ABSTRACT
Light chain amyloidosis has very rarely been reported in association with chronic lymphocytic leukemia (CLL). We reported on a 76-years-old female who presented with simultaneous kappa-restricted chronic lymphocytic leukemia (CLL) and a lambda-restricted multiple myeloma with plasma cells causing AL amyloidosis involving the heart. While monoclonal immunoglobulins occasionallyproduced by CLL have previously been implicated in AL amyloidosis, there only a few cases reported of AL amyloidosis resulting from a distinct plasma cell dyscrasia that is not clonally related to the concurrent CLL.
1. Introduction
Chronic lymphocytic leukemia (CLL) is often diagnosed at an early stage by the discovery of lymphocytosis on a routine blood cells count. In CLL, routine serum electrophoresis detects monoclonal Ig in only less than 5% of cases [Citation1], which may cause glomerulonephritis or other paraneoplastic phenomena [Citation2]. Immunoglobulin light chain amyloidosis (AL) is characterized by the progressive deposition of immunoglobulin light chains, leading to organ-wide amyloid fibril deposits and organ dysfunction. Light chain amyloidosis may be under-recognized in association with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
2. Case report
A 76-years-old Caucasian female with CLL (diagnosed five years ago with stable high lymphocyte count) and non-ischemic cardiomyopathy presented with worsening dyspnea on exertion. One year earlier she presented with dyspnea on exertion and mild bilateral lower extremity edema. Her initial EF was 50% by echocardiogram and her left heart catheterization did not show any evidence of coronary artery disease. A month prior to admission she developed progressive dyspnea with exertion and worsening lower extremity edema. At that time she also was found to have an EF of 25% (decreased from 50%) and hyponatremia. Her regimes of diuretics, beta-blocker, and ACEI were further optimized.
On admission, she was found to have borderline blood pressure (103/60 mmHg), and bilateral lower extremity edema. Upon further previous chart review, she had a persistent elevated serum free lambda light chain of about 870–1000 mg/L and faint lambda light chain monoclonal protein on SPEP (54–118 mg/dL) over the last 18 months. At this admission, the white blood cell count was 27.4 × 109/L with 70% lymphocytes (same range as her baseline). The red blood cell count was 3.90 × 1012/L, and the platelet count was 188 × 109/L. She also had worsening hyponatremia (sodium of 123 meq/L, baseline 130–133 meq/L). Creatinine was 1.2 mg/dL (baseline 1.0–1.2) and urine analysis was negative for protein.
SPEP and immunofixation analysis revealed lambda free light chain (Bence Jones) proteinemia and hypogammaglobulinemia involving IgG and IgA. Serum free lambda light chain was 724 mg/L, and kappa/lambda ratio was 0.01, indicating overproduction of lambda light chain. UPEP showed a spike in the beta region, 888.7 mg/24h. EKG was low voltage and CXR revealed bilateral small pleural effusion. Trans-thoracic echocardiogram (TTE) revealed normal left ventricular wall thickness, severe global systolic hypokinesis of the left ventricle, EF 25%, an inter-ventricular diastolic septal thickness of 8.0 mm, moderate right atria dilation and moderate low gradient aortic stenosis.
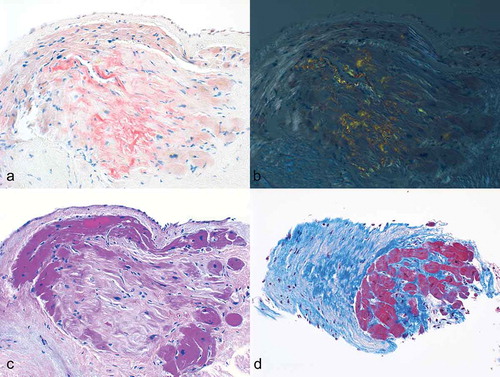
A right heart catheterization was performed revealing elevated right and left heart filling pressures (RA17 mmHg, PA 55/28 mmHg (mean 38 mmHg), PCW 28 mmHg). Cardiac index was low at 1.53 L/min/m2. Endo-myocardial biopsy showed focal patchy infiltration of the myocardium and fibrous tissue with Congo red stain positive for amyloid ().
