ABSTRACT
Adrenal collision tumors (ACTs), in which distinct tumors coexist without histological intermingling in the same adrenal gland, are rare and their actual prevalence is unknown. ACTs commonly consist of adrenal cortical adenoma, myelolipoma, or metastatic malignant tumor. We report a 58-year-old woman with a past history of breast cancer, who presented with a 1 month history of fevers, chills, and abdominal fullness. The physical examination and the laboratory data including endocrine studies were unremarkable. Computed tomography of the abdomen showed a right adrenal gland mass, and a laparoscopic right adrenalectomy was performed. Histological and immunohistochemical examinations revealed three distinct tumors: an adrenal cortical adenoma, a myelolipoma, and metastatic breast tumors. Breast cancer metastases are rare in the adrenal gland and exist as ACTs only in exceptionally rare cases. To our knowledge, this is the first reported case of coexisting metastatic breast tumors, adrenal adenoma, and myelolipoma in the same adrenal gland.
1. Introduction
Adrenal collision tumors (ACTs) are rare clinical entities referring to separate coexisting adjacent tumors involving an adrenal gland with a sharp demarcation between the two and without a substantial histological mixture at the interface [Citation1]. A composite tumor, which is sometimes interchangeably used in the literature with the collision tumor, arises from a common neoplastic source, whereas a collision tumor arises from different neoplastic sources [Citation2]. Invasive ductal carcinoma (IDC) of the breast primarily metastasizes to the lungs, bones, liver, pleura, gastrointestinal tract, brain, and adrenal gland at autopsy. The frequency of adrenal metastasis from breast adenocarcinoma is very low, being reported as 2.9%[Citation3]. We report a case of an ACT composed of a breast cancer metastasis, and a benign myelolipoma within a benign adrenal adenoma.
2. Case presentation
Our patient is a 58-year-old female who had a history of a lumpectomy for IDC of the left breast with metastasis present in one of three left axillary sentinel lymph nodes in January 2010. She underwent chemotherapy and radiation therapy after lumpectomy. The patient was noted in 2005 to have a small right adrenal mass of 3.2 cm (‘incidentaloma’), but she had had no specific follow-up imaging since then.
In January 2016, the patient developed episodes of fevers, chills, chest and back pain, and abdominal fullness for a month, which happened about once or twice a week. Her temperature maximum was 38–39ºC. She also had palpitations and headache during these episodes.
On examination, she was anemic and but had no lymphadenopathy or organomegaly. Laboratory data were as follows: white blood cell count 5.8 × 103 cells/µl (reference range 4.0–11.0 × 103 cells/µl), hemoglobin 9.8 g/dl (12.5–15.0 g/dl), platelets 383 × 103 cells/µl (150–450 × 103 cells/µl); alanine transaminase 7 U/l (4–31 U/l), aspartate transaminase 15 U/l (4–31 U/l), alkaline phosphatase 111 U/l (39–117 U/l), albumin 3.9 g/dl (3.2–5.2 g/dl), globulin 3.3 g/dl (2.0–3.7 g/dl), creatinine 0.93 mg/dl (0.4–1.1 mg/dl), erythrocyte sedimentation rate 105 mm/h (≤ 30 mm/h), C-reactive protein 10.2 mg/dl (≤ 0.5 mg/dl); antinuclear antibodies, anti-double-strain DNA, and anti-Smith antibodies all negative; rheumatoid factor 21 IU/ml (< 14 IU/ml), cortisol 4.3 µg/dl (4.0–22.0 µg/dl), thyroid-stimulating hormone 1.14 mIU/l (0.4–4.5 mIU/ml); iron 21 μg/dl (45–160 µg/dl), total iron-binding capacity 271 µg/dl (250–450 µg/dl), transferrin saturation 8% (11–50%), and ferritin 614 ng/ml (10–232 ng/ml). Urine culture, blood culture, and human immunodeficiency virus screening, and the QuantiFERON-TB Gold test for tuberculosis, were all negative. Contrast-enhanced computed tomography (CT) of the abdomen revealed a large mass (6.2 × 4.3 × 5.1 cm) with irregular enhancement in the right adrenal gland, which was significantly increased in size compared with the report from 2005 ( and ).
Figure 1. Axial computed tomography showing a low attenuating right adrenal mass, which proved to be an adrenal collision tumor on pathology.
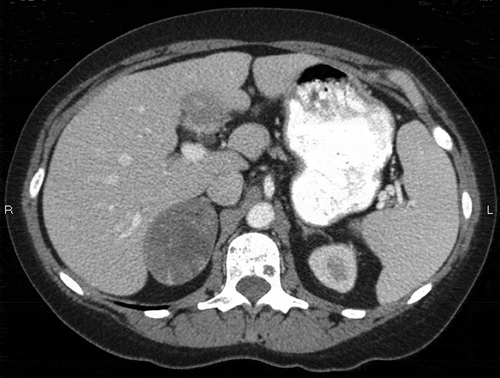
Figure 2. Coronal computed tomography showing a low attenuating right adrenal mass, which proved to be an adrenal collision tumor on pathology.

