ABSTRACT
Heparin Induced thrombocytopenia (HIT) is a rare, immune-mediated complication of heparin, associated with both thrombocytopenia and paradoxical thrombotic events. Initial diagnosis is made clinically when platelet count falls by 30% to <100 × 109cells/l or a > 50% decrease from baseline count in association with heparin therapy. Thromboembolic complications are seen in 50% of the cases. We present a case of acute pulmonary embolism (aPE) in a 65 year old male secondary to HIT while on unfractionated heparin for venous thromboprophylaxis. He was admitted to the hospital for severe acute exacerbation of asthma and was on heparin and venodyne boots for venous thrombo-prophylaxis. His chief presenting complaints improved until day 13, when he had severe pleuritic chest pain with worsening of shortness of breath and was desaturating while breathing ambient air. Computed tomography (CT) of the chest with intravenous contrast revealed aPE involving bilateral upper lobe segmental pulmonary arteries. Given the pattern and timing of thrombocytopenia prior to onset of his symptoms and acute thromboembolism, diagnosis of HIT was made which was later supported by positive platelet factor- ELISA and serotonin release assay (SRA) laboratory testing. Heparin and heparin-related products were promptly discontinued and argatroban was started. Later platelet count increased over 150 × 103/μL and argatroban was switched to warfarin prior to discharge. As heparin is extensively used, all physicians are required to be attentive of this life threatening complication. Discontinuing heparin while substituting with an alternative anticoagulant such as argatroban may become a life-saving strategy in such a case.
1. Introduction
HIT is an immune mediated adverse drug reaction to heparin exposure that occurs in up to 5 percent of patients exposed, regardless of the dose, schedule or route of administration. HIT is associated with thrombocytopenia but unlike other forms of thrombocytopenia, HIT is generally not marked by bleeding; instead, venous thromboembolism (example, deep venous thrombosis, pulmonary embolism) is the most common complication and may even precede the significant decline in platelet counts. For this reason, it is also called as Heparin induced thrombocytopenia and thrombosis (HITT). Mortality rate is as high as 20%, and earlier diagnosis and treatment have resulted in a better prognosis, with mortality and major morbidity of 6% to 10% [Citation1]. Herein we present a case of bilateral pulmonary embolism (PE), initially thought provoked by immobilization, but later found as being the thrombotic complication of HITT.
2. Case description
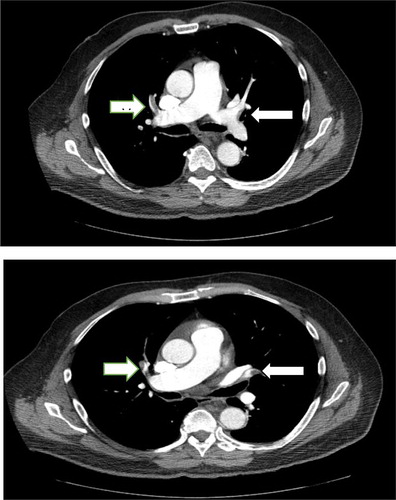
A 65 year old African American male who was admitted to our medical facility for acute exacerbation of asthma. Initially he was managed on the medical floor with intravenous steroids, antibiotics and inhaled bronchodilators. His past medical history was significant for bronchial asthma and chronic sinusitis with nasal polyps. On presentation, vitals were: Temperature 97.2, heart rate 110 bpm, RR 22/min, saturation of 96% on Venturi-mask and blood pressure 118/87 mmHg. Laboratory tests showed leukocytosis of 14 x 103/µL, hemoglobin 15.2g/dL and platelet count 377 x 103/µL. Comprehensive metabolic panel revealed normal electrolytes, kidney and liver function. Arterial blood gases done on 2L oxygen by nasal canula showed pH of 7.337, partial pressure of carbon dioxide 47.7 mmHg, partial pressure of oxygen 75.2 mmHg, and oxygen saturation 95.2 %. On the night of admission, he was found to be in severe respiratory distress, was intubated and mechanically ventilated and transferred to the intensive care unit (ICU) While in the ICU, he was treated with broad spectrum antibiotics, intravenous steroids, inhaled beta agonist-anticholinergics. He was also on venodyne compression boots and subcutaneous heparin for deep vein thromboprophylaxis. He gradually improved and was extubated on day 7 of the ICU stay. He was then transferred to the medical Stepdown unit for further management. Complete blood counts at the time of transfer out from ICU showed a white blood count of 11.3 X 103/µL, Hemoglobin 11.7 g/dl, platelet count of 217x 103/µL. He continued to have shortness of breath while in the Stepdown unit which delayed his discharge. On day 13 of hospitalization, the patient complained of right sided pleuritic chest pain and dyspnea even at rest. Vitals at this point were: heart rate 110 bpm, blood pressure of 114/77 mmHg, respiratory rate of 24 breaths per minute and oxygen saturation was 90% on room air. Arterial blood gas on room air showed pH 7.5, partial pressure of carbon dioxide 36.7mHg, partial pressure of oxygen 50.3mmHg, oxygen saturation 90.3%. A-a gradient was noted to be very high at 53. CT chest with intravenous contrast was done which revealed acute Pulmonary emboli involving bilateral upper lobe segmental pulmonary arteries (). 2D transthoracic echocardiogram showed dilated and hypokinetic right atrium and ventricles with a normal left ventricular function and a pulmonary artery systolic pressure of 39mmHg. The patient’s brain natriuretic peptide was 8.5 and cardiac biomarkers were negative.
