ABSTRACT
Group B streptococcus infections (GBSI) are commonly associated with neonates and pregnant women, but may also affect nonpregnant adults. Among its spectrum of manifestations, perinephric abscess (PA) is exceedingly rare. Comorbid conditions such as diabetes mellitus (DM) and immunosuppression increase the risk of GBSI. We describe a 61-year-old Vietnamese man with compensated alcoholic cirrhosis, who presented with acute encephalopathy following subacute, progressive abdominal pain. He was afebrile and hemodynamically stable. Laboratory data were remarkable for leukocytosis, thrombocytopenia, azotemia, and pyuria. He was found to have two right-sided PA measuring 15 × 10 × 11 cm and 4.6 × 2.7 × 7.8 cm, requiring interval placement of multiple percutaneous drains. Culture from abscesses revealed beta-hemolytic Group B streptococcus (GBS). His course was complicated by contiguous spread to abdominal wall and paraspinal musculature, as well as a new diagnosis of type 2 DM. Along with drainage, a prolonged course of intravenous antimicrobial treatment led to abscess resolution. Given the rising number of unusual clinical presentations of GBSI, this bacteria should be considered as a part of the microbiological differential diagnosis of PA, especially in conditions leading to immunosuppression.
1. Introduction
Group B streptococcus (GBS) or Streptococcus agalactiae is a facultative, gram-positive bacteria that predominantly causes disease in neonates and pregnant women. The incidence among nonpregnant adults has been concernedly increasing over the past two decades, now resulting in more than 66% of cases of invasive Group B streptococcus infections (GBSI) [Citation1]. The relative incidence has increased to 48% in adults less than 65 years of age and 20% in those above [Citation2]. The reason for this increment has not yet been elucidated but the presence of chronic morbid conditions and increased age has been implicated [Citation3].
The clinical spectrum of GBSI is broad and includes involvement of the skin and soft tissue, respiratory tract, bone and joints, cardiac and central nervous systems, and the urinary tract. Urinary tract disease comprises of 5–15% of cases, including acute cystitis, pyelonephritis, asymptomatic bacteriuria, and urosepsis [Citation4,Citation5]. However, only a few cases of GBS intra-abdominal abscesses have been published [Citation3,Citation6–Citation12].
2. Case report
A 62-year-old Vietnamese man with compensated alcoholic cirrhosis, psoriasis, and gastroesophageal reflux disease presented with abdominal pain. He described a 2-week history of insidious, intermittent, dull right lower quadrant abdominal pain with radiation to ipsilateral flank. Decreased appetite, constipation, and partial adherence to lactulose were also reported. He was afebrile and hemodynamically stable. Physical examination was remarkable for cachexia, hypovolemia, and right lower quadrant abdominal and right costovertebral angle tenderness. Digital rectal examination was negative for stool content. Laboratory data showed leukocytosis (11.4 [4.4–10.7 × 10E9 cells/L]) with neutrophilia (75% [44–73%]), azotemia (blood urea nitrogen 22 [7–21 mg/dL], creatinine 1.3 [0.5–1.3 mg/dL]), hypokalemia (3 [3.5–5.1 mmol/L]), hyperglycemia (211 [74–106 mg/dL]), and pyuria (WBC 20–50 [0–2 cells/HPF]). Abdominal X-ray showed retained stool. After potassium replacement in the emergency department, outpatient follow-up was recommended.
Eleven days later, he was brought in due to lethargy, nausea, non-bilious non-bloody emesis, anorexia, and progressive diffuse abdominal pain. He remained afebrile with stable vitals. Physical examination was remarkable for encephalopathy, worsened cachexia, and excruciating right-sided abdominal and flank tenderness. Laboratory data showed leukocytosis (10.8 [4.4–10.7 × 10E9 cells/L]) with bandemia (17% [0–11%]), mild thrombocytopenia (130 [153-416 × 10E9 cells/L]), azotemia (BUN 26 [7–21 mg/dL], creatinine 1.2 [0.5–1.3 mg/dL]), hyperkalemia (5.6 [3.5–5.1 mmol/L]), anion gap metabolic acidosis (16 [22–31 mmol/L]), hyperlactatemia (3.8 [0.7–2.1 mmol/L]), hypoalbuminemia (1.7 [3.4–5 g/dL]) with low prealbumin (<3.0 [20–40 mg/dL]), hyperammonemia (88 [<34 µmol/L]), and hyperglycemia (273 [74–106 mg/dL]). Hemoglobin A1c was elevated (10.8 [4.2–6.3%]), establishing a new diagnosis of diabetes mellitus (DM). Urinalysis showed persistent pyuria (WBC 20–50 [0–2 cells/HPF]). Prior urine culture was consistent with urogenital flora (<10,000 colony forming unit/mL).
