ABSTRACT
Coronary artery fistulas (CAFs) are found in 0.3–0.8% of patients who undergo coronary angiography. CAFs are defined as single or multiple, small or large direct communications that arise from one or more coronary arteries and enter into one of the four cardiac chambers or major vessels. We present two cases of multiple coronary artery fistulas arising from diagonal and left anterior descending (LAD) branches of left coronary artery draining into the left ventricle. In both the cases, No intervention was performed. Of the congenital fistulas, two major groups are identified: solitary CAFs or coronary artery-left ventricular multiple micro-fistulas (CALVMMFs). Noninvasive techniques such as transthoracic echocardiography, transesophageal echocardiography and magnetic resonance imaging are becoming increasingly popular for diagnosis and follow-up of CAFs. Despite the advent of these newer non-invasive modalities, coronary angiography remains the gold standard for diagnosis. Treatment of CAFs is indicated when the patients are symptomatic with left ventricular volume overload, myocardial ischemia, left ventricular dysfunction or in the presence of a large or increasing left-to-right shunt. If the fistula is small and hemodynamically insignificant, it can be managed with conservative management. Multiple left anterior descending to left ventricle (LV) fistulas are extremely rare and, as per our literature review, we noted only a few case reports of coronary artery fistulas between branches of LAD and left ventricle.
1. Introduction
Coronary artery fistulas are found in 0.3–0.8% of patients who undergo coronary angiography [Citation1]. They are reported very rarely in the adults. Coronary artery fistulas are defined as single or multiple, small or large direct communications that arise from one or more coronary arteries and enter into one of the four cardiac chambers or major vessels [Citation2]. They may be congenital or acquired, associated with various disorders or spontaneous. Patients are generally asymptomatic and the fistulas are discovered during cardiac catheterization for congenital heart anomalies or coronary artery disease. Rarely, they can result in heart failure, spontaneous intrapericardial rupture and tamponade or myocardial ischemia due to a coronary ‘steal’ phenomenon [Citation3]. We present two cases of multiple coronary artery fistulas arising from diagonal and left anterior descending (LAD) branches of left coronary artery draining into the left ventricle.
2. Case presentation
2.1. Case 1
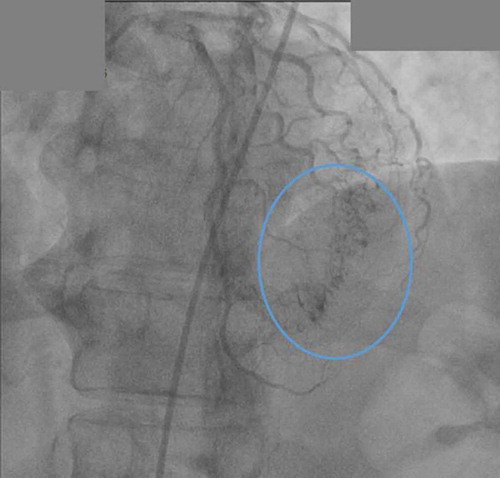
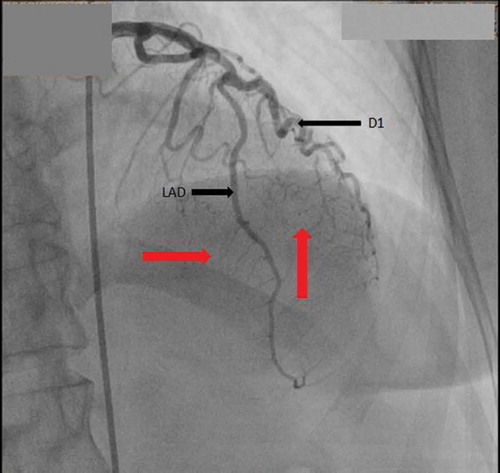
The patient is a 66 year old African American female with past medical history of primary hypertension, diabetes mellitus type 2, hyperlipidemia and ischemic cerebrovascular accident (CVA) with no residual weakness, presented to the cardiologist office with complaints of chest pain. The chest pain was left sided pressure, which was not exacerbated with exertion or relieved by rest. There was no association with position or food intake. She denied any exertional shortness of breath, palpitations, pedal edema, paroxysmal nocturnal dyspnea or orthopnea. Family history was significant for premature coronary artery disease in the sister at a very young age. She consumed alcohol socially but denied smoking cigarettes and illicit drug use. On presentation to office, vital signs were stable. Cardiovascular exam revealed regular rate and rhythm. Apical impulse was not displaced. First (S1) and second heart sound (S2) were heard, no S3 or S4 were auscultated. No evidence of murmurs rubs or gallops. Rest of the exam was unremarkable. She was sent from the office to the emergency department. On arrival to emergency room (ER), the patient still had some chest pain. Sublingual nitroglycerin was administered, which improved her chest pain. Vitals were stable and there was no change in physical exam. She was admitted to the hospital for further evaluation of chest pain. Electrocardiogram demonstrated left axis deviation, no pathological q waves, and ST segment depression in anteroseptal and anterior leads. The patient had a nuclear stress test that showed reversible ischemia in apical and anteroseptal walls with an ejection fraction of approximately 61%. Cardiac characterization was performed, which revealed right dominant system with no significant disease, and multiple coronary cavernous fistulas from the diagonal and distal part of LAD filling into the left ventricle ( and ). There was no significant atherosclerotic disease noted in the coronary arteries. No acute intervention was deemed necessary at this point as we wanted to monitor the patient’s response to medical therapy before pursuing surgical measures. The patient was discharged on antianginal medications including sublingual nitroglycerin as needed and isosorbide mononitrate tablets. On follow up 3 months later, patient was stable and did not have any further episodes of chest pain.
