ABSTRACT
Gastrointestinal stromal tumors (GISTs) are rare and current estimates range from 4,000 to 6,000 number of GIST cases in the USA annually. Imatinib, a tyrosine kinase inhibitor, has shown a survival benefit in GISTs, and the presence of KIT mutation status is predictive of response. The current case discusses rapidly progressive dyspnea and heart failure in an elderly male with metastatic GIST who was started on imatinib. Although reported as a rare and sporadic side effect of imatinib, the current case illustrates rapidity and the clinical significance of cardiotoxicity, with onset at 2 weeks.
Cases of imatinib-induced cardiotoxicity can range from being mild ventricular dysfunction to overt heart failure. Prior to starting imatinib, our patient had a history of hypertension. He subsequently ended up developing heart failure as acknowledged by the echocardiogram (ECHO). In general, elderly with preexisting cardiovascular comorbidity are at greater risk. The goal in such situations is immediate discontinuation or reduction of the imatinib dosage. The case prompts for awareness of imatinib cardiotoxicity. Moreover, a pretreatment cardiac assessment along with monitoring throughout therapy is therefore advisable. Also, imatinib-induced cardiotoxicity should be differentiated from imatinib-associated fluid retention, in which ECHO findings can be normal. This case report raises the concern for accelerated cardiotoxicity profile of imatinib. Further prospective studies with multidisciplinary input are needed to establish this association further.
1. Introduction
Imatinib, a tyrosine kinase inhibitor, has shown survival benefit in gastrointestinal stromal tumors (GISTs), and the presence of KIT mutation status is predictive of response. Toxicities of imatinib include fluid retention, diarrhea, nausea, fatigue, muscle cramps, abdominal pain, and rash. Congestive heart failure symptoms are seen in about 8.2% of the patients treated with imatinib for GISTs. However, few patients exhibit objective left ventricular dysfunction by echocardiography [Citation1].
We present a case of rapidly progressive dyspnea and heart failure in an elderly male with metastatic GIST who was started on imatinib. Baseline ejection fraction was normal. Although reported as a rare and sporadic side effect of imatinib, the current case illustrates the rapidity and clinical significance of very rapid progression to heart failure, with onset at 2 weeks.
2. Case
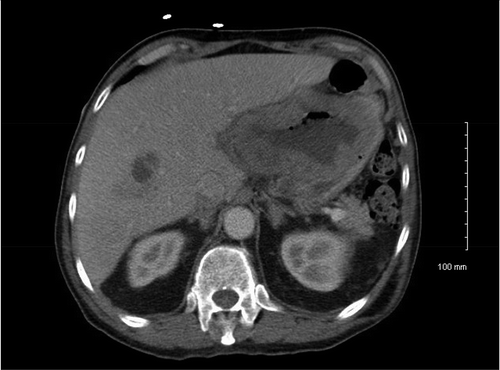
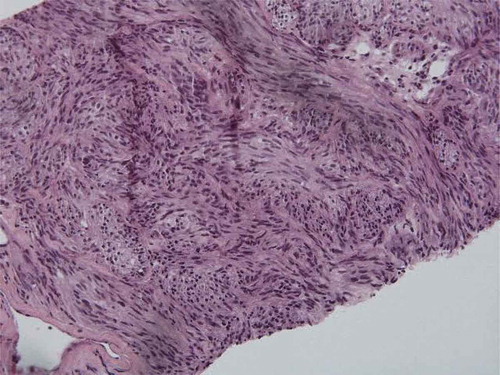
A 73-year-old African-American male with a history of benign hypertension presented with a 3-day history of dyspnea, cough, and fever. He also complained of weight loss, malaise, cough, and fatigue. During admission, imaging showed prostatic hyperplasia, midline retroperitoneal adenopathy, multifocal hypodense hepatic lesions, periportal adenopathy, and gastric wall thickening on abdominal and pelvic CT scan (). He underwent esophago-gastro-duodenoscopy and biopsy of a large antral mass, which returned negative for malignancy. Diagnostic laparoscopy with triplicate core biopsies returned positive for GIST with spindle cell type differentiation (). The patient was started on neoadjuvant imatinib 400 mg daily to reduce tumor size prior to possible surgical resection. Colonoscopy also revealed pan diverticulosis and a proximal rectal polyp, which was tubulovillous adenoma and superficial adenocarcinoma on pathological report.
Within 2 weeks of imatinib initiation, progressive dyspnea was noted. After 1 month, he returned to the emergency room. Physical exam revealed pulse, 108 bpm; respiratory rate, 24 breaths per minute; temperature, 98.5 F; and oxygen saturation, 100% on 6 l per minute of oxygen. There was no home oxygen requirement prior to presentation.
