ABSTRACT
Lactobacillus jensenii
is a gram-positive bacillus in the female genital tract believed to be a commensal organism that inhibits the growth of more virulent pathogens. Prevotella bivia is a gram-negative bacillus species also typically commensal in the female genital tract. Lactobacillus as the primary causative agent in perinephric abscesses and bacteremia has been documented, albeit very uncommon and opportunistic. Prevotella bivia is not classically associated with perinephric abscesses but has been implicated in rare cases of pelvic inflammatory disease and tubo-ovarian abscesses. In this report, we present a 26-year-old immunocompetent woman with a recent history of nephrolithiasis treated with lithotripsy, ureteral stent placement and removal, and antibiotics who was admitted for fever and severe right flank pain. Imaging showed a right-sided renal and perinephric abscesses colonized by Lactobacillus jensenii and Prevotella bivia. Blood cultures were also positive for Lactobacillus species. Per literature review, intravenous ceftriaxone and metronidazole were administered with successful resolution of abscesses and negative repeat blood cultures. To our knowledge, this is the first case of simultaneous renal system abscesses caused by Lactobacillus and Prevotella species. Nephrolithiasis and prior antibiotics likely contributed to the opportunistic pathogenesis in this otherwise immunocompetent patient.
1. Introduction
Lactobacillusis an anaerobic gram-positive, catalase-negative, non-sporulating rod-shaped bacteria found in the human oropharyngeal, gastrointestinal, and genitourinary systems. It is commonly seen as an additive in many yogurts, fermented foods, and probiotics. Lactobacillus jensenii is a commensal species normally found in the female genitourinary tract, with known protective activity against harmful pathogens including Candida and Gardnerella vaginalis [Citation1].
Although classically a benign member of the normal microflora, opportunistic infections due to Lactobacillus have been observed. The most common manifestation is endocarditis, although bacteremia, liver/pelvic/splenic abscesses, pyelonephritis, meningitis, pneumonia, and even septic arthritis secondary to Lactobacillus have been documented [Citation1–Citation5]. The most commonly implicated pathogenic species are L. casei, L. acidophilus, and L. leishmaniasis, but cases of L. jensenii-induced endocarditis, bacteremia, and pyelonephritis have been reported [Citation6,Citation7]. Lactobacillus infections are usually seen in older, immunocompromised individuals, and the gender distribution of L. jensenii favors women. Infections are typically associated with recent antibiotic use and/or comorbid immunosuppressive condition, such as prolonged neutropenia, malignancy, organ transplantation, steroid use, recent surgery, and critical illness [Citation2,Citation8]. Genitourinary instrumentation also appears to predispose to L. jensenii infection[Citation1]. In the setting of associated life-threatening comorbidities, mortality estimates in Lactobacillus bacteremia/endocarditis vary from 12% to 44%, although appropriate and timely antibiotic treatment has been postulated to significantly affect the mortality rate [Citation8,Citation9]. Data on the mortality of Lactobacillus infections at other sites are scare.
Similar to Lactobacillus, Prevotella bivia is also an organism that inhabits the female genitourinary microbiota. It is a gram-negative non-pigmented anaerobe that is known to be implicated uncommonly in bacterial vaginosis, endometritis, and pelvic inflammatory disease. Rarely, cases of P. bivia paronychia, chest wall abscess, emphysematous pyelonephritis, necrotizing fasciitis, osteomyelitis, and sepsis have been published [Citation10–Citation13]. Lactobacillus is a known inhibitor of Prevotella in the GU system, while the pathogenicity of Prevotella increases in the presence of aerobic bacteria [Citation10,Citation14]. Given the rarity of P. bivia infection, there is no current consensus on the antibiotic treatment of P. bivia infections, especially for inoculations outside of the genitourinary system.
In this vignette, we report a young immunocompetent woman with predisposing ureteral stenting and antibiotic use who was found to have Lactobacillus bacteremia in addition to renal and perinephric abscesses growing L. jensenii and P. bivia respectively.
2. Case description
We report a 26-year-old African-American woman with a history of stable renal cyst, no probiotic consumption, and no corticosteroid use who presented with fevers, chills, tachycardia, and one week of severe right flank pain. Four weeks prior, she underwent lithotripsy along with ureteral stent placement for acute right-sided nephrolithiasis and was prescribed ciprofloxacin by her urologist. The ureteral stent was removed after two weeks due to hematuria and intermittent fevers.
