ABSTRACT
Enteropathy-associated T-cell lymphoma (EATL) is a rare disease found in the small bowel and is seen most commonly in patients with refractory celiac disease (RCD). We present a case of an elderly male with celiac disease (CD) diagnosed in childhood with months of abdominal pain and diarrhea despite attempting to avoid gluten in his diet. After persistent symptoms for months, the patient was admitted for an acute abdomen and was found to have small bowel perforation due to a jejunal mass that was diagnosed as an EATL. In 2–5% of adult onset CD, serious complications such as RCD or malignancy develop. The clinical course for EATL is aggressive and generally has a poor prognosis. This case highlights the importance of early clinical suspicion for a small bowel malignancy in patients with a long-standing history of CD and acute worsening of symptoms. Early workup and diagnosis is vital in improving morbidity and mortality in patients with EATL.
1. Introduction
Lymphoid neoplasms are reported to be the fourth most common malignancy in the US and the sixth leading cause of cancer-related deaths [Citation1]. Extra-nodal lymphomas are seen most commonly in the skin, followed by stomach, oropharynx, and small intestine. The vast majority of gastrointestinal tract lymphomas are B-cell in origin and are found in the stomach, while only a small percentage are T-cell lymphomas and these are most commonly seen in the small bowel [Citation2]. Enteropathy-associated T-cell lymphoma (EATL) is a rare entity with approximately 50 new cases reported in 2016 [Citation1]. It is estimated that around 2–3% of patients with celiac disease (CD) develop an intestinal lymphoma, with 65% being of T-cell origin [Citation3,Citation4]. Early clinical suspicion for EATL in patients with CD who develop refractory diarrhea, abdominal pain, and malnutrition is crucial for treatment and survival.
We present a case of a 69 year-old-male with CD and a 9-month history of refractory diarrhea, abdominal pain, an unintentional 60-lb weight loss who was admitted for an acute abdomen due to a small bowel perforation secondary to a jejunal EATL.
2. Case overview
A 69-year-old male with a history of CD presented to his new primary care physician with a 4-week history of diarrhea, abdominal pain, and weight loss. His CD was diagnosed during childhood although he reported eating gluten later in life without any major symptoms. He had a colonoscopy 1 year ago that was unremarkable. He had no recent travel history, antibiotic use, new medications, sick contacts, or consumption of raw or undercooked foods. Recently, he noted worsening abdominal pain, weight loss, and loose stools, following eating and at night. Laboratory studies ordered were significant for normocytic anemia (Hgb 11.8 g/dl), low protein (albumin 2.4 g/dl), elevated gliadin IgA antibody of 121 units, and tissue transglutaminase IgA antibody value of 132 units.
A CT of his abdomen showed diffuse lymphadenopathy throughout the mesentery with numerous nodes measuring up to 10–12 mm. A CT-guided mesenteric lymph node biopsy was negative for metastatic tumor with no convincing evidence of malignancy. Gastric biopsies showed moderate gastritis and duodenal mucosa showed chronic inflammation, increased intraepithelial lymphocytes, and moderate-to-severe villous blunting consistent with CD, Marsh 3B-C class. Jejunal biopsies were not done during this procedure. As the patient spent a number of years asymptomatic while on a gluten diet, he was considered to have adult onset CD. The patient reinstituted a gluten-free diet (GFD) as he was educated about the importance of a GFD to prevent worsening of symptoms and to decrease his risk for small bowel carcinoma. If there was no improvement in symptoms, and his anemia persisted despite strict adherence to a GFD, a repeat EGD with capsule endoscopy would then be performed.
At a follow-up visit 3–4 months later, the patient was cachectic, and a capsule and CT enterography were done which showed increased prominence and number of mucosal folds of the ileum. In the jejunum, there were a decreased number of mucosal folds. No focal small bowel mass lesions or strictures were seen. A push and/or balloon endoscopy for further tissue sampling was scheduled and the patient was started on total parenteral nutrition for severe protein-calorie malnutrition.
