case of a young adult with no comorbidities, having abdominal pain, diagnosed with IM and found to have an underlying splenic infarct on CT scan.
2. Case report
A 25-year-old female with a BMI of 35 kg/m2 presented to the emergency department (ED) with complaints of abdominal pain rated 8/10 (at its worst) that had started 4 days prior to admission. It was accompanied with a sore throat that had started 1 week earlier, and was associated with generalised malaise and fatigue. Apart from her obesity and smoking one pack of cigarettes daily for eight years history (1 pack per day), her past medical history was unremarkable and she had no prior history of any trauma.
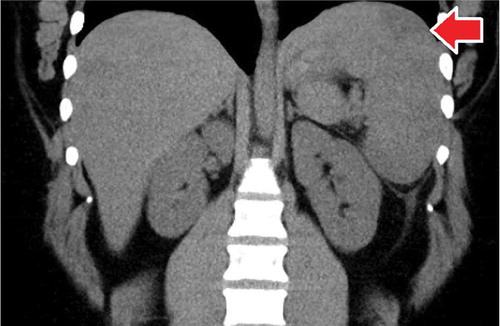
In the ED, she was afebrile, had a congested erythematous throat, cervical lymphadenopathy, and tenderness in the left upper quadrant of the abdomen on palpation. There was splenomegaly noted on examination but no guarding. Routine labs were remarkable for a lymphocytic predominance with a normal white blood cell count. Clotting profile (prothrombin time, activated partial thromboplastin time, and INR) was normal along with liver function tests. Hepatitis and HIV panels were also negative. Serological and polymerase chain reaction tests for Epstein Barr virus were positive and confirmed the diagnosis of IM. The EBV viral capsid Ag Ab IgM – 1.94 (normal <0.9). The EBV early Ag AB – 1.54 (normal <0.9). The EBV nuclear Ab was negative, and EBV DNA by PCR was 3548. The abdominal computed tomography scan showed an enlarged spleen measuring 15.3 by 6.0 by 14.5 cm with a wedge-shaped infarct (). Further evaluations with an electrocardiogram were unremarkable.
Subsequently, the patient was managed conservatively in the hospital and was discharged without any complication, with strict instructions to avoid any contact sports for at least 6 months.
3. Discussion
Patients with splenic infarct have a wide clinical spectrum ranging from being asymptomatic to fatal hemorrhage. Nores et al. Citation[1] explained that up to 30% of splenic infarcts may be asymptomatic. Those with symptoms may have left upper-quadrant pain which may radiate to the back or shoulder, nausea, vomiting, and/or fever. The best modality to diagnose the patients is with the help of a computed tomography scan, which has the best sensitivity among all imaging modalities Citation[2].
The most common test used historically to diagnose IM is a monospot test, since it is the most cost effective. Studies have shown that it can produce high false negative results early in the disease (up to 25% in the first week) Citation[3]. A positive test may indicate the presence of typical IM but does not confirm the presence of the disease and may be negative early in the disease course. Presence of IgM against the viral capsid antigen is the most sensitive test to confirm an acute infection. Antibodies to the nuclear antigen prove presence of chronic infection. shows the patterns of test results in acute infection versus old infection. A combination of two tests should be used to confirm the presence of an acute infection Citation[4].
Only 19 cases prior to this article (since 1961) have been reported in medical literature Citation[5]. The exact frequency of IM related infarction is unknown due to underreporting or underdiagnosis. Splenic infarct due to IM is rare. Only three cases were identified in our literature review that had histopathological findings, which showed reactive inflammatory changes [Citation6–Citation8]. Due to scarcity in the pathological study, the exact pathogenesis of the disease has not been determined. There have been a few hypotheses to explain the cause of splenic infarct in patients with IM. The most common explanation has been demand ischemia. Due to the enlargement of the spleen, which in some cases can be up to twice the size, the blood supplied by the arteries does not meet the demand of the organ as a result of the hypercellularity interrupting the blood flow. It is also unknown if the viral load has a role in the degree of hypercellularity or change in size of the spleen in the setting of an acute infection that may increase the chances of an infarction Citation[9]. Other causes revolve around the presence of a transient hypercoagulable state, caused by a decrease in the levels of protein C and protein S, compounded by an increase in the size of the spleen, resulting in an infarct [Citation10,Citation11]. However, activated protein C resistance was not checked Citation[12]. A case report was also seen to have positive antiphospholipid antibodies and another with elevated factor Ⅶ [Citation12,Citation13]. Hence, further studies will be needed to establish the hypothesis of transient hypercoagulable state leading to splenic infarct.
