ABSTRACT
Gastric cancer is the fifth most common cancer in the world and the third leading cause of cancer-related deaths. Signet-ring cell type is the most malicious subtype. We report a case of advanced stage gastric adenocarcinoma case post-radical gastrectomy who presented with nausea, vomiting, and diarrhea. Though there were no signs of bowel obstruction on abdominal CT and PET imagine studies, and the cytology of body fluid was initially negative, the patient had unilateral malignant pleural effusion, a moderate amount of ascites and bilateral hydronephrosis. After laparoscopic surgery, the patient was diagnosed with local cancer relapse causing jejunojejunal anastomosis obstruction and peritoneal carcinomatosis causing hydronephrosis. We urge broadening the indication of EGD in the evaluation of advanced stage gastric carcinoma to include mechanic bowel obstruction.
1. Introduction
Gastric cancer is the fifth most common cancer in the world and the third leading cause of cancer-related deaths. The peritoneum is considered the most common site for metastasis from gastric cancer, with peritoneal carcinomatosis detected in around 60% of patients with gastric cancer after initial curative treatment. Lung metastasis and malignant pleural effusion have a much lower incidence, ranging from 1% to 5% [Citation1,Citation2]. To the best of our knowledge, the combination of recurrent peritoneal carcinomatosis and malignant pleural effusion has rarely been documented. We herewith present the case of a young female patient with an unusual presentation of local relapse resulting in bowel obstruction complicated with peritoneal carcinomatosis, right-sided malignant pleural effusion and bilateral hydronephrosis after radical gastrectomy and splenectomy for gastric adenocarcinoma.
2. Case presentation
A 47-year-old Chinese female with a history of poorly differentiated gastric adenocarcinoma was admitted for worsening fatigue, vomiting and abdominal bloating for 3 months, and diarrhea for 10 days. She had been vomiting 6–8 times a day, with bilious non-bloody vomitus. She had persistent diarrhea, almost 6–7 times a day with loose stools that were watery, yellowish, non-bloody, odourless, and unrelated to meals. Of note, she has lost almost 40 pounds over the previous 6 months.
Physical examination demonstrated a cachectic woman, with diminished breath sounds on the right lung associated with dullness to percussion on the same side. Her abdomen was soft but distended with well-healed surgical scars in the midline of the abdomen and over the left upper quadrant area. There was mild tenderness on palpation with normal bowel sounds.
2.1. Disease course prior to admission
The patient underwent gastric mucosal biopsy and was diagnosed poorly differentiated gastric adenocarcinoma 6 months prior to presentation. She received radical gastrectomy, splenectomy with Roux-en-Y procedure () followed by three courses of hyperthermic intraperitoneal chemotherapy (HIPEC), and two courses of chemotherapy (Course1: Doxorubicin and Paclitaxel; Course2: Doxorubicin, Paclitaxel and oxalic platinum) in her home country. After completing this treatment, she returned to the U.S., where she began to experience nausea and vomiting. She was admitted to an outside hospital on three different occasions, the most recent being one month prior to current hospitalization, for suspected small-bowel obstruction due to an internal hernia. During that hospitalization, total parenteral nutrition was initiated. Tumor marker testing showed CA125 260 U/ml and CA19-9 822 U/ml. At the same time, the PET scan showed post-partial gastrectomy with moderate inflammatory changes in distal esophagus, no evidence of residual/recurrent tumour at the anastomotic sites, moderate right pleural effusion without hypermetabolism, moderate ascites with some peritoneal thickening without hypermetabolism, small-bowel obstruction, moderate bilateral hydronephrosis, mildly active non-enlarged upper right cervical lymph nodes, likely reactive (7 × 10 mm).
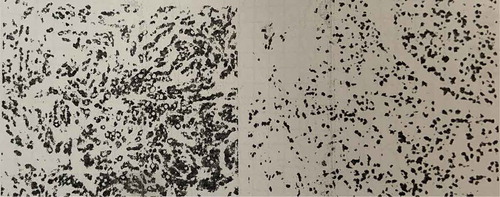
Figure 1. Original pathology report from her home country. (stomach) Diffuse invasive poorly differentiated adenocarcinoma, all layers of stomach tissues and some around fatty tissue were infiltrated. Spleen membrane was involved, not to parenchyma. Metastasis was found both in Less curvature lymph node 7/29 and Greater curvature lymph node 2/28, Immunochemistry result showed CD68(-), CK(+), CK20(+), CK7(-), Her-2(0), Ki67(+50%), SYN(-), Villain(+), Vimentin(-).
