ABSTRACT
Background: Contrast-induced acute kidney injury (CIAKI) following coronary angiography is frequently observed in the general population. End-stage liver disease (ESLD) patients are at a particularly increased risk for development of CIAKI following coronary angiography due to preexisting renal hypoperfusion.
Methods: We performed a retrospective study of 544 consecutive cardiac catheterizations in ESLD patients from December 2003 to May 2013 to calculate the incidence of CIAKI post-coronary angiography and to identify risk factors for CIAKI. CIAKI was defined as a serum creatinine increase of either ≥ 25% or ≥ 0.5 mg/dL from baseline within 72 hours. Multivariable and Cox regression analysis was performed for development of CIAKI and all-cause mortality, respectively.
Results: Overall, 179 cases of coronary angiography were included in the final analysis. CIAKI occurred in 23% of patients. All-cause mortality was 52% in the CIAKI group and 37% in the non-CIAKI group, with a mean follow-up of 2.2 ± 3.8 years. Multivariable analysis identified intensive care unit admission (OR 2.72, CI 1.05–7.01, p < 0.05) and baseline estimated glomerular filtration rate (OR 1.02, CI 1.002–1.035, p < 0.05) as independent predictors of CIAKI. Cox regression analysis identified pre-angiography beta-blocker use (HR 2.13, CI 1.04–4.38, p < 0.05), international normalized ratio (HR 1.37, CI 1.05–1.78, p < 0.05) and Mehran risk score (HR 1.13, CI 1.02–1.25, p < 0.05) as independent predictors of all-cause mortality.
Conclusions: CIAKI in ESLD patients undergoing coronary angiography occurs at a moderately elevated rate when compared to the general population.
1. Introduction
Contrast-induced acute kidney injury (CIAKI) following coronary angiography is a frequent complication, with incidence rates as high as 55% [Citation1]. The development of CIAKI following coronary angiography is an independent predictor of in-hospital mortality in chronic kidney disease (CKD) patients and long-term mortality in non-CKD patients [Citation2]. Of particular concern is the development of CIAKI following coronary angiography in end-stage liver disease (ESLD) patients, as this patient population has an increased risk of acute kidney injury (AKI) due to renal hypoperfusion in the setting of splanchnic vasodilation and systemic vasoconstriction [Citation3].
The incidence of AKI in hospitalized ESLD patients is as high as 70% [Citation4], with mortality rates up to 63% at 1 year [Citation5]. A small prospective study of ESLD patients undergoing abdominal computed tomography scans with contrast media showed no correlation between contrast administration and subsequent development of AKI [Citation6]. However, a retrospective cohort study evaluating the incidence of CIAKI in ESLD patients undergoing contrast computed tomography reported an incidence of 25% [Citation7]. The hemodynamic abnormalities that are common to ESLD patients make predicting CIAKI difficult.
To our knowledge, the incidence of CIAKI in ESLD patients undergoing coronary angiography has not been previously evaluated. Therefore, this study was performed with the aim of reporting the incidence of CIAKI following coronary angiography in ESLD patients and to identify risk factors for the development of CIAKI. In addition, we studied all-cause mortality and risk factors for death in the entire cohort of ESLD patients undergoing coronary angiography.
2. Subjects and methods
2.1. Study design and participants
This study was conducted at a large, academic, tertiary care center, in which we performed a retrospective review of 544 consecutive cardiac catheterizations in ESLD patients from December 2003 to May 2013. ESLD was defined as clinical findings consistent with cirrhosis, in addition to either (1) liver biopsy results with histologic evidence of regenerative nodules surrounded by fibrous bands or (2) radiologic evidence of inhomogeneous hepatic texture, enlarged caudate lobe, splenomegaly, or splanchnic collateral veins [Citation8]. Of the 544 cardiac catheterizations that were performed, 365 were excluded from subsequent analysis due to pre-procedure dialysis, lack of contrast use during catheterization, or absence of post-angiography serum creatinine (SCr) data within 72 hours. All 179 cases of coronary angiography included in the final analysis had at least 1 value for SCr before and 1 value within 72 hours after the procedure. Cox survival analysis of all-cause mortality was based on a sample size of 135 unique patients, as only the first encounter was used for patients with multiple encounters. Two non-ionic, iso-osmolar contrast media (ioversol and iopamidol) were used in the cardiac catheterization laboratory at the time of this study. Participants were followed for up to 38 ± 31 months post-coronary angiography until the study end date (13 February 2014) or death. The protocol was approved by Henry Ford Hospital’s Institutional Review Board.