2.2. Case 2
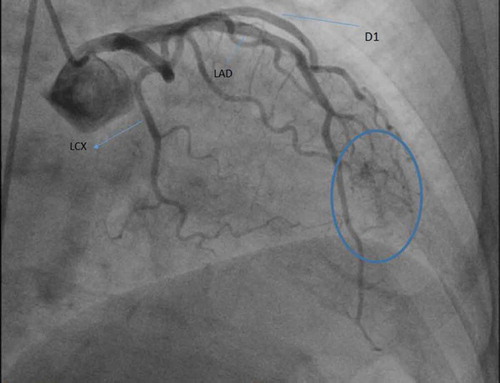
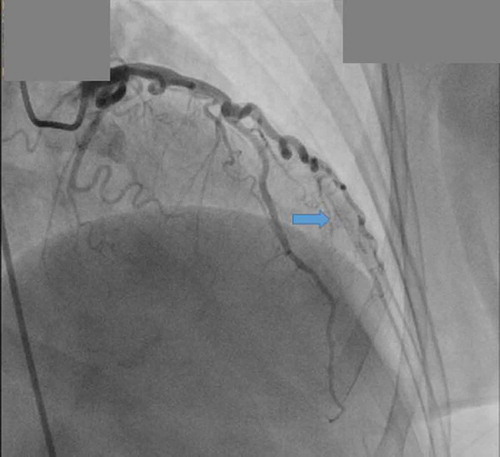
The patient is a 57 year old Hispanic female with past medical history of primary hypertension, hyperlipidemia, and transient ischemic attack, presents to the emergency department with complaints of chest pain. Chest pain was described as a pressure sensation, substernal in location and was exacerbated with exertion. There was no association with position or food intake. Family history was non-contributory. The patient is a lifelong non-smoker, consumed alcohol socially, with no recreational drug use. On presentation to the ER, vitals were stable. Cardiovascular exam revealed regular rate and rhythm and a nondisplaced apical impulse. First (S1) and second heart sound (S2) were heard and no S3 or S4 were auscultated. There was no evidence of murmurs rubs or gallops. EKG showed sinus rhythm with no ST-T wave changes. The patient was admitted to hospital for workup of exertional chest pain. Acute coronary syndrome was ruled out after three negative sets of troponin and the patient was eventually discharged on sublingual nitroglycerin as needed. However, she remained symptomatic with exertional chest pains. This prompted a cardiac catheterization, which showed non-obstructive coronary disease with multiple fistulas arising from the first diagonal branch filling the left ventricle directly ( and ). No intervention was planned and the patient was discharged on aspirin 81 mg, high intensity statin (Atorvastatin 40 mg), carvedilol 3.125 mg every 12 hours, lisinopril 5 mg daily, isosorbide dinitrate, and as needed sublingual nitroglycerin. At the 3 month follow-up visit, the patient was asymptomatic with no further episodes of chest pain.
3. Discussion
The majority of CAFs are congenital and not gender specific. Of the congenital fistulas, two major groups are identified: solitary CAFs or coronary artery-left ventricular multiple micro-fistulas (CALVMMFs). Contrary to the CA-LVMMFs, the solitary group may also have an acquired etiology, i.e. due to chest trauma or iatrogenic causes. Twenty percent of people with congenital CAFs have other concomitant cardiac anomalies, such as aortic and pulmonary atresia and patent ductus arteriosus [Citation2].