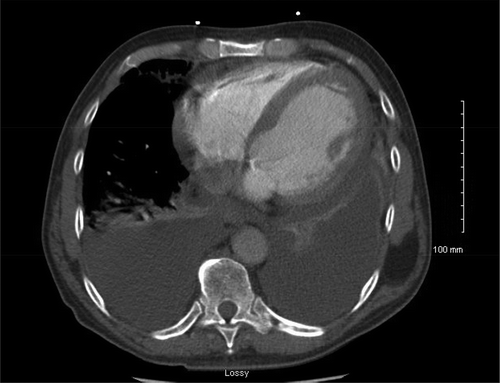
Chest X-ray showed bilateral pleural effusions, left greater than right, with atelectasis. Due to concern for pulmonary embolus, chest CT scan showed pericardial effusion and bilateral pleural effusions, worse on the left lung base (). Diagnostic thoracentesis and pleural fluid analysis resulted transudate and negative for malignant cells per cytology, favoring congestive heart failure diagnosis.
Figure 3. CT chest showing bilateral pleural effusions and moderate pericardial effusion with dilated cardiomyopathy.
Cardiology was consulted, and echocardiography demonstrated dilated left ventricle, moderate tamponade, stage 2 diastolic dysfunction, moderate pulmonary hypertension, and severe global hypokinesis, with an ejection fraction of 25–30%. Prior pretreatment echocardiography showed an ejection fraction of greater than 55% and normal left ventricle. Discussion with oncology and cardiology suggested new-onset systolic heart failure secondary to imatinib, which was discontinued. The patient was discharged on metoprolol, furosemide, and spironolactone. After 1 month, the patient was referred to another tertiary care facility and rechallenged with 100 mg of imatinib daily. Gradual escalation to full dose of 400 mg was done over 4 months with reduction of tumor size while remaining asymptomatic. Three months later, repeat ECHO showed ejection fraction (EF) of 45–50%.
3. Discussion
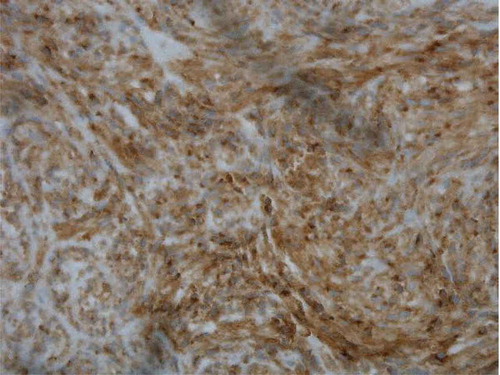
GIST diagnosis and treatment is based both on tumor morphology and on immunohistochemistry. Thus, biopsy is needed for further analysis, in which markers such as cluster of differentiation (CD) 117, CD 34, desmin, S-1000, and smooth muscle actin are examined. Such markers were diffusely positive in the core biopsies of the gastric mass in the current case (). First-line therapy for primary disease is surgical resection [Citation2]. Unfortunately, in the current case, proximal metastasis to retroperitoneal lymph nodes with tumor size approximately 8 cm on CT imaging was noted.
Multiple studies have shown that oral neoadjuvant therapy with imatinib at 400 mg daily has improved outcome in incomplete resection rates [Citation2,Citation3]. After the current patient was started on neoadjuvant therapy of imatinib at 400 mg orally, he manifested rapid progressive dyspnea and symptoms of heart failure, confirmed on echocardiography. Of note, baseline echocardiography ejection fraction was normal.
Imatinib toxicity usually occurs within the first 2 years. Side effects include fluid retention, edema, fatigue, and liver profile abnormalities, commonly reversible after drug cessation. Imatinib’s toxicity, in general, has been associated with higher dose exposure and long-term usage [Citation4]. In most cases, management includes imatinib discontinuation, and alternative tyrosine kinase inhibitors are considered. The current patient had rapidly worsening heart function after starting imatinib, which improved on discontinuation.
In general, literature suggests that patients who are prone to develop imatinib cardiotoxicity are older (i.e., greater than 65 years of age) with cardiac risk factors. Overt heart failure remains rare [Citation4]. These literature assertions contradict findings by Kim et al, who suggest that fluid retention in imatinib treatment for GIST can either be acute, associated with drug initiation or dose escalation, or chronic. Acute fluid retention in Kim’s series was noted at 0.3–4.0 months (median ~2 months) after dose escalation or imatinib initiation. In contrast to literature assertions, acute fluid retention presentations were more severe, and more likely in younger patients with fewer comorbidities [Citation5,Citation6]. In general, elderly with preexisting cardiovascular comorbidities might be more prone to chemotherapeutic toxicity; but, in the current case, dose-dependent toxicity seems more likely. The goal in such situations is immediate discontinuation or reduction of the imatinib dosage [Citation7,Citation8].
According to one report on 10 patients with congestive heart failure after imatinib initiation, histology revealed toxic cardiomyopathy, but patients had preexisting cardiovascular risk factors [Citation9]. Presentations ranged from asymptomatic to life-threatening overt congestive heart failure and acute coronary syndrome. Only 1% of cases suffered arrhythmias, congestive heart failure, or acute coronary syndrome. However, as noted, 8.2% of imatinib-treated GIST patients may experience edema and effusions [Citation1].