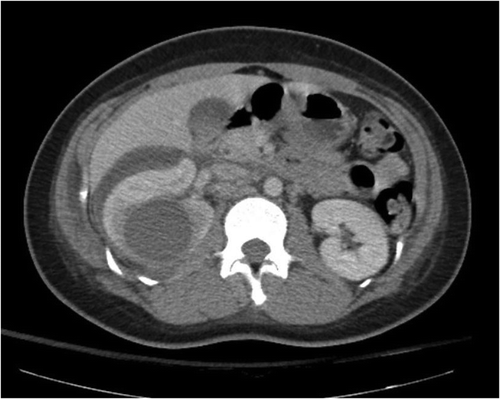
In the emergency department, she presented with fevers (38.3 ⁰C) and tachycardia (117). Significant right abdominal and costovertebral angle tenderness were present on the exam. Complete blood count showed mild leukocytosis (11.4). Urinalysis showed rare bacteria with negative leukocyte esterase, and urine culture was negative. CT abdomen/pelvis with contrast showed enlarged right kidney with a 4 cm renal cyst, as well as a complicated right-sided perinephric fluid collection, suspicious for abscess or hematoma (). She was admitted for sepsis and started on cefepime and vancomycin. A percutaneous drain was placed into the renal cyst, and purulent fluid was removed. Cultures of the drained fluid grew Lactobacillus, Streptococcus viridans species, and ‘mixed anaerobes’. Blood cultures grew Lactobacillus jensenii on anaerobic media. This was confirmed by both rapid ANA sequencing and MALDI-TOF Mass Spectrometry.
Figure 1. Renal cyst of 4.6 cm seen in right kidney (arrow), with perinephric fluid collection, also observed.
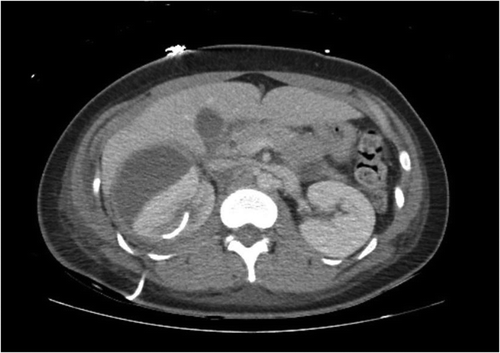
Per literature review, she was transitioned to intravenous ceftriaxone 2 g daily and metronidazole 500 mg every 8 h for the duration of her admission. Echocardiography revealed no cardiac vegetations and repeat blood cultures were negative, ruling out endocarditis. HIV testing was negative. CT abdomen/pelvis with contrast was repeated one week later, showing new right perinephric fluid collections (). These collections were subsequently drained and found to be purulent as well. Cultures of the fluid grew P. bivia, which was confirmed with MALDI-TOF Mass Spectrometry. CT urogram was performed, showing no fistulous tracts between the genitourinary and gastrointestinal systems. Pelvic ultrasound revealed a complex ovarian cyst, but gynecological evaluation ruled this to be a benign, unrelated finding.
Figure 2. Repeat CT Abdomen with intravenous contrast showing growing perinephric collection (arrow) and resolved prior kidney cyst.
With the above antibiotics and drains, the patient gradually experienced symptomatic improvement. She was discharged home on intravenous ceftriaxone via peripherally-inserted-central catheter, oral metronidazole, and follow up with outpatient infectious disease physician. Once the patient experienced complete clinical resolution, both ceftriaxone and metronidazole were terminated to conclude a total of four weeks of therapy. Drains were removed at the time of antibiotic discontinuation and CT scan showed complete resolution of all abscesses.
Of note, two months later, our patient has been diagnosed with Gardnerella-positive bacterial vaginosis. Testing at this time reveals undetectable levels of Lactobacillus species in the vaginal flora.
3. Discussion
Renal and perinephric abscess are commonly caused by E. coli, S. aureus, and K. pneumonia, and in less typical cases by P. aerogenosa, Enterococcus, Enterobacter, and Candida [Citation15,Citation16]. Persons predisposed to renal and perinephric abscesses include those with a history of kidney stones, diabetes mellitus, renal cysts, structural abnormalities of the kidneys, and urological procedures. They are classically associated with pyelonephritis secondary to ascending urinary tract infections, but can also arise from hematogenous spread from extrarenal primary infections [Citation16,Citation17]. During our literature search, most reviews on renal/perinephric abscesses did not even mention Lactobacillus or Prevotella.
