ABSTRACT
Introduction: Sarcoidosis is an inflammatory granulomatous multisystem disease with an unknown etiology. Neurosarcoidosis is a cryptogenic neuroinflammatory manifestation of sarcoidosis.
Case report: We describe a case of neurosarcoidosis with initial presentation as confusion. Our patient fits the picture of probable neurosarcoidosis as per the Zajicek et al criteria, with the clinical presentation of neurosarcoidosis, evidence of inflammation in the CNS, evidence of systemic sarcoidosis and exclusion of other diseases. He was started on steroids with subsequent improvement in mental status.
Discussion and conclusion: The diagnosis of neurosarcoidosis becomes challenging when neurosarcoidosis presents as isolated clinical neurological symptoms. We want to highlight the highly variable nature of neurosarcoidosis and underscore the fact that for cryptogenic neuroinflammatory diseases, neurosarcoidosis should be considered after excluding common infections and autoimmune causes.
Abbreviations: CXR: Chest X-ray; CTA: Computed tomography angiography; PCR: Polymerase chain reaction; DNA: Deoxyribonucleic acid; MRI: Magnetic resonance imaging; ACE: Angiotensin converting enzyme; HRCT: High Resolution computed tomography
1. Introduction
Sarcoidosis is an inflammatory granulomatous multisystem disease with an unknown etiology. It has an incidence of approximately 10 and 20 per 100,000 population [Citation1]. Neurosarcoidosis is a cryptogenic neuroinflammatory manifestation of sarcoidosis. The clinical manifestations of neurosarcoidosis are seen only in about 5–10% of cases, even though the neurological localizations of sarcoidosis are seen in up to 50% of patients in autopsic studies [Citation2,Citation3]. We describe a case of neurosarcoidosis which presented as confusion.
2. Case presentation
A 76-year-old man was admitted due to increasing confusion. His family reported no baseline dementia or cognitive impairment. He was recently treated for an E. faecalis urinary tract infection complicated by obstructive uropathy due to a ureteral stone at the ureteropelvic junction and underwent J-stent placement two weeks prior to this admission. On physical examination, he was somnolent, oriented to person and place only with a Glasgow Coma Scale score of 13/15. Cranial Nerves II to XII were grossly intact, normal bulk, tone, and strength bilaterally; there was no pronator drift. Light touch was intact bilaterally in upper and lower extremities. Reflexes were 2+ and symmetric at the biceps, triceps, knees, and ankles. Plantar responses were flexor. There was no dysmetria on finger-to-nose and heel-to-shin test. Also, new skin lesions, lymphadenopathy or asterixis were absent on physical examination. Initial workup showed anemia (10.4 g/dl; 12.0–16.0 g/dL), deranged kidney function with an elevated blood urea nitrogen (BUN) (36 mg/dL; 8.0–25.0 g/dL) and creatinine (4.7 mg/dL; 0.60–1.50 g/dL), and hypercalcemia (corrected calcium of 11.8 mg/dL; 8.5–10.5 g/dL). The patient had developed another episode of obstructive uropathy despite recent stent placement, thus a ureteral lithotripsy and stone extraction was performed with resolution of obstruction. His renal function, however, did not improve nor did his mental status. On analysis, the ureteral stone was calcium oxalate. We believed that his altered mental status could not be attributed to his hypercalcemia as the hypercalcemia was mild and resolved within the first two days of admission.