In contrast to previous reports and studies discussed above, our patient underwent less than 1 month of treatment; most literature reported that cases had longer duration of therapy. In cases of chronic myelogenous leukemia (CML) treated with imatinib, average duration before cardiotoxicity was 162 days [Citation10]. Length of imatinib treatment, dose, and cumulative dose may pose significant cardiotoxicity risk. Our patient’s baseline cardiac ejection function was normal pretreatment. Higher doses of imatinib, with more rapid tumor debulking, may pose the greatest risk of cardiotoxicity. Toxicity can range from mild left ventricular dysfunction to overt heart failure. Although our patient’s previous history was only significant for hypertension, heart failure was documented by echocardiography after dose initiation.
Although a rare toxicity of imatinib, cardiotoxicity of imatinib therapy should be taken seriously. Pretreatment cardiac assessment along with monitoring throughout the therapy should be implemented. Moreover, imatinib-induced cardiotoxicity should be differentiated from imatinib fluid retention, in which echocardiography findings are normal.
In one study of imatinib fluid retention, comparison of baseline and post-treatment echocardiography demonstrated normal cardiac function. Thus, the authors concluded that fluid retention was not associated with imatinib cardiotoxicity [Citation5]. Incidence of cardiotoxicity was cited as similar to onset of heart failure incidence in the general population, estimated at 0.2% per year, as evidenced by new-onset heart failure or left ventricular dysfunction [Citation5]. While the impact of imatinib cardiotoxicity for GIST has been debated, molecular re-engineering of anticancer C-kit kinase inhibitors and cellular data on mitochondrial dysfunction and adenosine triphosphate (ATP) production deficit in murine models suggest a relationship, despite advocates for imatinib safety [Citation11–Citation14]. Prospective data on cardiac function and long-term follow-up data are needed to confirm murine observation in humans. Kim et al and Trent et al. both have described a rapidly progressive heart failure with imatinib-treated GIST in a small series of patients, consistent with the current case in which dyspnea manifested just 2 weeks after dose initiation [Citation5,Citation6].
Our case illustrates the importance of monitoring cardiac function before and during imatinib therapy. While patient’s age and comorbidities should be taken into consideration, younger patients with absence of comorbidities may also be at risk for cardiotoxicity. Toxicity can be rapidly progressive and can occur within a few weeks, despite previous reports and studies suggesting delayed onset. Additionally, imatinib-induced cardiotoxicity should be differentiated from imatinib-related fluid retention. In the latter, echocardiography is normal without objective findings of left ventricular (LV) dysfunction. Further prospective studies are needed to establish the association of accelerated cardiotoxicity with imatinib usage.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Rammohan A, Sathyanesan J, Rajendran K, et al. A gist of gastrointestinal stromal tumors: a review. World J Gastrointest Oncol. 2013;5(6):102–112.
- Rutkowski P, Przybył J, Zdzienicki M. Extended adjuvant therapy with imatinib in patients with gastrointestinal stromal tumors. Mol Diagn Ther. 2013;17(1):9–19.
- Blay JY, von Mehren M, Blackstein ME. Perspective on updated treatment guidelines for patients with gastrointestinal stromal tumors. Cancer. 2010;116(22):5126–5137.
- Mughal TI, Schrieber A. Principal long-term adverse effects of imatinib in patients with chronic myeloid leukemia in chronic phase. Biologics. 2010;4:315–323.
- Kim KW, Shinagare AB, Krajewski KM, et al. Fluid retention associated with imatinib treatment in patients with gastrointestinal stromal tumor: quantitative radiologic assessment and implications for management. Korean J Radiol. 2015;16(2):304–313.
- Trent JC, Patel SS, Zhang J, et al. Rare incidence of congestive heart failure in gastrointestinal stromal tumor and other sarcoma patients receiving imatinib mesylate. Cancer. 2010;116:184–192.
- Ran HH, Zhang R, Lu XC, et al. Imatinib-induced decompensated heart failure in an elderly patient with chronic myeloid leukemia: case report and literature review. J Geriatr Cardiol. 2012;9(4):411–414.
- Jain VV, Pawade H, Gupta OP. Cardiac failure following imatinib treatment–a rare but fatal complication. J Indian Acad Clin Med. 2011;12(3):243–245.
- Kerkelä R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12(8):908–916.
- Atallah E, Durand JB, Kantarjian H, et al. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood. 2007;110(4):1233–1237.
- Fernandez A, Sanguino A, Peng Z, et al. An anti-cancer C-kit kinase inhibitor is re-engineered to make it more active and less cardiotoxic. J Clin Invest. 2007;117(12):4044–4054.
- Chambers TP, Santiesteban L, Gomez D, et al. Sab mediates mitochondrial dysfunction involved in imatinib mesylate-induced cardiotoxicity. Toxicology. 2017;382:24–35.
- Perik PJ, Rikhof B, de Jong FA, et al. Results of plasma N terminal pro B-type natriurectic peptide and cardiac troponin monitoring in GIST patients do not support the existence of imatinib-induced cardiotoxicity. Ann Oncol. 2008;19:359–361.
- Ami EB, Demetri GD. A safety evaluation of imatinib mesylate in the treatment of gastrointestinal stromal tumor. Expert Opin Drug Saf. 2016;15(4):571–578.