Computerized tomography (CT) of abdomen and pelvis with contrast revealed a massive multiloculated right perinephric abscess (PA) arising from the lower pole and invading adjacent abdominal wall, measuring 15 × 10 × 11 cm. A second PA arising from the upper pole of the right kidney was also disclosed, measuring 4.6 × 2.7 × 7.8 cm (). Intravenous vancomycin and piperacillin-tazobactam were initiated. A 12-French pigtail drainage catheter was placed into the first PA and a size 10-French was placed into the second. Partial defervescence ensued. The purulent output sent for culture grew GBS.
Figure 1. CT of abdomen and pelvis with intravenous contrast revealing right-sided PA with multiloculation and contiguous spread, as shown by the arrows in the coronal (left) and sagittal (right) sections.
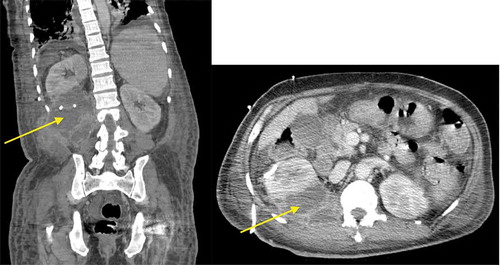
Repeat urine culture remained consistent with urogenital flora (<10,000 CFU/mL). Blood culture was negative. Repeat CT showed a persistent multiloculated PA along the posteroinferior aspect of the right kidney. Paraspinal musculature involvement was seen, but magnetic resonance imaging of lumbar spine was without evidence of bone involvement. A third 12-French pigtail drainage catheter was inserted. Antimicrobial treatment was deescalated to intravenous ceftriaxone. A 4-week course leads to complete clinical recovery.
3. Discussion
Clinical manifestations of GBSI are diverse. Commonly associated conditions are DM, cirrhosis, immunosuppression, cerebrovascular accident, breast cancer, decubitus ulceration, and neurogenic bladder [Citation13–Citation18]. One study involving 19,512 patients revealed the presence of at least one risk factor in 88% of patients with GBSI [Citation19]. Risk factors also seem to vary between younger and older adults (≥65 years). DM, malignancy, and skin disease were commonly encountered in younger adults, whereas cardiac disease, bedridden state, and nursing home residence were more frequently seen in older patients [Citation20]. In contrast, nephrolithiasis and prior urologic surgery were strong independent risk factors for retroperitoneal abscesses (RPAs), according to one 26-year retrospective study [Citation21]. Among invasive GBSI, PA/RPA has been much less described ().
Table 1. Case reports of GBS intra-abdominal abscesses.
The treatment of PA involves antimicrobial therapy and surgical drainage. For small abscesses (size ≤3 cm), successful medical treatment has been reported in a few cases [Citation22,Citation23]. Abscess size, obstructive uropathy, severe vesicoureteral reflux, gas-producing bacteria, DM, and old age have been associated with poor response to medical treatment [Citation24,Citation25]. In one study comprising of 65 patients, medical treatment alone was performed in only 9% and associated mortality rate reached 33% [Citation26]. Multiple and multiloculated abscesses should undergo open surgical drainage [Citation27]. However, successful percutaneous and laparoscopic-assisted drainage in multiloculated abscesses have been reported [Citation11,Citation12]. As a mere diagnostic tool, drainage appears to be fundamental in identifying GBS as the offending microorganism since urine and blood cultures show positive growth in less than half the cases [Citation26]. Based on two retrospective studies, antibacterial drug selection has been stable over the years as GBS has remained susceptible to all penicillins and cephalosporins. Nonetheless, resistance to clindamycin has been increasing, up to 23% in one study [Citation19,Citation28–Citation30].
Preventive strategies for GBSI in nonpregnant adults are evolving. Oral hygiene may potentially reduce invasive GBSI. GBS has been detected in dental plaques at a higher rate in hospitalized and bedridden patients with swallowing or speech disorders [Citation31]. Another study showed reduction of GBS health-care-associated pneumonia in patients undergoing daily oral hygiene [Citation32]. Moreover, after promising Phase I trial, GBS conjugate vaccination in high-risk older adults may be possible [Citation33].