2.2. Study variables
CIAKI was defined as a SCr increase of either ≥25% or ≥0.5 mg/dL from baseline within 72 hours, with the highest SCr within 72 hours being used to determine whether CIAKI occurred [Citation9]. The most recent SCr within a 3-month period before contrast exposure was defined as the baseline SCr and used to calculate the baseline estimated glomerular filtration rate (eGFR). eGFR was determined based on the 4 variable Modification of Diet in Renal Disease (MDRD) Study equation [Citation10]. Henry Ford Hospital Laboratory began using isotope dilution mass spectrometry for SCr determination in 2012, so the MDRD equation used for our eGFR calculations was adjusted appropriately for values of SCr obtained after that date [Citation11]. Mortality was confirmed by public documents available via the Social Security Death Index. In addition to demographic characteristics, data were collected on variables included in the Mehran risk score [Citation1], Model for End-Stage Liver Disease score [Citation12], and pre-angiography medications. Comorbidity was identified by physician review of electronic medical records pertaining to the eligible patients.
2.3. Study outcomes
The primary outcomes were the incidence of CIAKI following coronary angiography in ESLD patients and the risk factors for the development of CIAKI. Secondary outcomes included all-cause mortality and risk factors for all-cause mortality in all ESLD patients who underwent coronary angiography.
2.4. Statistical analysis
The incidence of CIAKI was calculated by counting the number of patients who developed CIAKI from December 2003 to May 2013 divided by the total number of patients who received coronary angiography during that time. Univariable analysis of risk factors was done by comparing the 2 main study groups (CIAKI and non-CIAKI) using 2-sample t-tests for normally distributed numeric variables, Wilcoxon rank sum tests for non-parametric distributed numeric variables, chi-square tests for non-sparse categorical variables, and Fisher exact tests for sparse categorical variables. Multivariable logistic regression analysis was used to identify independent risk factors for CIAKI with group comparisons (p < 0.2) (New York Heart Association congestive heart failure class III/IV, intra-aortic balloon pump use, intensive care unit (ICU) admission, inotrope use, mechanical ventilation, Mehran risk score, baseline eGFR, international normalized ratio (INR), and angiotensin-converting enzyme inhibitor and non-steroidal anti-inflammatory medication use). In addition, multivariable Cox regression analysis was used to evaluate the same risk factors as predictors of all-cause mortality over the complete follow-up period. P-values <0.05 were considered statistically significant.
3. Results
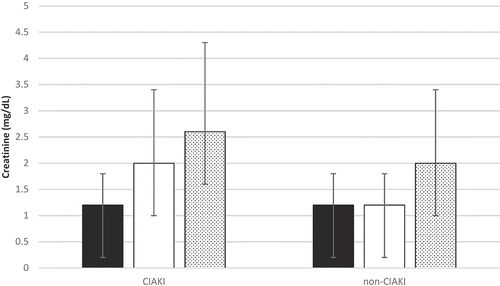
Of the 544 cardiac catheterizations that were performed on ESLD patients from December 2003 to May 2013, 179 were analyzed after exclusion of those cardiac catheterizations that did not meet inclusion criteria. All-cause mortality analysis was based on a sample size of 135 unique patients, as only the first encounter was used for patients with multiple encounters. Baseline characteristics of the CIAKI and non-CIAKI groups are reported in . The 2 cohorts were similar with regard to age, sex, race, and body mass index. In both groups, nearly two-thirds of the patients were male. Both groups had similar rates of diabetes, smoking, and CKD. New York Heart Association class III/IV congestive heart failure was present in 41.5% of patients in the CIAKI group versus 26.8% in the non-CIAKI group. Acute coronary syndrome as an indication for coronary angiography was present in 46% of the CIAKI group and 40% of the non-CIAKI group. CIAKI occurred in 22.9% of ESLD patients following coronary angiography. All-cause mortality was 37% in the CIAKI group and 52% in the non-CIAKI group, with a mean follow-up of 2.2 ± 3.8 years (). When categorized by the use of beta-blockers prior to coronary angiography, all-cause mortality was 74% in those with and 26% in those without pre-angiography beta-blockers (). In comparing short- and long-term creatinine trends between the CIAKI and non-CIAKI group, both groups had similar baseline SCr values, but the peak SCr within 72 hours and 1 year was nearly 80% and 20% greater in the CIAKI cohort, respectively ().
Table 1. Baseline characteristics of the ESLD cohorts.