For further evaluation of the adrenal mass, more tests were performed, with the following results: aldosterone 6 ng/fl (upright 8.00–10.00am ≤ 28 ng/fl), plasma renin activity (PRA) 3.24 ng/ml/h (0.25–5.82 ng/ml/h), aldosterone/PRA 1.9 (0.9–28.9), free normetanephrine 234 pg/ml (≤ 148 pg/ml), free metanephrine < 25 pg/ml (≤ 57 pg/ml), and metanephrine total (24 h) 234 pg/ml (≤ 205 pg/ml), indicating that the right adrenal mass was hormonally inactive.
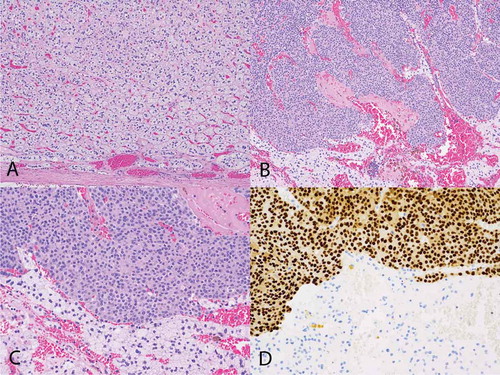
Laparoscopic right adrenalectomy was performed on 4 April 2016. On histological examination of the entirely embedded adrenal gland, greater than 95% of the right adrenal mass was composed of an adrenocortical adenoma reminiscent of the zona fasciculata. The mass was centrally hemorrhagic with race calcifications and cystic changes. A 4.0 mm myelolipoma was also present within the adrenocortical adenoma. In addition, there were scattered atypical cells within this adrenocortical adenoma, representing approximately 2% of the volume of the mass. Immunohistochemical statins showed that the zona fasciculate type cells were positive for inhibin and negative for GATA-3, while the atypical cells were positive for GATA-3, CKAE1/AE3, CK7, estrogen receptor, and progesterone receptor, and negative for inhibin, CK20, and SOX10 (). HER-2/Neu was equivocal. These findings were consistent with metastatic breast cancer.
Figure 3. Histological findings of the patient. (A) Hematoxylin & eosin stain showing adrenal cortical adenoma with a capsule of adenoma noted at the bottom of the image (100×). (B) Metastatic breast carcinoma (top of image) and adrenal cortical adenoma (bottom) (100×). (C) Metastatic breast carcinoma (top of image) and adrenal cortical adenoma (bottom) (200×). (D) GATA-3 immunohistochemical stain showing metastatic breast carcinoma (top of image) and adrenal cortical adenoma (bottom). The metastatic breast carcinoma shows positive nuclear staining (brown) with the GATA-3 antibody consistent with breast carcinoma, while the cells of the adrenal cortical adenoma are negative for that stain (200×).
Post-surgery, positron emission tomography/computed tomography (PET/CT) of the whole body revealed diffuse osseous metastases throughout all osseous structures including both humeri and femurs, which corresponded to diffuse sclerotic changes. Additional areas of fluorodeoxyglucose (FDG)-avid metastases were demonstrated in the left supraclavicular region and possibly within both hila.
The patient declined aggressive treatment options and only agreed to aromatase inhibitor therapy. However, her disease gradually progressed and she decided to be under hospice care and died about 10 months from the diagnosis of the metastatic disease.
3. Discussion
Collision tumors can occur in various organs such as the lungs, liver, and genitourinary tract. An ACT is a rarely reported tumor entity. The actual prevalence of ACT is unknown, despite the relatively high incidence of both benign and metastatic lesions of adrenal glands.An ACT may consist of two benign or two malignant lesions. The most commonly reported ACT comprises an adenoma and a myelolipoma [Citation3,Citation4]. Other reported cases include metastases within adenomas or myelolipomas and pheochromocytomas within adenomas or myelolipomas [Citation5]. The metastatic tumors are usually lung, breast carcinoma, or melanoma [Citation6]. Our patient’s ACT had three components: adenoma, breast cancer metastasis, and myelolipoma. To our knowledge, this is the first case report with triple components in an ACT.
The pathogenesis of ACT is unclear. Various mechanisms have been postulated, including a single carcinogenic stimulus altering a particular region of the adrenal gland, allowing two separate tumors to occur in close proximity; and the presence of one tumor altering the local environment, providing a fertile ground for the development of the other tumor [Citation1,Citation7].
Diagnosis of ACT can be very challenging. The presence of a clinical syndrome related to elevated serum levels of an adrenal hormone (hyperfunctioning adenoma) is helpful for the diagnosis of adrenal adenoma. However, most adrenal adenomas do not produce any hormones (non-functioning). A non-functioning adrenal adenoma can be difficult to distinguish from a metastatic lesion. If a mixed benign and malignant collision tumor is biopsied and only the benign component identified, suboptimal treatment may be delivered. This will have a significant impact on prognosis.