As per the literature review, the right coronary artery, or its branches, is the site of the fistula in about 55% of cases; the left coronary artery in about 35%, and both coronary arteries in 5%. Over 90% of the fistulas drain into the venous circulation. Small, asymptomatic fistulas have been seen to arise much more commonly from the left coronary system (87%) [Citation4]. Low-pressure structures are the most common sites of drainage of the coronary fistula. These include right-sided chambers, pulmonary artery, superior vena cava, and coronary sinus. Fistulous communication to the left-sided chambers is less frequent (10%) [Citation3].
The size of the fistulas and the difference between the systemic and receiving chamber resistance determines the volume of the shunt. Regardless of these variables, flow moves from the coronary arteries to the lower pressure chambers. Most coronary artery fistulas are small and consequently myocardial blood flow is not compromised and the patient is usually asymptomatic. In some cases, however, coronary artery steal does occur with consequent development of ischemia in myocardial segments perfused by the coronary artery distal to the fistula [Citation5].
Patients with multiple fistulas arising from LAD coronary artery and its branches to the left ventricle usually present with typical or atypical anginal symptoms. Most patients experience the first anginal attack at adulthood despite assumed congenital origin of the fistulas. Symptoms of heart failure are rarely reported in this entity but, when observed, are seen in association with atrial fibrillation or apical hypertrophic cardiomyopathy. In contrast, patient with single fistulas present more commonly with heart failure. The clinical symptoms in such cases have been attributed to coronary steal phenomenon due to shunting of blood via low resistance fistulas. A loud continuous murmur usually located at the lower sternal border can be useful in identifying patients with coronary artery fistula. The clinical diagnosis of LAD to left ventricle (LV) fistulas is difficult because clinical presentation, laboratory and ECG manifestations are non-specific [Citation6].
In general, three types of fistulous communication have been identified in association with this entity: 1) the arterial-luminal type, which is the most common, where a coronary artery enters directly the cardiac chamber; 2) the arterio-sinusoidal type, where communication is through a myocardial sinusoidal network; and 3) the arterio-capillary type, where the arterial vessel drains into the capillaries. The former two forms bypass capillaries for oxygen delivery to the myocardium and may give rise to myocardial ischemia. Since the normal circulation usually impacts a greater resistance to flow than the fistulas, coronary steal may be essential in the pathogenesis of myocardial ischemia [Citation7]. Of note, both the patients mentioned in this case series had multiple arterial-luminal type of CAF.
Coronary angiography remains the gold standard for diagnosis. Angiography helps define the artery of origin, recipient vessel, or chamber and the site of communication. Noninvasive techniques such as transthoracic echocardiography, transesophageal echocardiography and magnetic resonance imaging are becoming increasingly popular for diagnosis and follow-up of CAFs [Citation2]. Combined two-dimensional and pulsed Doppler echocardiography demonstrates a dilated coronary artery, turbulent flow in the fistula and the recipient chamber. Transthoracic color Doppler echocardiography with a high frequency transducer allows multiple coronary arteries – left ventricular micro-fistulas to be visualized [Citation6]. Transesophageal echocardiography is used intraoperatively to identify the precise location of the site of drainage of the fistulas, which could not be accurately revealed with preoperative coronary arteriography. Magnetic resonance imaging and multidetector computed tomography have also become the alternative methods to evaluate the anatomy, flow, and function of CAF [Citation8].
Treatment of LAD to LV fistula is similar to other coronary fistula. It is indicated when the patients are symptomatic with left ventricular volume overload, myocardial ischemia, left ventricular dysfunction or in the presence of a large or increasing left-to-right shunt. The frequency of symptoms and the incidence of complications such as aneurysm formation, infective endocarditis (IE), myocardial infarction (MI), pericardial tamponade, aneurysm formation in the fistulas, and rupture of the aneurysm; increase with age. As per data from available literature, all symptomatic patients should undergo closure of medium or large coronary artery fistulas. Trivial to small (not clinically discernible), asymptomatic fistulas do not require a specific procedure for closure simply because they are detected incidentally. Closure of even small fistulas may be recommended if the patient is at high risk for endocarditis, if longitudinal follow-up is not feasible or prior to invasive cardiac procedure. Additionally, single fistula is more amenable to percutaneous and surgical repair in comparison to multiple fistulas [Citation9]. In our patients, even though they were initially symptomatic with chest pain and had multiple fistulas, it was decided to monitor them on medical therapy as their symptoms improved with sublingual nitroglycerin and they were relatively asymptomatic prior to undergoing cardiac catheterization.