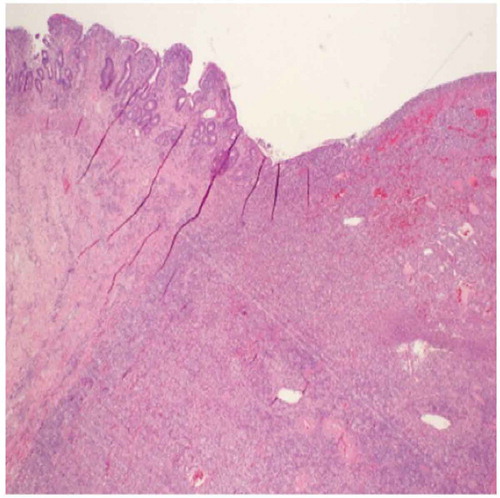
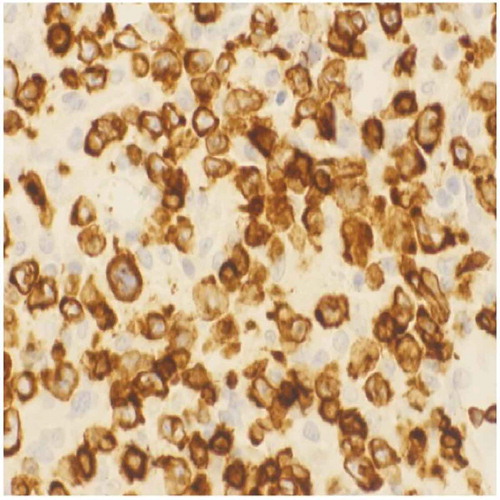
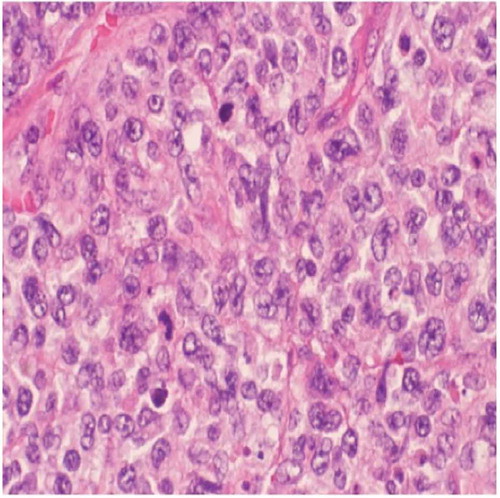
Subsequently, he presented to the emergency department with an acute abdomen and underwent an exploratory laparotomy with resection of a perforated jejunum (). The resected jejunal segment showed an ulcerated mass composed of medium to large cells with pale-staining cytoplasm, ovoid nuclei, and prominent nucleoli ( and ). Immunohistochemical stains revealed the tumor to be positive for CD3, CD30, CD43, and LCA. The biopsy was consistent with EATL arising in the background of CD. The tumor invaded and perforated the small bowel wall.
The patient was started on CHOEP chemotherapy (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone) after discharge. He completed six cycles of CHOEP therapy with near complete remission of the lymphoma. Following this, he underwent an autologous stem cell transplant and was free of disease for approximately 6 months. His diarrhea reoccurred and was admitted to the hospital for dehydration, hypotension, and severe malnutrition. A colonoscopy showed multiple punctate ulcerative lesions of varying sizes and biopsies confirmed EATL involving the colon and terminal ileum. The patient made the decision to not pursue further treatments and he died shortly thereafter.
3. Discussion
The incidence of CD is reportedly on the rise in Northern America [Citation5]. EATL is known to present more commonly in the fifth and sixth decades of life with a slightly greater predominance in men [Citation6]. In 2–5% of adult onset CD, serious complications develop such as refractory celiac disease (RCD) or malignancies. RCD patients can be divided into RCD Type I and II. While Type II patients harbor abnormal phenotypic intraepithelial T-cell populations, Type I patients have normal phenotypic intraepithelial lymphocytes. Approximately 50–60% of patients with RCD Type II develop EATL within 5 years [Citation6]. As EATL is closely linked to CD, it is more commonly encountered among individuals of northern European descent. EATL generally follows a highly aggressive clinical course and has a poor prognosis. Outcome is poor with a 2-year survival rate of 15–25%. It is one of the main causes of death in patients with CD diagnosed in adulthood [Citation7].
Patients with EATL typically present with diarrhea, abdominal pain, anemia, and weight loss, and other symptoms that include fevers, night sweats, and vomiting. There are two types of EATL, with classic EATL (Type I) being more common and associated with CD. EATL Type II is seen in 10–20% of all cases and is not associated with CD. In EATL, there is multifocal involvement of the small intestine, most commonly in the jejunum followed by the ileum and duodenum [Citation8]. Gastric and colonic involvement are uncommon. Intestinal ulcers, stenosis, and perforation of the small bowel are typical findings. EATL can also present as a disseminated process, with involvement of sites such as mesenteric lymph nodes, liver, spleen, lung, or skin [Citation3].
In most reported cases, EATL is a complication of CD, and it is usually preceded by a short history of adult CD. In some patients, EATL presents in individuals with established CD who have previously responded well to a GFD but are now symptomatic due to developing RCD and/or classic EATL [Citation9]. Other patients present acutely with a small bowel obstruction or perforation, and this can be seen in individuals with prior undiagnosed CD or without a history of CD (EATL Type II). Interestingly, our patient presented with an acute obstruction and perforation following adult CD with refractory symptoms after being symptom-free for many years.