Of the 19 cases, six underwent follow up imaging with ultrasound and a CT scan was done in three cases each with follow-up ranging from 30 to 120 days [Citation11,Citation14,Citation15]. All cases showed improvement. Imaging was done earlier in two cases at day 7 with a CT scan, and day 10 with an MRI, respectively, showing no changes to mild aggravation in the latter [Citation10,Citation16]. Hence, we recommend a follow up in 4–6 weeks based on the limited follow-up imaging results. Earlier imaging can be obtained if the clinical suspicion for deterioration is high.
4. Conclusion
In our patient, the coagulopathy tests and EKG were normal. The EKG did not show any atrial fibrillation. We hypothesize that due to obesity and smoking, combined with increased size of the spleen due to hypercellularity may have starved the blood flow resulting in an infarct. Since the CT scan did not show rupture or hemorrhage, it was decided to treat the patient conservatively, which led to improvement in symptoms after 3 days and it was determined that it was safe for her to go home. It was recommended that she continued to rest for at least a week before returning to her routine activities with restrictions (e.g. avoiding sports, etc.) and to obtain a follow up CT scan of the abdomen in one month.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Nores M, Phillips EH, Morgenstern L, et al. The clinical spectrum of splenic infarction. Am Surg. 1998;64:182–188.
- Antopolsky M, Hiller N, Salameh S, et al. Splenic infarction: 10 years of experience. Am J Emerg Med. 2009;27:262–265.
- Monospot MS. FamilyPracticeNotebook.com. Available from: http://www.fpnotebook.com/ID/Lab/Mnspt.htm rev. 11/32008, cited 12/8/08.
- www.cdc.gov/epstein-barr/laboratory-testing.html
- Naviglio S, Abate MV, Chinello M, et al. Splenic infarction in acute infectious mononucleosis. J Emerg Med 2016;50(1):e11–113.
- Chevat H, Aulong C, Demaille A, et al. Splenic infarct and infectious mononucleosis. Lille Med. 1961;6:314–317.
- Garten AJ, Mendelson DS, Halton KP. CT manifestations of infectious mononucleosis. Clin Imaging. 1992;16:114–116.
- Suzuki Y, Shichishima T, Mukae M, et al. Splenic infarction after Epstein–Barr virus infection in a patient with hereditary spherocytosis. Int J Hematol. 2007;85:380–383.
- Heo D-H, Baek D-Y, Sang-Min O, et al. Splenic infarction associated with acute infectious mononucleosis due to Epstein–Barr virus infection. J Med Virol. 2017;89:332–336.
- Breuer C, Janssen G, Laws HJ, et al. Splenic infarction in a patient hereditary spherocytosis, protein C deficiency and acute infectious mononucleosis. Eur J Pediatr. 2008;167:1449–1452.
- Gang MH, Kim JY. Splenic infarction in a child with primary Epstein–Barr virus infection. Pediatr Int. 2013;55:e126–e128.
- Machado C, Melo Salgado J, Monjardino L. The unexpected finding of a splenic infarction in a patient with infectious mononucleosis due to Epstein–Barr virus. BMJ Case Rep. 2015. DOI:10.1136/bcr-2015-212428
- Cull E, Stein BL. Splenic infarction, warm autoimmune hemolytic anemia and antiphospholipid antibodies in a patient with infectious mononucleosis. Int J Hematol. 2012;95:573–576.
- van Hal S, Senanayake S, Hardiman R. Splenic infarction due to transient antiphospholipid antibodies induced by acute Epstein–Barr virus infection. J Clin Virol. 2005;32:245–247.
- Kobe D, Nakatani T, Fujinaga Y, et al. A case of infectious mononucleosis with splenic infarction. Nihon Shokaki- Byo Gakkai Zasshi. 2013;110:1461–1467.
- Li Y, Pattan V, Syed B, et al. Splenic infarction caused by a rare co infection of Epstein-Barr virus, cytomegalovirus, and Mycoplasma pneumoniae. Pediatr Emerg Care. 2014;30:636–637.