2.2. Laboratory and radiological workup on the current admission
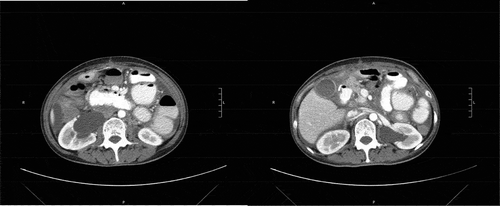
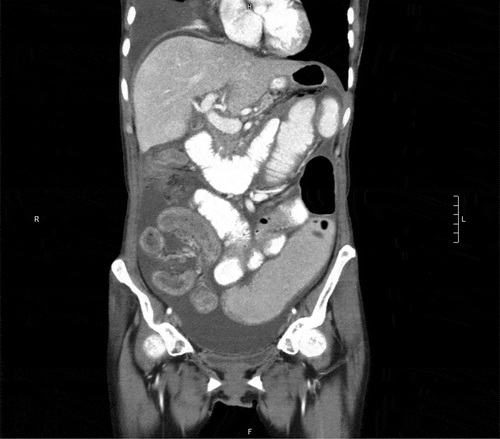
Complete blood count showed normocytic anemia with hemoglobin 11.1 g/dL, MCV 90.7. Chemistry tests were noted for hypoalbuminemia 3.1 g/dL. All stool studies and cultures were negative. Tumor markers were increased compared to previous values, with CEA 5.5 ng/ml, CA 125 345 U/ml, CA 19–9 3228 U/ml. Chest X-ray showed large right simple pleural effusion with a near complete collapse of the right lower lobe (). Abdominal CT with contrast showed moderate volume of ascites without obvious carcinomatosis demonstrated. Diffuse small bowel ileus was suspected, as no discrete transition point was definitively demonstrated (). Severe right greater than left hydroureteronephrosis is noted on cross-sectional view ().
Figure 2. Chest x-ray showed large right pleural effusion with near complete collapse of the right lower lobe.
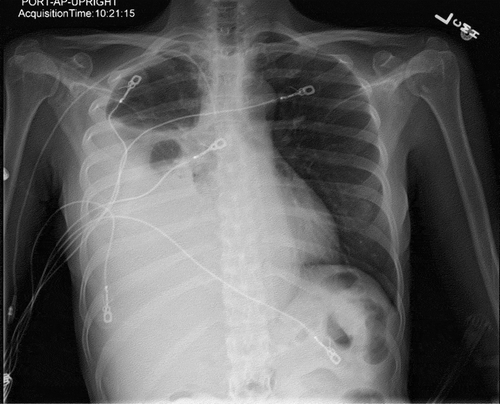
Figure 3. Abdominal CT coronal section. Distended gallbladder with wall edema. Postsurgical changes of total gastrectomy with Roux-en-Y gastrojejunostomy. No obvious leak. Moderate volume simple ascites. No obvious carcinomatosis demonstrated. Diffuse small bowel wall thickening most pronounced in the distal small bowel and possibly extending into the right colon. Compatible with enterocolitis. No pneumatosis, free air, or fluid collections. Likely diffuse small bowel ileus as no discrete transition point is definitively demonstrated.
2.3. Hospital course and advanced investigation
Chest tube placement and diagnostic paracentesis were performed. A large volume of yellowish serous pleural effusion and ascites with similar features were noted. Body fluid total protein 3.9 g/dL, albumin 1.8 g/dL, LDH 322 units/L, the serum LDH 316 units/L, and serum total protein 6.1 g/dL. These results met Light's Criteria for an exudative pleural effusion [Citation3], suggesting an exudative pleural effusion. The serum-ascites albumin gradient (SAAG) is 0.7; indicating peritoneal fluid was not due to portal hypertension [Citation4]. Cytology of peritoneal fluid was negative for malignant cells.