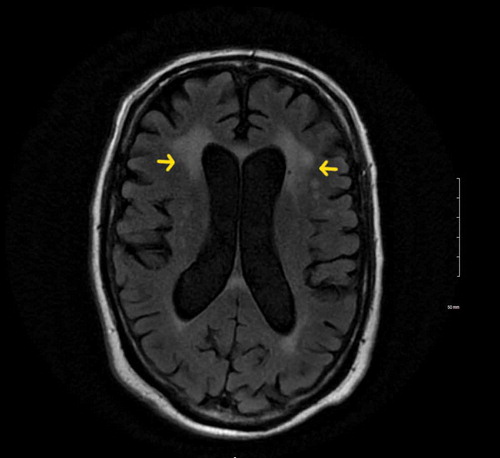
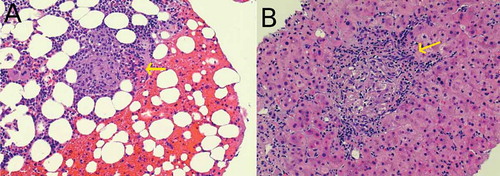
On further investigation, Chest X-ray (CXR) (), Computed tomography angiography (CTA) chest and Computed tomography (CT) head were unremarkable. Due to a mild elevation of transaminases and elevated ammonia level, a right upper quadrant ultrasound was performed which was consistent with liver cirrhosis. Hepatitis panel revealed positive Hepatitis C antibody but Polymerase chain reaction (PCR) for Hepatitis C Ribonucleic acid (RNA) was negative. Alcohol and toxins as a cause of cirrhosis were ruled out with a negative urine drug screen and serum alcohol level, and collateral history from the patient’s family. Autoimmune hepatitis was less likely as he had negative anti-smooth muscle antibodies, anti-liver/kidney microsomal antibodies and gamma globulin. The antinuclear antibodies were positive which led to further testing to evaluate for autoimmune diseases. Anti–neutrophil cytoplasmic antibodies, JO-1 antibodies, SS-A/Ro and SS-B/La antibodies, Anti-Smith antibodies, RNP antibodies, Anti-Scl-70 antibodies, Anti-double stranded DNA antibodies, Anti-chromatin antibodies, Anti-centromere antibodies, and antimitochondrial antibodies were negative. The patient was subsequently started on lactulose with eventual resolution of ammonemia but no improvement in mental status. Magnetic resonance imaging (MRI) of the brain showed bilateral periventricular, centrum semiovale, and subcortical white matter high signal intensity on T2-Weighted and FLAIR sequences (). Lumbar puncture done showed mildly elevated proteins (60 mg/dl), normal glucose (46 mg/dl), WBC 1 cell, RBC 0 cells and presence of oligoclonal bands. Serum and urine protein electrophoresis showed high levels of lambda (131.81 mg/L; Reference Range 5.70–26.30 mg/L) and kappa (114.95 mg/L; 3.30–19.40 mg/L) light chains but he had less than 10% plasma cells in bone marrow which ruled out multiple myeloma. A bone and liver biopsy showed non-caseating granulomas (). Angiotensin converting enzyme (ACE) (92 U/L; 8–53 U/L) and 1, 25- dihydroxy vitamin D levels (89.3 pg/mL; 18–64 pg/mL) were also elevated. 25- hydroxy vitamin D levels (46.4 ng/mL; 20–50 ng/mL) were within normal limits. Based on these findings, the patient was diagnosed with probable neurosarcoidosis and started on steroids. He was initially started on intravenous methylprednisolone 40 mg every six hours for 3 days with subsequent improvement in mental status and was then switched to oral prednisone 40 mg daily. He was discharged on oral prednisone tablet 40 mg daily for one month. His family was involved in his care and assured adherence to his medication regimen. The patient tolerated the oral steroids without major side effects. On one and three month follow-up he showed a return to his baseline mental status. Repeat MRI was not done after steroid therapy to evaluate improvements in white matter lesions as the patient returned to his baseline mental status.
3. Discussion
Clinical manifestations of neurosarcoidosis are reported in 5% to 10% of sarcoidosis patients [Citation2,Citation3]. Diagnosis may be particularly challenging when neurosarcoidosis occurs in isolation. In a meta-analysis of 1088 patients, neurological symptoms were the first clinical manifestation of sarcoidosis in 52% of the patients while pulmonary involvement was present in 67% of the cases.Chest X-ray showed 60% of the patients and chest CT showed 70% of the patients had findings consistent with pulmonary or lymph node sarcoidosis [Citation4,Citation5]. Neurosarcoidosis can present in a number of manifestations, involving any part of the central or peripheral nervous system. Common manifestations include, cranial mononeuropathy, with facial nerve palsy being the most common (25–50%) [Citation6,Citation7] and visual, vestibular and auditory dysfunction due to optic and cranial nerve VIII neuropathy [Citation8], neuroendocrine dysfunction as a result of hypothalamus inflammation which can present as libido, sleep, temperature, appetite and thirst disturbances [Citation9], generalized seizure or a generalized or restricted encephalopathy due to perivascular granulomatous inflammation with the encephalopathy presenting as cognitive and behavioral problems and/or focal neurological deficits [Citation6,Citation7], meningeal involvement presenting as aseptic or chronic meningitis [Citation10], communicating or noncommunicating hydrocephalus [Citation11], myelopathy or radiculopathy due to granulomatous inflammation of the spinal cord [Citation12], and peripheral neuropathy presenting as mononeuropathy, mononeuritis multiplex, and sensory, sensorimotor, motor and autonomic polyneuropathies [Citation13]. Our patient had a generalized encephalopathy.