Figure 1. Deposition of amyloid in the myocardium. a–b The amyloid deposits display characteristic apple-green birefringence by polarized light microscopy (Congo red stain 100X). c (hematoxylin-eosin stain 100X) and d (Masson trichrome stain 100X) shows the amyloid deposit in the myocardium.
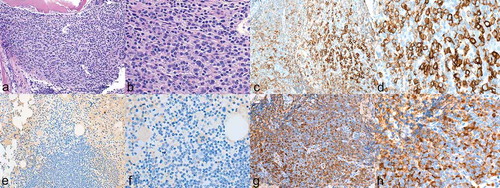
Bone marrow biopsy revealed hypercellular marrow that was replaced by nodules of small lymphocytes and patchy sheets of plasma cells. Plasma cells comprise approximately 50% of the cellularity. Immunohistochemistry stains highlighted the plasma cells that were lambda light chain-restricted. Flow cytometry showed 63% monoclonal B-cells that express CD5 and kappa light chain-restricted as well as clonal plasma cells that express lambda light chain, demonstrating that she had two separate clones. A Congo red stain did not show definitive amyloid deposition ().
Figure 2. a–b Bone marrow with dense aggregates of plasma cells consistent with multiple myeloma. (a hematoxylin-eosin stain 100X; b 200X). c–d Immuno-histochemical staining of CD138 positive plasma cells comprising 50% of marrow cellularity. CD138 (syndecan-1) was found to be a specific marker for plasma cells. (c 100X; d 200X).e–f Stain for kappa light chain was negative (e 100X; f 200X). g–h stain for lambda light chain was positive (g 100X; h 200X).
Patient’s home medications of beta-blocker and ACE inhibitor were discontinued. She was treated with furosemide intravenously and her weight decreased to 48.4 kg from 50.5 kg. Patient was maintained on furosemide and spironolactone with plans for chemotherapy. However, she was readmitted for refractory heart failure symptoms requiring inotropic support. Ultimately, she chose a palliative approach.
3. Discussion
Immunoglobulin light chain (AL) amyloidosis is a systemic disorder characterized by widespread deposition of amyloid fibrils derived from monoclonal immunoglobulin light chains in organs and soft tissues. AL is typically caused by an underlying plasma cell clone disorder and occurs in 6–15% of patients with multiple myeloma [Citation3]. Both light chain amyloidosis (AL) and non-AL amyloidosis have rarely been reported in association with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) [Citation4].
In CLL, monoclonal light chains are detected in the serum, and the surfaces of lymphocytes and glomeruli are predominantly kappa type, which is much less predisposed to form the β-pleated sheet structure. Lambda light chains are more amyloidogenic than kappa light chains, resulting in lambda AL being twice as common as kappa AL [Citation5]. Serum free light chain (FLC) levels are usually low in CLL patients. The predominant kappa presentation and low level of FLC explain why there are up to one-third of CLL patients who have abnormal serum FLC ratios, but rarely have reported systemic amyloidosis [Citation6].
Another known observation is that in CLL/SLL lymph nodes and bone marrow, polyclonal B-cells share the same micro-environmental interactions as CLL tumor cells [Citation6]. CLL cells producing clonally restricted FLCs coexist with lymphocytes expressing non-clonal FLCs in lymph nodes and bone marrow [Citation6]. This ongoing B-cell activation, which is demonstrated by the overproduction of polyclonal FLC, indicates the pathogenesis of several inflammatory and autoimmune conditions, which are associated with an increased risk of malignancy transformation [Citation7]. CLL may function as a cofactor in the neighborhood of B-cells generating light chains and predisposing for transformation to multiple myeloma. The Mayo clinic case series study revealed that in CLL patients, AL amyloidosis is more commonly caused by a coexisting plasma cell clone (14 cases out of 18 cases of AL). Among these 14 patients, six had both a plasma cell clone and a CLL clone that shared the same light chain. These cases of AL amyloidosis were almost equally divided between kappa and lambda restriction [Citation4].