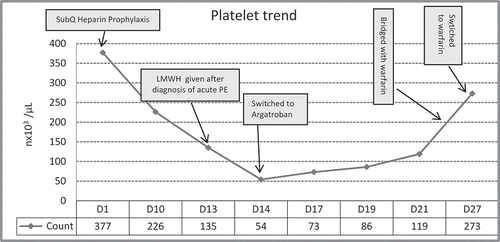
The subcutaneous heparin prophylaxis was discontinued and the patient was started on low molecular weight heparin (LMWH). Following the first dose of LMWH, a precipitous drop in the platelet count to 54 x 103/µL from 135 x 103/µL was noted on day 14. The platelet trend is depicted in . With a 4Ts score of 8 and the clinical picture of documented venous thromboembolism, HITT was strongly suspected. Although it is not always possible to cite HIT as the cause for PE as opposed to treatment for PE causing HIT, in our patient the drop in platelet count was significant even before the appearance of symptoms of PE and the fact that he was on subcutaneous heparin and venodyne compression stocking all the while in hospital, made PE more likely to be a complication of HITT.
All heparin products were held and on day 14 patient was started on IV infusion of argatroban, a direct thrombin inhibitor, 2 microgram per kilogram per minute, and the dose was adjusted to keep his activated partial thromboplastin time at 1.5–2.5x control (47–90 sec). Platelet factor antibody-ELISA test (platelet factor 4-heparin- immunosorbent assay) and C14-serotinin release assay were performed. Both were strongly positive. The PFA-ELISA titer value was 2.674 ODI (positive is over 0.4 units) and SRA is 78% (cutoff point is above 20%). The platelet count rose to 273 x 103/µL on the 13th day after starting argatroban infusion. Bridging with warfarin was started after the platelet count was above 150 x 103/µL and continued to maintain international normalized ratio above 3 and subsequently, patient was discharged home on Warfarin 7.5mg daily with follow up appointment in hematology clinic.
3. Discussion
HIT has been reported in 1–5% of patients exposed to heparin for more than 4 days [Citation2,Citation3]. Thrombocytopenia is the most common feature of HIT but in 25% of patients, it can be preceded by development of thrombosis [Citation4]. The most common complication of HIT is venous thrombosis and represents itself as a DVT and/or PTE in 17% to 55% of patients with a mortality rate from 18.8 to 50% [Citation5–Citation7].
Factors that increase the frequency of development of HIT include female gender [Citation8], the duration of heparin use [Citation2], the patient population with higher frequency in surgical patients [Citation9–Citation11] and type of heparin used with highest risk associated with use of unfractionated heparin [Citation3,Citation10]. Two forms of HIT are established, of which only one is clinically significant. HIT type 1 affects up to 10 % of patients and is not associated with increased risk of thrombosis. It usually occurs within the first two days of heparin treatment, due to non-immune platelet aggregation, leading to transient drop in platelet count (rarely <100 x 103/µL) which resolves over discontinuation of heparin products. HIT type II is a rare but clinically significant syndrome occurring due to immune-mediated complication of heparin therapy caused by IgG antibodies against heparin-platelet factor 4 associated with thrombosis and thrombocytopenia. It typically develops 5 days after starting heparin therapy but can occur earlier with recent heparin exposure (within 24 hours) or rarely have a delayed onset (3 weeks after cessation) [Citation5,Citation6].