Figure 1. All-cause mortality at 1 year and at the end of follow-up based on contrast-induced acute kidney injury (CIAKI) status and beta-blocker use. Stippled pattern, CIAKI; downward diagonal pattern, non-CIAKI; solid black, beta-blocker; solid white, non-beta-blocker.
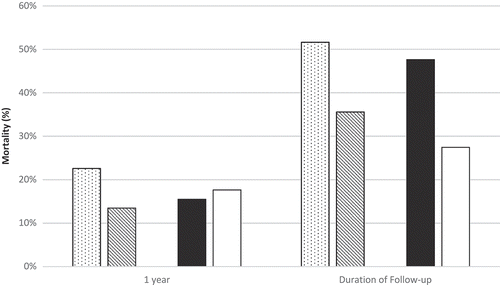
Figure 2. Creatinine trends before and after coronary angiography based on contrast-induced acute kidney injury (CIAKI) status. Bars indicate mean and standard deviation for each parameter. Solid black, baseline serum creatinine (SCr); solid white, peak SCr within 72 hours; stippled pattern, peak SCr within 1 year.
3.1. Independent predictors of CIAKI
Univariable analysis for risk of CIAKI detected statistically significant differences with the use of intra-aortic balloon pumps, inotropes, and mechanical ventilation, as well as with ICU admission. Multivariable logistic regression analysis for risk of CIAKI showed that ICU admission (odds ratio [OR] 2.78, p < 0.05) and baseline eGFR (OR 1.019, p < 0.05) were statistically significant risk factors (). A baseline eGFR threshold of 93 mL/min/1.73 m2 was found to have the strongest association with the development of CIAKI. No differences were found with regard to contrast volume, contrast ratio [Citation13,Citation14], or contrast-to-eGFR ratio [Citation15].
Table 2. Independent predictors of CIAKI and all-cause mortality for the duration of follow-up.
3.2. Independent predictors of all-cause mortality
Multivariable Cox regression analysis for risk of death revealed that pre-coronary angiography beta blocker use (hazard ratio [HR] 2.13, p < 0.05), INR (HR 1.37, p < 0.05) and the Mehran risk score (HR 1.13, p < 0.05) predicted all-cause mortality at a mean follow-up of 2.2 ± 3.8 years (). An INR of 1.3 and a Mehran risk score of 6 were found to have the strongest association with all-cause mortality. CIAKI conferred a trend toward increased mortality (HR 1.78, p = 0.08).
4. Discussion
To our knowledge, this is the first study to evaluate the incidence of CIAKI in ESLD patients who have undergone coronary angiography. The cohort of subjects analyzed were all hospitalized patients with ESLD, a disease state that commonly compromises kidney function due to decreased renal perfusion [Citation16]. Based on observational data, the development of AKI in hospitalized patients with ESLD has been associated with poor prognosis [Citation17]. Our results suggest that the incidence of CIAKI in ESLD patients following coronary angiography is elevated in comparison to the general inpatient population, with ICU admission and baseline eGFR < 93 mL/min/1.73 m2 as independent predictors of developing CIAKI.
The pathophysiology of CIAKI in patients with ESLD is not well studied, but the unique hemodynamic compromise in these patients likely plays a significant role. The prevailing theory of CIAKI involves (1) toxic ischemic injury to the renal tubules by reactive oxygen species and (2) afferent arteriolar vasoconstriction caused by decreased nitric oxide production and decreased prostacyclin activity [Citation3]. The risk of decreased afferent arteriolar perfusion is likely exaggerated in ESLD patients due to decreased systemic vascular resistance secondary to portal hypertension, which subsequently leads to increased nitric oxide generation within the splanchnic system and increased endogenous cannabinoid production [Citation5]. In addition, ESLD patients have concomitant vasoconstrictor activation due to increased sympathetic nervous system activity and renin-angiotensin-aldosterone axis activation, both of which further compromise renal artery perfusion [Citation5].