Our case report is relevant for multiple reasons. It reinforces the association, though not well understood, between GBSI and DM. Abnormalities in phagocyte function and decreased intracellular killing have been identified in patients with DM [Citation34,Citation35]. Yet, when testing the function of neutrophils against type III GBS in vitro, an intrinsic defect in phagocytosis and killing was not observed [Citation36]. Cirrhosis also plays a significant role in our case via its associated immunosuppression, but the pathogenesis again remains speculative [Citation3]. Secondly, invasive GBSI may be race-dependent. Regardless of age, its incidence is higher in African–Americans than Caucasians (ratio of 2:1). A study with one of the highest burden of reported GBSI showed a more common occurrence in aboriginal Indian Ocean than Black descent [Citation37]. The relative prevalence of GBSI in Asians compared to other races has not been described. In one recent Chinese study of 98 patients with PA, GBS was indeed not isolated [Citation38]. Thirdly, we report successful use of percutaneous drainage to treat large multiloculated PA. This minimally invasive approach decreases morbidity associated with open surgical drainage, although some patients may still need more invasive treatment [Citation12,Citation39]. Finally, this case illustrates the destructive nature of GBSI, which is more recognized in other beta-hemolytic streptococcal infections, especially Group A.
Our case report is not without limitations. GBS serotyping was not obtained, as further molecular testing would have been meaningful. One study consisting of nonpregnant pediatric and adult patients found serotype V to be the most predominant (31%), followed by serotypes Ia (24%), II (12%), and III (12%) [Citation2]. Older adults with GBS carriage are more likely to harbor serotype V [Citation40]. GBS colonization rate in adults is estimated to be 22%. In our patient, rectal specimens were not collected. In fact, recurrent episodes of GBSI have been reported in up to 4% of carriers where the interval between episodes was 43 weeks on average [Citation41].
4. Conclusion
GBSI impose significant morbidity and mortality in children and pregnant adults. In older adults, the estimated incidence has increased. Urinary GBSI, including PA, is rare. Immunosuppression related with DM and cirrhosis has been identified as an important contributing factor. The diagnostic modality of choice is culture of the abscess itself, as urine and blood cultures may remain negative. Along with administration of antimicrobials, successful treatment can be achieved using percutaneous drainage, avoiding open-laparotomy-related complications.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Farley MM, Strasbaugh LJ. Group B streptococcal disease in nonpregnant adults. Clin Infect Dis. 2001;33(4):556–561.
- Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the USA, 1999-2005. Jama. 2008;299(17):2056–2065.
- Crum-Cianflone NF. An unusual case of a large, sporadic intra-abdominal abscess due to group B streptococcus and a review of the literature. Infection. 2015;43(2):223–227.
- Farley MM, Harvey C, Stull T, et al. A population-based assessment of invasive disease due to group B streptococcus in nonpregnant adults. N Engl J Med. 1993;328:1807–1811.
- Schwartz B, Schuchat A, Oxtoby MJ, et al. Invasive group B streptococcal disease in adults a population-based study in metropolitan Atlanta. Jama. 1991;266(8):1112–1114.
- Ulett KB, Shuemaker JH, Benjamin WH Jr, et al. Group B streptococcus cystitis presenting in a diabetic patient with a massive abdominopelvic abscess: a case report. J Med Case Rep. 2012;6:237–241.
- Tyan P, Abi-Khalil E, Dwarki K, et al. First described case of group B streptococcus pelvic abscess in a patient with no medical comorbidities. Case Rep Obstet Gynecol. 2016;2016:1–4.
- Baumgardner DJ. Perinephric abscess caused by group B streptococcus. Am Fam Physician. 2004;69(12):2764–2766.
- Santoro-Lopes G, Halpem M, Goncalves RT. Perinephric abscess caused by streptococcus agalactiae after renal transplantation. J Infection. 2005;51(3):145–147.
- Jernelius H, Tollig H. Renal abscess caused by streptococcus group B (Article in Swedish). Lakartidningen. 1982;79(42):3832.
- Lawlor BT, Orenstein SB, Sardella WV. Laparoscopic-assisted drainage of a massive retroperitoneal abscess caused by group B streptococcus. Surg Infect (Larchmt). 2015;16(1):110–111.
- Ishizu K, Yamaguchi S, Naito K. A case of multiloculated retroperitoneal abscess successfully treated by percutaneous drainage with a Malecot catheter. Hinyokika Kiyo. 1999;45(2):103–105.
- Jackson LA, Hilsdon R, Farley MM, et al. Risk factors for group B streptococcal disease in adults. Ann Intern Med. 1995;123:415–420.
- Ko MC, Chiu AW, Liu CC, et al. Effect of diabetes on mortality and length of hospital stay in patients with renal or perinephric abscess. Clinics (Sao Paulo). 2013;68(8):1109–1114.
- Ko MC, Liu CC, Liu CK, et al. Incidence of renal and perinephric abscess in diabetic patients: a population-based national study. Epidemiol Infect. 2011;139(2):229–235.