In a systematic review and meta analysis of 1088 patients of neurosarcoidosis the main presenting neurological symptoms were cranial nerve palsies (55%), headache (32%), sensory abnormalities like hypoaesthesia, paraesthesia, and neuropathic pain (29%) and gait abnormalities (28%). Visual impairment, hemiparesis and paraparesis, ataxia, vertigo, seizures, psychiatric symptoms, nystagmus and papilledema also occurred frequently [Citation5]. Confusion without any other neurological symptoms, as was present in our patient, is a unique presenting symptom. It is present in sporadic case reports in combination with other neurological symptoms like hemiparesis and gait disturbances [Citation14,Citation15]. We used the Zajicek et al criteria to diagnose our patient with neurosarcoidosis [Citation16]. This criteria divided neurosarcoidosis into confirmed neurosarcoidosis, probable neurosarcoidosis and possible neurosarcoidosis. Our patient fit the picture of probable neurosarcoidosis with clinical presentation of neurosarcoidosis with evidence of inflammation in the central nervous system, and evidence of systemic sarcoidosis and exclusion of other diseases. Marangoni et al. recently proposed some changes to the existing diagnostic criteria by adding a High Resolution computed tomography (HRCT) instead of a CXR and measuring the CD4/CD8 T cell ratio in the Cerebrospinal fluid (CSF) [Citation17]. However, it is still unclear what the sensitivity and specificity of the diagnostic criteria of zajicek et al is and how manrangoni et al changes may affect the existing criteria.
Cerebrospinal fluid (CSF) abnormalities occur commonly in patients with neurosarcoidosis, however, the findings are non- specific. In approximately 10 percent of patients, increased CSF opening pressure is noted. The total protein is elevated (up to 250 mg/dL) in approximately two-thirds of patients. 50 percent of patients can have pleocytosis, predominantly mononuclear cells. Mononuclear oligoclonal bands can be present in some cases. Glucose can be decreased or normal [Citation6,Citation7]. It is important to recognize aseptic meningitis on the CSF analysis. These patients have diffuse leptomeningeal gadolinium enhancement on MRI and high cell counts ≥50 cells/μl, total protein ≥200 mg/dl, and significantly lower glucose levels ≤40 mg/dl in the majority of patients [Citation10]. Our patient had mildly elevated proteins (60 mg/dl) and oligoclonal bands, with no leptomeningeal enhancement on the MRI pointing more towards generalized encephalopathy due to perivascular granulomatous inflammation rather than aseptic meningitis. On serology, our patient also had antinuclear antibodies, which were attributed to sarcoidosis. Studies have shown a higher prevalence of antinuclear antibodies in sarcoidosis patients as compared to the general population [Citation18].
There are no randomized placebo controlled trials to direct treatment in neurosarcoidosis. Currently the main treatment for sarcoidosis remain glucocorticoids. Majority of the patients usually respond to glucocorticoids. Our patient was treated with 1 mg/kg prednisone. For patients with rapid progressive disease or inadequate response high dose oral prednisone, or IV methylprednisolone can be used. For patients who are unable to tolerate the side effects of steroid therapy, immunosuppressive agents like methotrexate, cyclophosphamide, chloroquine and hydroxychloroquine can be used. Recently, infliximab has been used with varying success for steroid and immunosuppressive refractory patients [Citation4,Citation19,Citation20].
3.1. Conclusion
As of yet neurosarcoidosis is an evolving subject and is poorly understood. The problem is compounded by unavailability of a standardized diagnostic criteria and lack of randomized controlled trials guiding treatment. We want to highlight the highly variable nature of neurosarcoidosis and underscore the fact that for cryptogenic neuro inflammatory diseases, neurosarcoidosis should be considered after excluding common infections and autoimmune cause [Citation4,Citation21].
3.2. Key points
The diagnosis of neurosarcoidosis becomes challenging when neurosarcoidosis presents as isolated clinical neurological symptoms in a patient with no known history of sarcoidosis.
Pulmonary involvement is present in only 67% of the cases.
Main presenting neurological symptoms were Cranial Nerve palsies, headache, sensory abnormalities like hypoaesthesia, paraesthesia, and neuropathic pain and gait abnormalities.
Confusion without any other neurological symptoms, as was present in our patient, is a unique presenting symptom .
As of yet neurosarcoidosis is an evolving subject and is poorly understood. The problem is compounded by unavailability of a standardized diagnostic criteria and lack of randomized controlled trials guiding treatment.
We want to highlight the highly variable nature of neurosarcoidosis and underscore the fact that for cryptogenic neuro inflammatory diseases, neurosarcoidosis should be considered after excluding common infections and autoimmune cause.
3.3. Declarations
‘This research was supported (in whole or in part) by HCA and/or an HCA affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA or any of its affiliated entities.’
Written informed consent, and a copy of the consent will be available if requested.