For amyloidosis, the most commonly affected organs that result in symptoms are the heart, kidney, skin, peripheral nerves and liver. The most common presentations of heart are normal ejection fraction with diastolic dysfunction, and left ventricular hypertrophy with low voltage EKG [Citation5].
Evaluation of cardiac involvement is very important since the major determinant of outcome in amyloidosis is the extent of cardiac involvement. Cardiac biomarkers, such as natriuretic peptides, particularly N-terminal pro-B-type natriuretic peptide (NT-pro BNP) and cardiac troponin-T, play a role for the assessment of prognosis in AL amyloidosis [Citation8,Citation9].
Echocardiography is one of the main diagnostic modalities used in patients with suspected cardiomyopathy, including cardiac involvement in AL amyloidosis. Recent studies showed that tissue Doppler imaging (TDI)-derived myocardial systolic strain is associated with overall prognosis in AL amyloidosis. Increased left ventricular (LV) wall thickness and decreased fractional shortening, a granular sparkling appearance, longitudinal left ventricular (LV) function, diastolic relaxation abnormalities and right ventricular dysfunction with valvular thickening have all been shown to be associated with prognosis [Citation10–Citation12].
4. Conclusion
This unusual patient presented with clonally unrelated CLL and multiple myeloma plasma cells, the latter causing AL amyloidosis. It becomes challenging to diagnose multiple myeloma and other plasma cell disorders in the setting of CLL and other chronic leukemias. Clinicians must pay attention to subtle clinical and laboratory abnormalities to support further diagnostic testing.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med 2005;352:804–815.
- Moulin B, Ronco PM, Mougenot B, et al. Glomerulonephritis in chronic lymphocytic leukemia and related B-cell lymphomas. Kidney Int. 1992;42:127–135.
- Friman C, Pettersson T. Amyloidosis. Curr Opin Rheumatol. 1996;8:62–71.
- Kourelis TV, Gertz M, Zent C, et al. Systemic amyloidosis associated with chronic lymphocytic leukemia/small lymphocytic lymphoma. Am J Hematol. 2013;88:375–378.
- Dispenzieri A, Gertz MA, Buadi F. What do I need to know about immunoglobulin light chain (AL) amyloidosis? Blood Rev. 2012;26:137–154.
- Morabito F, De Filippi R, Laurenti L, et al. The cumulative amount of serum- free light chain is a strong prognosticator in chronic lymphocytic leukemia. Blood. 2011;118:6353–6361.
- Gottenberg JE, Aucouturier F, Goetz J, et al. Serum immunoglobulin free light chain assessment in rheumatoid arthritis and primary Sjogren’s syndrome. Ann Rheum Dis. 2007;66(1):23–27.
- Kristen AV, Giannitsis E, Lehrke S, et al. Assessment of disease severity and outcome in patients with systemic light-chain amyloidosis by the high-sensitivity troponin T assay. Blood. 2010;116(14):2455–2461.
- Palladini G, Campana C, Klersy C, et al. Serum N-terminal pro-brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation. 2003;107(19):2440–2445.
- Klein AL, Hatle LK, Taliercio CP, et al. Serial Doppler echocardiographic follow-up of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1990;16(5):1135–1141.
- Buss SJ, Emami M, Mereles D, et al. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical and biochemical markers. J Am Coll Cardiol. 2012;60(12):1067–1076.
- Koyama J, Falk RH. Prognostic significance of strain Doppler imaging in light- chain amyloidosis. JACC Cardiovasc Imaging. 2010;3:333–342.