One retrospective study evaluated ESLD patients who underwent computed tomography scans with intravenous contrast and found the incidence of CIAKI to be 25%, similar to what was reported in our study [Citation7]. Interestingly, this study also found that the presence of diabetes and the Model for End-Stage Liver Disease score both lacked predictive power for the development of CIAKI [Citation7]. In contrast to these results, a small prospective study did not find an association between contrast exposure and development of CIAKI [Citation6]. This study, however, excluded patients on diuretic therapy, whereas our study more closely approximated usual clinical practice, with nearly half of all subjects prescribed some form of diuretic prior to coronary angiography. For the inpatient population in general, the incidence of CIAKI following coronary angiography has been well documented and has been associated with increased short- and long-term mortality [Citation2,Citation18,Citation19]. The development of CIAKI has been linked to the presence of multiple comorbidities [Citation1,Citation20], but of particular importance in chronically vasodilated ESLD patients is the finding that hemodynamic compromise contributes to the development of CIAKI and increases the risk for all-cause mortality. CIAKI has been correlated with increased cardiac mortality [Citation19], and it has been postulated from animal data that ischemic AKI as seen in CIAKI may increase subendocardial myocardial ischemia by diminishing coronary vessel reactivity and inducing a myocardial oxygen supply/demand mismatch [Citation21]. More recent theories have implicated a discordance in the activity of endothelial and smooth muscle progenitor cells in patients with mild CKD, leading to increased atherosclerosis and thus cardiovascular mortality [Citation22]. However, this phenomenon has not been evaluated in subjects with CIAKI. The individual aspects of these studies highlight the relevance of identifying risk factors for the development of CIAKI in ESLD patients undergoing coronary angiography, as they represent a particularly susceptible cohort of patients for whom vigilant pre- and post-angiography care may reduce the risk of CIAKI and all-cause mortality.
Of particular concern is the potential for underestimating the incidence of CIAKI in ESLD patients, as the SCr is commonly diminished in this patient population due to malnutrition and muscle wasting [Citation23]. However, the definition of CIAKI as used in this study, in which proportional increases in SCr were required, should reduce this underestimation.
The univariable analysis suggested that differences between ESLD patients with and without CIAKI were, not surprisingly, indicators of critical illness. After accounting for potential confounders, multivariable analysis was able to identify ICU admission and baseline eGFR as independent predictors for developing CIAKI. A baseline eGFR threshold of 93 mL/min/1.73 m2 was found to have the strongest association with CIAKI, but as previously mentioned, this value may overestimate the true GFR in the ESLD population [Citation24,Citation25]. GFR may be overestimated in the ESLD population due to protein-calorie malnutrition and muscle wasting causing decreased creatinine production [Citation26]. In addition, ESLD patients may have impaired liver synthesis and increased tubular secretion of creatinine, further contributing to disproportionately decreased SCr [Citation25,Citation27]. Previous studies have shown that the MDRD equations may overestimate true GFR in ESLD patients by 16 ± 22 mL/min/1.73 m2, suggesting that even lower GFRs may confer an increased risk of developing CIAKI [Citation24]. More accurate measurements of GFR by inulin or iothalamate clearance could be implemented, but most centers would be limited by the cost, complexity, and lack of availability of these tests.
The Mehran risk score was formulated in 2004 as a means to quantify the risk of developing CIAKI after percutaneous coronary intervention [Citation1]. This risk score was subsequently found to be useful in predicting mortality in patients with acute myocardial infarction [Citation28,Citation29]. As the Mehran risk score proved to have an association with development of CIAKI and mortality in the general population, we wanted to evaluate its usefulness in the ESLD population. The Mehran risk score did not predict the development of CIAKI in our study, but multivariable analysis revealed that a risk score > 6 was an independent predictor of all-cause mortality. This finding supports the association of increased mortality with increasing frequency of comorbidities and hemodynamic instability [Citation18]. Similarly, elevated INR was also predictive of all-cause mortality, which likely reflects the association of coagulopathy with worsening liver function. This finding also supports the well-documented significance of INR in predicting poor survival in ESLD patients [Citation30]. Pre-angiography use of beta-blockers was also an independent predictor of all-cause mortality. A majority (61%) of the patients within the overall cohort were prescribed beta-blockers prior to coronary angiography. Interestingly, of the patients who died during follow-up, 75% were administered periprocedural beta-blockers, nearly 70% of which were beta-1-selective. Causal relationships cannot be generally inferred from observational data, but beta-blocker use may be a surrogate for severity of liver disease, as ESLD patients with esophageal varices are commonly prescribed beta-blockers [Citation31]. Alternatively, beta-blockers may potentiate renal hypoperfusion during coronary angiography by decreasing cardiac output [Citation32]. Previous studies in patients with ESLD and refractory ascites have demonstrated a deleterious effect of beta-blockers on mortality [Citation33]. CIAKI showed a trend toward increased mortality for the duration of follow-up. Our sample size may have limited our ability to demonstrate a significant difference between the CIAKI and non-CIAKI cohorts, therefore stronger conclusions regarding the relationship between CIAKI and mortality will likely require further investigation.