However, Lactobacillus-induced renal infections have been documented in case reports. Chazan et al. described a 59-year-old woman with fever, chills, and flank pain following ureteral stent placement for a kidney stone found to have both L. jensenii pyelonephritis and bacteremia; she recovered with high-dose ampicillin [Citation7]. In another more morbid case, a 51-year-old woman with end-stage renal disease, diabetes mellitus, cirrhosis, and multiple recent urinary infections requiring broad-spectrum antibiotics was diagnosed with emphysematous pyelonephritis and bacteremia secondary to Lactobacillus species; she underwent nephrectomy but eventually died secondary to sepsis/critical illness [Citation18]. These cases illustrate the opportunistic pathogenicity of Lactobacillus infections: the first case involved urinary flow obstruction by ureteral stent and the second involved heavy antibiotic use along with multiple immunocompromising comorbidities.
For the L. jensenii renal abscess observed in our case, we suspect there could have been multiple possible routes of infection. Given the association of renal and perinephric abscesses with pyelonephritis, an ascending infection from a potentially contaminated ureteral stent could have led to direct inoculation. It is also possible that the patient had pyelonephritis in the weeks prior to admission treated successfully with ciprofloxacin; however, this likely cleared competing flora and, in combination with decreased urinary flow, allowed for a favorable environment for ascending opportunistic renal infection/abscess formation with or without extension into the bloodstream. Pre-existing renal cysts, like that seen in our patient, can also predispose to renal abscess formation [Citation15]. Another alternative possibility is that Lactobacillus may have entered the bloodstream during the prolonged two weeks of hematuria experienced by our patient after ureteral stent placement, with competing flora reduced by ciprofloxacin use. This bacteremia may have then spread hematogenously to the renal and perinephric sites (although the lack of confirmed P. bivia bacteremia does challenge the likelihood of hematogenous spread.)
Like L. jensenii, P. bivia is a genitourinary opportunistic pathogen. It is even more rarely associated with bacteremia than Lactobacillus and requires specific culture systems to grow [Citation19,Citation20]. To our knowledge, this is the first case report to document a perinephric abscess containing this organism. P. bivia and other bacterial vaginosis-associated organisms are actually inhibited by Lactobacillus [Citation14]. Therefore, clearance of Lactobacillus with ceftriaxone may have directly led to the P. bivia abscesses observed. The only other case in which there has been documented involvement of P. bivia in the kidney is a 54-year-old patient with P. bivia emphysematous pyelonephritis who required nephrostomy and four weeks of antibiotic therapy with metronidazole and cephalosporin, before recovering with no complications [Citation13].
Given the scarcity of documented Lactobacillus and Prevotella infections of the kidney, there are no defined treatment guidelines in the literature. Prior to presenting to the hospital, our patient was treated with ciprofloxacin, which has been shown to be a poor choice for L. jensenii and instead likely cleared competing flora [Citation9]. Our patient was treated with ceftriaxone given its documented effectiveness on L. jensenii (and S. viridans) at a low MIC [Citation9] and metronidazole given the presence of other mixed anaerobes in the culture. Ceftriaxone and metronidazole were continued for a total of four weeks until complete clinical resolution was reached. In one study, P. bivia was shown to be particularly susceptible to metronidazole, imipenem, and piperacillin-tazobactam [Citation21], suggesting that the former played a critical role in resolving the P. bivia-associated abscesses in our patient.
Interestingly, several months after treatment, our patient has been diagnosed with bacterial vaginosis secondary to G. vaginalis in the setting of undetectable Lactobacillus levels. Like inhibiting P. bivia, Lactobacillus also normally inhibits G. vaginalis [Citation1,Citation14], and our patient’s current disease could very well be a side effect of her extended antibiotic therapy.
This case is unique because of several reasons. Firstly, it describes a rare case of Lactobacillus infection and even rarer case of P. bivia infection simultaneously in the renal system. Secondly, many opportunistic infections, especially Lactobacillus, are commonly implicated in patients with significant illness or immunocompromised state; our case, however, illustrates risk factors (e.g., pre-existing renal cyst, genitourinary instrumentation, urinary flow obstruction, recent antibiotic use, hematuria, etc.) that can lead to pathogenesis in even a young and healthy woman. Thirdly, we outline the effectiveness of a literature-guided antibiotic regimen that, along with interventional drainage, led to successful clinical resolution of an infectious process associated with high mortality. Lastly, we discuss bacterial vaginosis as a possible side effect of antibiotic treatment. Limitations of our paper include the limited speciation of Lactobacillus and ‘mixed anaerobes’ in first renal abscess culture (no mass spectrometry confirmation was performed), and the inherent lack of generalizability of conclusions from only a single case. However, we hope this clinical vignette can be an influential resource for treating patients with Lactobacillus and P. bivia infections and help significantly reduce the mortality associated with them.