The typical imaging of a collision tumor consists of a combination of the two lesions. An adrenal adenoma usually has a low density on an unenhanced CT, representing a lipid-rich region, whereas the metastatic lesion has a high density. These metastatic lesions may mimic atypical adrenal adenomas that contain lipid-poor regions. A collision tumor should be considered if the adrenal lesion is suspected of having changes in appearance and size suggesting an atypical adenoma [Citation6]. A collision tumor should also be suspected in a patient with a history of malignancy with the imaging revealing a heterogeneous adrenal mass.
CT with contrast is often the first modality used in detecting adrenal masses. Magnetic resonance imaging (MRI) is indicated for characterization of adrenal masses that show atypical findings on CT. Chemical- shift MRI, PET/CT, and adrenal radiocholesterol scintigraphy may also used to discriminate between benign and malignant components of the tumor mass. Images obtained with chemical-shift imaging and gadolinium-enhanced techniques are very useful in differentiating various tumor components [Citation1,Citation8,Citation9]. PET/CT study can provide additional functional characteristics of these tumors and guide the percutaneous biopsy. Studies have demonstrated the high sensitivity (94–100%) and specificity (80–100%) of FDG-PET in differentiating benign and malignant adrenal lesions [Citation10,Citation11]. Adrenal radiocholesterol scintigraphy is also considered to be very accurate in distinguishing benign adrenal adenomas from extra-adrenal lesions [Citation12].
A fine-needle aspiration biopsy of the tumor can be attempted under CT guidance to confirm the diagnosis. However, the sensitivity of this method has never been determined in patients with ACT owing to the rarity of these lesions [Citation13]. In our patient, the history of malignancy, the increase in the size of the adrenal adenoma, and the heterogeneous appearance on the contrast-enhanced CT make ACT highly likely. In cases like this, surgical resection is the most appropriate choice.
4. Conclusion
An ACT composed of an adrenal adenoma, myelolipoma, and metastatic breast cancer is a rare tumor. Diagnosis can be challenging as metastatic lesions and non-functional benign adrenal tumors can have the same characteristics on unenhanced CT imaging. Newer imaging modalities such as chemical-shift MRI, PET/CT, and high-resolution CT help to differentiate the various components of the tumor. Heterogeneous adrenal masses in a patient with a past history of malignancy should raise high suspicion for a metastatic lesion and should be confirmed with histological and immunohistochemical studies.
Acknowledgements
We thank the Department of Radiology, Dr Charles D. Yim MD, and Jennifer Wang, library director at Greater Baltimore Medical Center, for their continued support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Schwartz LH, Macari M, Huvos AG, et al. Collision tumors of the adrenal gland: demonstration and characterization at MR imaging. Radiology. 1996;201:757–760.
- Sung CT, Shetty A, Menias CO, et al. Collision and composite tumors; radiologic and pathologic correlation. Abdom Radiol. 2017 Jun 16. (Epub ahead of print).
- Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23(3):175–180.
- Otal P, Escourrou G, Mazerolles C, et al. Imaging features of un-common adrenal masses with histopathologic correlation. Radio-Graphics. 1999;19:569–581.
- Ukimura O, Inui E, Ochiai A, et al. Combinedadrenal myelolipoma and pheochromocytoma. J Urol. 1995;154:1470.
- Siddiqi AJ 1, Miller FH, Kasuganti D, et al. Adrenal hemangioma-adenoma: an exceedingly rare adrenal collision tumor. J Magn Reson Imaging. 2009;29(4):949–952.
- Anderson SB, Webb MD, Banks KP. Adrenal collision tumor diagnosed by F-18 fluorodeoxyglucose PET/CT. Clin Nucl Med. 2010;35(6):414–417.
- Elsayes KM, Mukundan G, Narra VR, et al. Adrenal masses: MR imaging features with pathologic correlation. Radiographics. 2004;24(Suppl 1):S73–S86.
- Katabathina VS, Flaherty E, Kaza R, et al. Adrenal collision tumors and their mimics: multimodality imaging findings. Cancer Imaging. 2013;13(4):602–610.
- Blake MA, Slattery JM, Kalra MK, et al. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy – initial experience. Radiology. 2006;238:970–977.
- Yun M, Kim W, Alnafisi N, et al. 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. J Nucl Med. 2001;42:1795–1799.
- Rubello D, Bui C, Casara D, et al. Functional scintigraphy of the adrenal gland. Eur J Endocrinol. 2002;147(1):13–28.
- Thorin-Savouré A, Tissier-Rible F, Guignat L, et al. Collision/composite tumors of the adrenal gland: a pitfall of scintigraphy imaging and hormone assays in the detection of adrenal metastasis. J Clin Endocrinol Metab. 2005 Aug;90(8):4924–4929.