Laboratory testing of HIT- associated antibodies is diagnostically important. PF4-ELISA is the most accessible test with high sensitivity (99%) but low specificity (<50%) (10). In most instances, a positive ELISA requires further evaluation with the more specific SRA (sensitivity and specificity >98 and 95% respectively) [Citation12]. Our patient tested positive on both the ELISA and SRA. The finding of acute venous thrombosis, prior exposure to heparin, positive laboratory testing and a high 4T score confirmed the diagnosis of HITT.
HITT requires immediate treatment based on clinical suspicion, even before confirmatory laboratory tests are made available. For individuals with a strong clinical suspicion of HIT, therapy includes discontinuation of all heparin with initiation of a non-heparin anticoagulant, such as a argatroban, direct thrombin inhibitor. Argatroban is administered at a usual starting dose of 1 to 2 mcg/kg per min via continuous infusion, adjusted to maintain the APTT of 1.5–3.0 times patient’s baseline (with dose-reduction for patients with hepatic dysfunction, heart failure, post-cardiac surgery and anasarca) [Citation13]. Dose adjustment is not required in the presence of renal impairment as argatroban is mostly metabolized by the liver [Citation14]. Bivalirudin is another direct thrombin inhibitor approved, with reduced doses, managing the subset of HIT patients with renal and/or hepatic failure [Citation15,Citation16] and patients undergoing percutaneous coronary intervention [Citation17]. Usually patients are continued on these anticoagulants until recovery of platelet count (>150,000) before transitioning to warfarin. Our patient was safely transitioned to Warfarin after 14 days of argatroban administration with excellent platelet recovery and no complications at 3-month follow-up.
In conclusion, since both heparin and LMWH are widely used in medicine, all physicians need to be aware of the possibility of aPE as a life-threatening complication of heparin treatment-HITT spectrum. It also helps to focus on serial platelet count monitoring in patients on heparin and to initiate stepwise investigations if any significant changes occur. High clinical suspicion of HIT mandates immediate cessation of all heparin therapy and substituting it with an alternative anticoagulant, which can significantly improve the morbidity and mortality rate in such patients as is evident in our case.
Acknowledgments
None.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- LA L, Al D, Moores LK, et al. Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e495S–530S.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005 Oct;106(8):2710–2715.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332(20):1330.
- Greinacher A, Farner B, Kroll H, et al. Clinical features of heparin-induced thrombocytopenia including risk factors for thrombosis. A retrospective analysis of 408 patients. Thromb Haemost. 2005 Jul;94(1):132–135.
- Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e 495S.
- Bircan HA, Alanoglu EG. Massive pulmonary embolism in a patient with heparin induced thrombocytopenia: successful treatment with dabigatran. Eurasian J Med. 2016;48(1):65–68.
- Kelton JG. Heparin-induced thrombocytopenia: an overview. Blood Rev. 2002 Mar;16(1):77–80.
- Warkentin TE, Sheppard JI, Sigouin CS, et al. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108(9):2937–2941.
- Warkentin TE, Roberts RS, Hirsh J, et al. An improved definition of immune heparin-induced thrombocytopenia in postoperative orthopedic patients. Arch Intern Med. 2003;163:2518–2524.
- Gruel Y, Pouplard C, Nguyen P, et al.; French Heparin-Induced Thrombocytopenia Study Group. Biological and clinical features of low-molecular-weight heparin-induced thrombocytopenia. Br J Haematol. 2003 Jun;121(5):786–792.
- Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. Am J Med. 1996 Nov;101(5):502–507.
- Raschke RA, Curry SC, Warkentin TE, et al. Improving clinical interpretation of the anti-platelet factor 4/heparin enzyme-linked immunosorbent assay for the diagnosis of heparin-induced thrombocytopenia through the use of receiver operating characteristic analysis, stratum-specific likelihood ratios, and Bayes theorem. Chest J. 2013;144(4):1269–1275.
- Cuker A, Crowther M. Clinical practice guideline on the evaluation and management of adults with suspected heparin-induced thrombocytopenia (HIT). Am Soc Hematol. 2013;1:1–4.
- Swan SK, Hursting MJ. The pharmacokinetics and pharmacodynamics of argatroban: effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy. 2000;20:318.
- Wisler JW, Washam JB, Becker RC. Evaluation of dose requirements for prolonged bivalirudin administration in patients with renal insufficiency and suspected heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2012;33:287.
- Kiser TH, Fish DN. Evaluation of bivalirudin treatment for heparin-induced thrombocytopenia in critically ill patients with hepatic and/or renal dysfunction. Pharmacotherapy. 2006;26:452.
- Kleinschmidt S, Stephan B, Pindur G, et al. Argatroban: pharmacological properties and anesthesiologic aspects. Der Anaesth. 2006;55(4):443–450.