Initial diagnostic catheterization is needed to assess the hemodynamic significance of the fistulas and to provide a detailed anatomy of them, in particular the size, the origin, the course, presence of any stenosis, and the drainage site. This helps to plan the appropriate treatment. Percutaneous embolization is a procedure that has emerged as an alternative to surgical closure in the last two decades [Citation10]. The basic technique of transcatheter closure is to advance a delivery catheter to the most distal portion of the fistula (past any branches that may feed normal myocardium) and place the occluding device in that location Embolization materials include standard coils, controlled-release coils, micro coils (standard, GDC, or IDC) and an Amplatzer® duct occluder. A covered stent may be a good device to close some coronary artery fistulas. Complications of percutaneous closure of the fistula include transient ‘t’ wave changes and transient bundle branch block, and inadvertent migration of the coils due to the high flow in the fistula or using undersized coils. If the remaining fistula is small and hemodynamically insignificant it can be managed with conservative management. Surgical closure with a coronary artery bypass is used most of the times [Citation10,Citation11].
Multiple coronary artery fistulas arising from LAD emptying into the LV are extremely rare and little is known about the anatomic and clinical features, and the hemodynamic consequences of multiple left-to-left-shunts [Citation7]. As per our literature review, we noted only a few case reports of coronary artery fistula between branches of LAD and left ventricle [Citation3,Citation6,Citation12]. We presented two cases of CAFs, both of which were symptomatic but they underwent no intervention as the fistulas are small and multiple, and it was decided that the patients would be followed up closely for any other developing complications.
Conflict of interest
The authors reported no conflict of interest and no funding was received on this work. All authors reviewed and approved the final version of the manuscript. The manuscript has not been published and is not being considered for publication elsewhere in whole or in part in any language.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Vitarelli A1, De Curtis G, Conde Y, et al. Assessment of congenital coronary artery fistulas by transesophageal color Doppler echocardiography. Am J Med. 2002 Aug 1;113(2):127–133.
- Raju MG, Goyal SK, Punnam SR, et al. Coronary artery fistula: A case series with review of the literature. J Cardiol. 2009;53:467—472.
- Papazoglou PD, Mitsibounas D, Nanas JN. Left anterior descending coronary artery–left ventricular fistula presenting as unstable angina and syncope. Int J Cardiol. 2004;96:121–122.
- Gowda RM, Vasavada BC, Khan IA. Coronary artery fistulas: clinical and therapeutic considerations. Int J Cardiol. 2006;107:7–10.
- Gupta NC, Beauvais J. Physiologic assessment of coronary artery fistula. Clin Nucl Med. 1991;16(1):40–42.
- Hong GR, Choi SH, Kang SM, et al. Multiple coronary artery-left ventricular microfistulae in a patient with apical hypertrophic cardiomyopathy: a demonstration by transthoracic color doppler echocardiography. Yonsei Med J. 2003 Aug;44(4):710–714.
- Stierle U, Giannitsis E, Sheikhzadeh A, et al. Myocardial ischemia in generalized coronary artery–left ventricular microfistulae. Int J Cardiol. 1998;63:47–52.
- Iida R, Yamamoto T, Suzuki T, et al. The usefulness of intraoperative transesophagealechocardiography to identify the site of drainage of coronary artery fistula. Anesth Analg. 2005;101:330–331.
- Angelini P. Coronary artery anomalies- Current clinical issues - Definitions, classification, incidence, clinical relevance, and treatment guidelines. Texas Heart Inst J. 2002;29(4):271–278.
- Armsby LR, Keane JF, Sherwood MC, et al. Management of coronary artery fistulae. Patient selection and results of transcatheter closure. J Am Coll Cardiol. 2002 Mar 20;39(6):1026–1032.
- McMahon CJ, Nihill MR, Kovalchin JP, et al. Coronary artery fistula - Management and intermediate-term outcome after transcatheter coil occlusion. Texas Heart Inst J. 2001;28(1):21–25.
- Said SAM, Schiphorst RHM, Derksen R, et al. Coronary-cameral fistulas in adults. World J Cardiol. 2013 Sep 26;5(9):329–336. ISSN 1949-8462.