Authors’ contributions
Ateeq Mubarik: concept, design
Syed Moin Hassan: literature search, manuscript preparation, manuscript editing.
Monicka Felix: manuscript editing, and manuscript review.
Salman Muddassir: manuscript editing, and manuscript review.
Furqan Haq: manuscript editing, and manuscript review.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Thomas KW, Hunninghake GW. Sarcoidosis. JAMA. 2003 Jun 25;289(24):3300–3303.
- Nozaki K, Judson MA. Neurosarcoidosis. Curr Treat Options Neurol. 2013 Aug;15(4):492–504.
- Segal BM. Neurosarcoidosis: diagnostic approaches and therapeutic strategies. Curr Opin Neurol. 2013 Jun;26(3):307–313.
- Ibitoye RT, Wilkins A, Scolding NJ. Neurosarcoidosis: a clinical approach to diagnosis and management. J Neurol. 2017 May;264(5):1023–1028.
- Fritz D, van de Beek D, Brouwer MC. Clinical features, treatment and outcome in neurosarcoidosis: systematic review and meta-analysis. BMC Neurol. 2016 Nov 15;16(1):220.
- Joseph FG, Scolding NJ. Neurosarcoidosis: a study of 30 new cases. J Neurol Neurosurg Psychiatry. 2009 Mar;80(3):297–304.
- Pawate S, Moses H, Sriram S. Presentations and outcomes of neurosarcoidosis: a study of 54 cases. QJM. 2009 Jul;102(7):449–460.
- Imran TF, Nizami S, Eyzner I, et al. Vertigo as a predominant manifestation of neurosarcoidosis. Case Rep Med. 2015 Apr;2(2015):397046.
- Bihan H, Christozova V, Dumas J-L, et al. Sarcoidosis: clinical, hormonal, and magnetic resonance imaging (MRI) manifestations of hypothalamic-pituitary disease in 9 patients and review of the literature. Medicine. 2007 Sep;86(5):259–268.
- Wengert O, Rothenfusser-Korber E, Vollrath B, et al. Neurosarcoidosis: correlation of cerebrospinal fluid findings with diffuse leptomeningeal gadolinium enhancement on MRI and clinical disease activity. J Neurol Sci. 2013 Dec 15;335(1–2):124–130.
- Brouwer MC, de Gans J, Willemse RB, van de Beek, D. Neurological picture. Sarcoidosis presenting with hydrocephalus. J Neurol Neurosurg Psychiatry. 2009 May;80(5):550–551.
- Reda HM, Taylor SW, Klein CJ, et al. A case of sensory ataxia as the presenting manifestation of neurosarcoidosis. Muscle Nerve. 2011 Jun;43(6):900–905.
- Zuniga G, Ropper AH, Frank J. Sarcoid peripheral neuropathy. Neurology. 1991 Oct;41(10):1558–1561.
- Mitchell MS, Chalikonda MD, Kotler – the medicine forum MD, 2017. Neurosarcoidosis: granulomatous disease presenting with confusion and gait disturbance.jdc.jefferson.edu. [Internet]. 2017; Available from: http://jdc.jefferson.edu/tmf/vol18/iss1/9/
- Bhat R, Ahad R, Ahmed I, et al.Neurosarcoidosis presenting as acute inflammatory polyneuropathy (P6.142). Neurology. 2016Apr5;8616 Suppl:Internet]. Available fromhttp://www.neurology.org/content/86/16_Supplement/P6.142.abstract
- Zajicek JP, Scolding NJ, Foster O, Rovaris M, Evanson J, Moseley IF, et al. Central nervous system sarcoidosis–diagnosis and management. QJM. 1999 Feb;92(2):103–117.
- Marangoni S, Argentiero V, Tavolato B. Neurosarcoidosis clinical description of 7 cases with a proposal for a new diagnostic strategy. J Neurol. 2006 Apr;253(4):488–495.
- Kobak S, Yilmaz H, Sever F, et al. The prevalence of antinuclear antibodies in patients with sarcoidosis. Autoimmune Dis. 2014 Dec;17(2014):351852.
- Ungprasert P, Matteson EL. Neurosarcoidosis. Rheum Dis Clin North Am. 2017 Nov;43(4):593–606.
- Tana C, Wegener S, Borys E, et al. Challenges in the diagnosis and treatment of neurosarcoidosis. Ann Med. 2015 Oct 15;47(7):576–591.
- Flanagan EP, Kaufmann TJ, Krecke KN, et al. Discriminating long myelitis of neuromyelitis optica from sarcoidosis. Ann Neurol. 2016 Mar;79(3):437–447.