Prior presentations
SGIM 2019.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Suárez-García I, Sánchez-García A, Soler L, et al. Lactobacillus jensenii bacteremia and endocarditis after dilatation and curettage: case report and literature review. Infection. 2012;40(2):219–222.
- Antony SJ. Lactobacillemia: an emerging cause of infection in both the immunocompromised and the immunocompetent host. J Natl Med Assoc. 2000;92(2):83–86.
- Sherid M, Samo S, Sulaiman S, et al. Liver abscess and bacteremia caused by lactobacillus: role of probiotics? Case report and review of the literature. BMC Gastroenterol. 2016;16(1):138.
- Sherman ME, Albrecht M, DeGirolami PC, et al. Lactobacillus: an unusual case of splenic abscess and sepsis in an immunocompromised host. Am J Clin Pathol. 1987;88(5):659–662. .
- Chanet V, Brazille P, Honore S, et al. Lactobacillus septic arthritis. South Med J. 2007;100(5):531–532.
- Fradiani PA, Petrucca A, Ascenzioni F, et al. Endocarditis caused by Lactobacillus jensenii in an immunocompetent patient. J Med Microbiol. 2010;59(5):607–609.
- Chazan B, Raz R, Shental Y, et al. Bacteremia and pyelonephritis caused by Lactobacillus jensenii in a patient with urolithiasis. Isr Med Assoc J. 2008;10(2):164–165.
- Husni RN, Gordon SM, Washington JA, et al. Lactobacillus bacteremia and endocarditis: review of 45 cases. Clin Infect Dis. 1997;25(5):1048–1055.
- Salminen MK, Rautelin H, Tynkkynen S, et al. Lactobacillus bacteremia, species identification, and antimicrobial susceptibility of 85 blood isolates. Clin Infect Dis. 2006;42(5):e35–44.
- Mirza A, Bove JJ, Litwa J, et al. Mixed Infections of the Paronychium with Prevotella bivia. J Hand Microsurg. 2012;4(2):77–80.
- Hsu GJ, Chen CR, Lai MC, et al. Chest wall abscess due to Prevotella bivia. J Zhejiang Univ Sci B. 2009;10(3):233–266.
- Salman SA, Baharoon SA. Septic arthritis of the knee joint secondary to Prevotella bivia. Saudi Med J. 2009;30(3):426–428.
- Jain D, Elhwairis H, Campe J. Emphysematous pyelonephritis due to Prevotella bivia. Infect Dis Clin Pract. 2013;21. DOI:10.1097/IPC.0b013e3182639f51
- Atassi F, Brassart D, Grob P, et al. Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol Med Microbiol. 2006;48(3):424–432.
- Liu XQ, Wang CC, Liu YB, et al. Renal and perinephric abscesses in West China Hospital: 10-year retrospective-descriptive study. World J Nephrol. 2016;5(1):108–114.
- Gardiner RA, Gwynne RA, Roberts SA. Perinephric abscess. BJU Int. 2011;107(s3):20–23.
- Tsukagoshi D, Dinkovski B, Dasan S, et al. Perinephric abscess secondary to a staghorn calculus presenting as a subcutaneous abscess. CJEM. 2006;8(4):285–288.
- Morgan M, Hunter LK. Lactobacillus sepsis and emphysematous pyelonephritis. Infect Med. 2004;21(2):79–82.
- Boggess KA, Trevett TN, Madianos PN, et al. Use of DNA hybridization to detect vaginal pathogens associated with bacterial vaginosis among asymptomatic pregnant women. Am J Obstetrics Gynecology. 2005;193(3 Pt 1):752–756.
- Mueller-Premru M, Jeverica S, Papst L, et al. Performance of two blood culture systems to detect anaerobic bacteria. Is there any difference? Anaerobe. 2017;45:59–64.
- Byun JH, Kim M, Lee Y, et al. Antimicrobial susceptibility patterns of anaerobic bacterial clinical isolates from 2014 to 2016, including recently named or renamed species. Ann Lab Med. 2019;39(2):190–199.