- Sunkara B, Bheemereddy S, Lorber B, et al. Group B streptococcus infections in non-pregnant adults: the role of immunosuppression. Int J Infect Dis. 2012;16(3):182–186.
- Cho SY, Kang CL, Kim J, et al. Association of liver cirrhosis with group B streptococcal bacteremia in non-pregnant adults. J Infec. 2013;67(6):617–619.
- Kortsalioudaki C, Breathnach A, Heath PT. Invasive group b streptococcal (GBS) infection in London adults. J of Infec. 2008;57(6):425.
- Skoff TH, Farley MM, Petit S, et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin Infect Dis. 2009;49:85–92.
- Tyrrell GJ, Senzilet LD, Spika JS, et al. Invasive disease due to group B streptococcal infection in adults: results from a Canadian, population-based, active laboratory surveillance study 1996. J Infect Dis. 2000;182:168–173.
- Manjon CC, Sanchez AT, Lara JDP, et al. Retroperitoneal abscesses-analysis of a series of 66 cases. Scand J Urol Nephrol. 2003;37(2):139–144.
- Dalla Palma L, Pozzi-Mucelli F, Ene V. Medical treatment of renal and perirenal abscesses: CT evaluation. Clin Radiol. 1999;54(12):792–797.
- Siegel JF, Smith A, Moldwin R. Minimally invasive treatment of renal abscess. J Urol. 1996;155(1):52–55.
- Fulla J, Storme O, Fica A, et al. Renal and perinephric abscesses: a series of 44 cases. Rev Chilena Infectol. 2009;26(5):445–451.
- Yen DH, Hu HC, Tsai J, et al. Renal abscess: early diagnosis and treatment. Am J Emerg Med. 1999;17:192–197.
- Ferreira-Coelho R, Schneider-Monteiro ED, Borges MJL, et al. Renal and perinephric abscesses: analysis of 65 consecutive cases. World J Surg. 2007;31:431–436.
- El-Nahas AR, Faisal R, Mohsen T, et al. What is the best drainage method for a perinephric abscess? Int Braz Urol. 2010;36(1):29–37.
- Murdoch DR, Reller LB. Antimicrobial susceptibilities of group B streptococci isolated from patients with invasive disease: 10-year perspective. Antimicrob Agents Chemother. 2001;45:3623–3624.
- Björnsdóttir ES, Martins ER, Elendsdóttir H, et al. Changing epidemiology of group B streptococcal infections among adults in Iceland: 1975–2014. Clin Microbiol Infect. 2016;22(4):379.e9-e16.
- Tazi A, Morand PC, Reglier-Poupet H, et al. Invasive group B streptococcal infections in adults, France (2007-2010). Clin Microbiol Infect. 2011;17:1587–1589.
- Tada A, Shiiba M, Yokoe H, et al. Relationship between oral motor dysfunction and oral bacteria in bedridden elderly. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2204;98(2):184–188.
- El-Solh AA, Pietrantoni C, Bhat A, et al. Colonization of dental plaques: a reservoir of respiratory pathogens for hospital-acquired pneumonia in institutionalized elders. Chest. 2004;126:1575–1582.
- Palazzi DL, Rench MA, Edwards MS, et al. Use of type V group B streptococcal conjugate vaccine in adults 65–85 years old. J Infect Dis. 2004;190:558–564.
- Rajagopalan S. Serious infections in elderly patients with diabetes mellitus. Clin Infect Dis. 2005;40:990–996.
- Harris MI, Hadden WC, Knowler WC, et al. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20-74yr. Diabetes. 1987;36(4):523–534.
- Mazade MA, Edwards MS. Impairment of type III group B streptococcus–stimulated superoxide production and opsonophagocytosis by neutrophils in diabetes. Mol Genet Metab. 2001;73:259–267.
- Camuset G, Picot S, Jaubert J, et al. Invasive group B streptococcal disease in non-pregnant adults, Reunion Island, 2011. Int J Infec Dis. 2015;35:46–50.
- Liu XQ, Wang CC, Liu YB, et al. Renal and perinephric abscesses in West China Hospital: 10-year retrospective-descriptive study. World J Nephrol. 2016;5(1):108–114.
- Meng MV, Mario LA, McAninch JW. Current treatment and outcomes of perinephric abscesses. J Urol. 2002;168(4):1337–1340.
- Edwards MS, Rench MA, Palazzi DL, et al. Group B streptococcal colonization and serotype-specific immunity in healthy elderly persons. Clin Infect Dis. 2005;40(3):352–357.
- Harrison LH, Ali A, Dwyer DM, et al. Relapsing invasive group B streptococcal infection in adults. Ann Intern Med. 1995;123:421–427.