Our study limitations include the retrospective nature of the analysis, which does not prove a causal association between contrast exposure and the development of CIAKI. However, many previous analyses including randomized controlled trials, have demonstrated the association between contrast exposure and AKI. Additionally, many of our patients were excluded from the CIAKI analysis due to the absence of post-angiography SCr data. However, there were no obvious systematic differences between the ESLD groups with and without post-angiography SCr measurements; therefore we are confident the patient sample included for analysis is representative of the ESLD cohort undergoing coronary angiography.
5. Conclusion
The incidence of CIAKI in ESLD patients undergoing coronary angiography in our study was moderately high at 23%. Risk factors for CIAKI in our population of ESLD patients were different than those previously developed for the general population.
Transparency declarations
None to declare.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–1399.
- Neyra JA, Shah S, Mooney R, et al. Contrast-induced acute kidney injury following coronary angiography: a cohort study of hospitalized patients with or without chronic kidney disease. Nephrol Dial Transplant. 2013;28(6):1463–1471.
- Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361(13):1279–1290.
- Warner NS, Cuthbert JA, Bhore R, et al. Acute kidney injury and chronic kidney disease in hospitalized patients with cirrhosis. J Investig Med. 2011;59(8):1244–1251.
- Fede G, D’Amico G, Arvaniti V, et al. Renal failure and cirrhosis: a systematic review of mortality and prognosis. J Hepatol. 2012;56(4):810–818.
- Guevara M, Fernandez-Esparrach G, Alessandria C, et al. Effects of contrast media on renal function in patients with cirrhosis: a prospective study. Hepatology. 2004;40(3):646–651.
- Lodhia N, Kader M, Mayes T, et al. Risk of contrast-induced nephropathy in hospitalized patients with cirrhosis. World J Gastroenterol. 2009;15(12):1459–1464.
- Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371(9615):838–851.
- Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl. 2006;100:S11–5.
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470.
- Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772.
- Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470.
- Cigarroa RG, Lange RA, Williams RH, et al. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med. 1989;86(6 Pt 1):649–652.
- Marenzi G, Assanelli E, Campodonico J, et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann Intern Med. 2009;150(3):170–177.
- Nyman U, Almen T, Aspelin P, et al. Contrast-medium-Induced nephropathy correlated to the ratio between dose in gram iodine and estimated GFR in ml/min. Acta Radiol. 2005;46(8):830–842.
- Slack A, Yeoman A, Wendon J. Renal dysfunction in chronic liver disease. Crit Care. 2010;14(2):214.
- Moreau R, Lebrec D. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. Hepatology. 2003;37(2):233–243.
- Dangas G, Iakovou I, Nikolsky E, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95(1):13–19.
- Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105(19):2259–2264.
- Bartholomew BA, Harjai KJ, Dukkipati S, et al. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93(12):1515–1519.
- Kingma JG Jr., Vincent C, Rouleau JR, et al. Influence of acute renal failure on coronary vasoregulation in dogs. J Am Soc Nephrol. 2006;17(5):1316–1324.
- Jie KE, Zaikova MA, Bergevoet MW, et al. Progenitor cells and vascular function are impaired in patients with chronic kidney disease. Nephrol Dial Transplant. 2010;25(6):1875–1882.
- Figueiredo FA, Dickson ER, Pasha TM, et al. Utility of standard nutritional parameters in detecting body cell mass depletion in patients with end-stage liver disease. Liver Transpl. 2000;6(5):575–581.
- Francoz C, Prie D, Abdelrazek W, et al. Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: impact on the model for end-stage liver disease score. Liver Transpl. 2010;16(10):1169–1177.
- Caregaro L, Menon F, Angeli P, et al. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994;154(2):201–205.
- Pirlich M, Schutz T, Spachos T, et al. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology. 2000;32(6):1208–1215.
- Selberg O, Bottcher J, Tusch G, et al. Identification of high- and low-risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology. 1997;25(3):652–657.
- Sgura FA, Bertelli L, Monopoli D, et al. Mehran contrast-induced nephropathy risk score predicts short- and long-term clinical outcomes in patients with ST-elevation-myocardial infarction. Circ Cardiovasc Interv. 2010;3(5):491–498.
- Wi J, Ko YG, Shin DH, et al. Prediction of contrast-induced nephropathy with persistent renal dysfunction and adverse long-term outcomes in patients with acute myocardial infarction using the mehran risk score. Clin Cardiol. 2013;36(1):46–53.
- Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–871.
- Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922–938.
- Llach J, Gines P, Arroyo V, et al. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology. 1988;94(2):482–487.
- Serste T, Melot C, Francoz C, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52(3):1017–1022.
