ABSTRACT
This article aims at raising clinical awareness about pyoderma gangrenosum especially when presenting in primary care settings. Due to its initial manifestation as a nonspecific ulcer, physicians with relatively less dermatology experience usually misdiagnose PG as cutaneous infection or vascular disease. This usually leads to inappropriate treatment with subsequent worsening of condition and devastating effects on patients’ lives.
1. Introduction
‘Pyoderma Gangrenosum’ (PG) originated as a term in 1930 when incorrectly described as a purulent ‘streptococcal’ skin infection (‘Pyoderma’) leading to necrosis of tissue (‘Gangrenosum’) [Citation1]. This description was later disapproved as it is proven to be a noninfectious condition. However, the use of the misnomer ‘Pyoderma Gangrenosum’ has persisted in medical literature due to its classical manifestation as single or multiple painful ulcers usually seen on the lower extremities. In its real essence, PG is a chronic inflammatory form of neutrophilic dermatosis characterized by accumulation of neutrophils in the skin with rare involvement of the internal organs. It is estimated to occur in every 3–10 individuals/million and is usually seen in middle age with women being more affected. Childhood PG can be seen in up to 4% of total cases [Citation2].
2. Pathophysiology
Although determined to have a noninfectious background, the exact mechanism involved in PG is still not understood. It is mainly postulated to occur due to inflammatory cytokines production such as TNF-α, IL6 and IL8 with subsequent neutrophilic dysfunction and chemotaxis leading to recurrent sterile inflammation in the skin and associated organs [Citation3]. This theory is also supported by the fact that approximately 50% of cases of PG occur in the context of other systemic inflammatory diseases (). Various genetic factors are also considered to play a role in the development of this disorder especially in the setting of various syndromes such as PAPA (Pyogenic Arthritis, PG and Acne) and PASH (PG, Acne and Suppurative Hidradenitis).
3. Clinical presentation
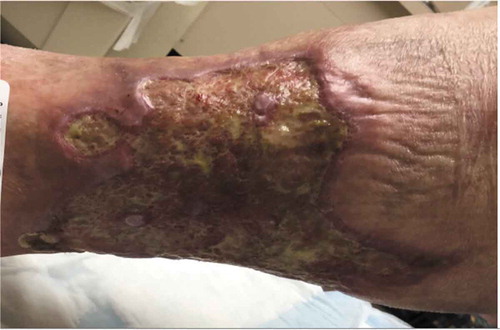
PG presents as a painful pustule or papule on lower extremities which rapidly progresses to an extremely painful enlarging ulcer. The ulcer is classically described as a centrifugally creeping lesion with central oozing, undermined violaceous necrotic borders and surrounding raised erythematous region. Associated constitutional symptoms include fever, malaise, weight loss and myalgias. Healing of the ulcer leads to the formation of an atrophic cribriform scar and considerable disfiguration.
The pretibial region is most commonly affected () although lesions can also occur on hands, abdomen, head/neck and genital region. In atypical cases, there can be involvement of peristomal skin, surgical sites and internal organs such as kidney and lungs.
The aforementioned features are seen in classical ulcerative PG. However, there are three additional clinical variants as described next [Citation3]:
Bullous PG is seen in various lymphoproliferative and hematological disorders. Patients present with grouped vesicles on arms, hands or head which coalesce to form large bullae with subsequent ulceration.
Pustular PG is characterized by multiple painful pustules with surrounding erythema seen on trunk and extensor surface of limbs. This variant is commonly associated with Inflammatory bowel disease.
Vegetative PG is characterized by a solitary superficial plaque present on head or neck region with lack of classical violaceous border. This variant of PG is usually uncommon and less frequently associated with a systemic disease.
4. Diagnosis
Diagnosing PG is usually a challenge especially in non-dermatological primary care setting. This is because many other conditions can mimic PG leading to initial misdiagnosis and subsequent unsuccessful treatment ().
In the absence of a definitive test, it is recommended that PG should be diagnosed based on clinical criteria (). For this purpose, two major along with at least two minor criteria need to be fulfilled.
Table 3. Proposed diagnostic criteria for pyoderma gangrenosum [Citation5].
This necessitates that a physician should start off with a detailed history to look for significant clues such as initial lesion as a papule/vesicle, its rapid progression, pain, any underlying systemic disorder and symptoms associated with pathergy. Pathergy is a hallmark phenomenon seen in up to 30% of patients with PG when the lesions develop or worsen after any kind of trauma in form of a surgery or debridement. Initial history and physical examination should be followed by complete work up including urine examination, immunological testing, gammopathy screening and skin biopsy.
Skin biopsy is an important part of the diagnostic process as it helps to exclude other common ulcerative diseases such as infection. Histopathology of the ulcer usually demonstrates epidermal/dermal necrosis with neutrophilic infiltrates, micro-abscesses and leukocytoclasia. Vasculitic changes and giant-cell formation can also be seen [Citation6]. However, it should be kept in mind that none of these pathological findings are pathognomonic and depend on the temporal course of the disease at the time of skin biopsy.
5. Treatment
Management of a patient with PG includes wound care, topical/systemic immunosuppression while treating any underlying disorder [Citation7]. This therapy carries quite high risk thus making it imperative that a correct diagnosis is made before initiation of therapy.
Optimized wound care includes routine wound cleansing and moist sterile dressings along with pain control and monitoring for signs of secondary wound infection. Occlusive dressings, compression stockings and surgical debridement should be avoided as these interventions can cause worsening of the ulcers secondary to pathergy. Skin grafting and hyperbaric oxygen therapy can be attempted in recalcitrant ulcers although no definite data still exists [Citation8].
For mild or localized disease, topical corticosteroids and calcineurin-inhibitors such as cyclosporine/tacrolimus are used. In case of severe and more generalized disease, first-line therapy includes systemic corticosteroids while steroid-sparing agents such as cyclophosphamide, cyclosporine, azathioprine and methotrexate can be used after remission is achieved. For refractory cases, combination therapy comprising of two or more of the previously mentioned drugs is used while biological agents such as Adalimumab have also been shown to have a good response. These interventions can help treat an associated auto-inflammatory disease such as IBD. However, immunosuppression needs to be at a minimum in cases of malignancy. Although PG can present at any time during the course of these disorders, treating them leads to better control of PG itself.
6. Conclusion
Pyoderma gangrenosum may be a diagnosis of exclusion but as primary care physician, we need to be aware about its presentation as an ulcerative condition with a wide differential diagnosis. This fact necessitates appropriate clinical workup with a multidisciplinary approach toward management of the disease leading to better outcomes and improved quality of life.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Farhi D. The clinical and histopathological description of geometric phagedenism (pyoderma gangrenosum) by Louis Brocq one century ago. Arch Dermatol. 2008;144(6):755.
- Wollina U. Pyoderma gangrenosum - a review. Orphanet J Rare Dis. 2007;2:19.
- Gameiro A, Pereira N, Cardoso JC, et al. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol. 2015;8:285–293.
- Weenig RH, Davis MD, Dahl PR, et al. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347(18):1412.
- Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43(11):790–800.
- Ruocco E, Sangiuliano S, Gravina AG, et al. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23(9):1008–1017.
- Wu XR, Shen B. Diagnosis and management of parastomal pyoderma gangrenosum. Gastroenterol Rep (Oxf). 2013;1(1):1–8.
- Davis JC, Landeen JM, Levine RA. Pyoderma gangrenosum: skin grafting after preparation with hyperbaric oxygen. Plast Reconstr Surg. 1987;79(2):200.