In patients where there is a suspicion for EATL, workup studies such as ENT evaluation, video capsule enteroscopy (VCE), double-balloon enteroscopy for biopsy, CT with 18F-FDG-PET scan, and magnetic resonance enteroclysis are important in order to make a timely diagnosis. A complete workup improves the accuracy of detecting early EATL or recurring EATL [Citation6]. Our patient initially had a CT of the abdomen done, followed by CT-guided biopsy of mesenteric lymph nodes that were negative for malignancy. He subsequently had an EGD with biopsy of the gastric and duodenal mucosa. Following persistent symptoms and over 60 lb of weight loss, the patient did have a VCE done with a plan for balloon enteroscopy. Further workup, however, was precluded because of an acute small bowel perforation with resection. Biopsy of small bowel perforated mass showed EATL Type I. It is reported in the literature that while EATL is almost always found in the small bowel, it is most commonly seen in the jejunum, followed by the ileum, then duodenum. Our patient’s initial biopsy was done solely in the duodenum. For RCD or EATL, it may be useful to biopsy the jejunum or ileum along with the proximal small bowel to evaluate for tissue abnormalities.
Interestingly, our patient had childhood CD, followed by many symptom-free years on a gluten diet, and later presented with adult onset CD. After this diagnosis, he attempted to follow a GFD; however, following nutritional consultation for persistent symptoms, it was discovered that his diet was not completely gluten free. In patients with CD, approximately 70% of patients have symptomatic improvement within 2 weeks after starting a GFD [Citation10]. However, gluten is present in many processed foods because wheat flour is used as a thickener in many items. In retrospect, this patient actually did have EATL regardless of his percentage of gluten intake in the months prior, but more importantly, clinically his health was significantly deteriorating. He had continued diarrhea, significant weight loss, abdominal pain, vitamin and protein malnutrition, and anemia. It should be highlighted that RCD is a diagnosis of exclusion, and it is defined as severe enteritis that doesn’t respond to at least 6 months of a strict GFD and is not related to other causes of enteropathy or to overt intestinal lymphoma. The patient’s inadvertent gluten diet may have diminished suspicion for other causes of his symptoms, including malignancy.
While the exact timeline of when the patient’s T-cell lymphoma developed is unknown, he seemed to have exhibited alarm signs early in his presentation. Given his history of CD, gender, ethnicity, age, and clinical picture of persistent diarrhea, anemia, abdominal pain, and continued weight loss, a heightened suspicion for a small bowel lymphoma should have been considered. The difficulty of trying to establish a GFD may have impeded a thorough workup of EATL earlier on. A negative biopsy of gastric and duodenal tissue without looking at the jejunum may have erroneously appeased suspicions of a small bowel lymphoma.
The clinical course for EATL is aggressive and generally has a poor prognosis. The reported survival rates are less than 10% at 5 years and 10–25% at 2 years [Citation6,Citation11]. The mainstay of therapy for EATL is usually chemotherapy and surgical resection. Stem cell transplant is an option for individuals who have achieved complete or near complete remission of disease. Our patient underwent six cycles of CHEOP therapy followed by an autologous stem cell transplant. While his symptoms initially did improve with therapy, EATL was found to involve his colon 2 years after his initial diagnosis in the jejunum. The importance of early clinical suspicion for a small bowel malignancy in patients with a long-standing history of CD and acute worsening of symptoms cannot be understated. Early workup and diagnosis is vital in improving morbidity and mortality in patients with EATL.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–459.
- Rand A, Cardona DM, Proia A, et al. Clinically undiagnosed enteropathy associated T-cell lymphoma type II presenting with prolonged lower gastrointestinal tract symptoms: report of an autopsy case and review of diagnostic challenges and clinicopathological correlation. J Gastrointest Oncol. 2013;4(1):103–108.
- Arps DP, Smith LB. Classic versus type II enteropathy-associated T-cell lymphoma: diagnostic considerations. Arch Pathol Lab Med. 2013;137(9): 1227–1231. Delabie J, Holte H, Vose JM, et al. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood. 2011;118(1):148–155.
- Malamut G, Murray JA, Cellier C. Refractory celiac disease. Gastrointest Endosc Clin N Am. 2012;22(4):759–772.
- Ludvigsson JF, Rubio-Tapia A, Van Dyke CT, et al. Increasing incidence of celiac disease in a North American population. Am J Gastroenterol 108. 2013;5:818.
- Van de Water JMW, Cillessen SAGM, Visser OJ, et al. Enteropathy associated T-cell lymphoma and its precursor lesions. Best Pract Res Clin Gastroenterol. 2010;24(1):43–56.
- Biagi F, Corazza GR. Defining gluten refractory enteropathy. Eur J Gastroenterol Hepatol. 2001 May;13(5):561–565.
- Eigner W, Bashir K, Primas C, et al. Dynamics of occurrence of refractory coeliac disease and associated complications over 25 years. Aliment Pharmacol Ther. 2017;45(2):364–372.
- Delabie J, Holte H, Vose JM, et al. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood. 2011;118(1):148–155.
- Farrell RJ, Kelly CP. Celiac sprue. New England J Med. 2002;346(3):180–188.
- Al-Toma A, Verbeek WH, Hadithi M, et al. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007 Oct;56(10):1373–1378.