During the hospital stay, the patient’s stool volume decreased. The frequency of her vomiting increased up to 20 times per day despite multiple anti-emetic and prokinetic agents . Though no evidence of carcinomatosis was found, and no mechanical obstruction was identified on any imaging study, the symptoms were highly suggestive of bowel obstruction. EGD was performed, showing that the efferent limb of the jejunojejunal anastomosis was obstructed ().
Figure 5. EGD showed normal esophagus, healthy appearing mucosa at esophago-jejunal anastomosis. The efferent limb of the jejunojejnuo anastomosis appears obstructed.
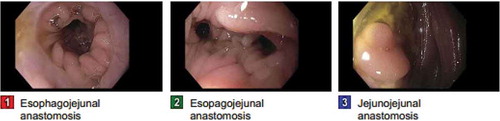
Repeated cytology examination of the pleural fluid demonstrated malignant cells, consistent with metastatic poorly differentiated adenocarcinoma, signet-ring cell type. The patient then received diagnostic laparoscopy, evacuation of ascites, a biopsy of peritoneal nodules x3, and pelvic peritonectomy. Pathology showed widespread metastasis, poorly differentiated, signet-ring carcinoma. The patient was deemed a poor candidate for additional chemotherapy or immunotherapy, and was discharged home with hospice support for palliative care.
3. Discussion
Despite being the fifth most common cancer in the world, gastric cancer is rare in the U.S.; the highest incidence is in East Asia, and specifically Japan, China, and South Korea [Citation5]. It is twice more common in men compared to women [Citation5]. The high prevalence led many countries with high incidence, including Japan and South Korea, to establish national screening programs for gastric cancer [Citation6]. The challenge in screening is that early stage tumors are usually asymptomatic, and that most patients present with advanced disease. Even after initial curative resection, patients often develop metastatic recurrences. Peritoneal carcinomatosis is the most common pattern of metastasis and is usually associated with poor prognosis in gastric cancer patients.
Despite advances in the treatment of gastric cancer, whether with cytoreductive surgery or HIPEC, there is a high recurrence rate, with a 5-year overall survival rate of only 24.5% in Europe [Citation7] and 40%-60% in Asia [Citation8]. The most common reason for treatment failure following surgery is peritoneal carcinomatosis, mostly by seeding of cancer cells through the peritoneum. Patients with this complication have a median overall survival rate of 3–6 months [Citation9].
Our patient had radical gastrectomy followed by three cycles of HIPEC and two cycles of chemotherapy before she decompensated due to development of recurrent peritoneal carcinomatosis, complicated by bilateral hydronephrosis and right-sided malignant pleural effusion. Interestingly, the previous PET from the prior hospital, and CT abdomen with contrast on presentation did not show evidence of mechanical obstruction. Persistent diarrhea and vomiting were attributed to bowel wall edema, as evidenced on the CT scan. However, EGD demonstrated evidence of mechanical obstruction at the jejunojejunal anastomosis. The sensitivity of the abdominal CT scan in detecting intestinal obstruction is 84 − 95%, depending on the degree of obstruction[Citation10]. This could explain why obstruction was not seen on the CT abdomen in our case. Also, the sensitivity of cytology in pleural fluid is only 60%, making it fairly common to have a negative cytology in cases of recurrent cancer [Citation11]. Given the presence of persistence of abdominal symptoms, EGD should be considered as the next step in evaluation and management.
Different strategies are involved in the palliative care of metastatic gastric cancer. One of the most common complications of metastatic gastric cancer and peritoneal carcinomatosis is bowel obstruction resulting in nausea, vomiting, and abdominal distension. The preferred method for the treatment of these symptoms is the endoscopic placement of a self-expanding stent. The development of malignant ascites would worsen the symptoms of bowel obstruction, as what occurred in our patient. This procedure is usually performed to patients with life expectancy less than 2–6 months; the technical and clinical success rates are 97% and 89% [Citation12]. Otherwise, patients may benefit from pro-kinetic agents, dopamine antagonists, or corticosteroids [Citation13].
4. Conclusion
The overall outcome for signet-ring cell cancers is very poor. Advanced stage carcinomatosis may be complicated with bowel obstruction, pleural effusion, malignant ascites, peritoneal metastasis, and hydronephrosis. In such patients presenting with nausea and vomiting, EGD should be considered to rule out obstruction, even though signs of obstruction are not evident on imaging studies. Despite a negative cytology of the peritoneal fluid, EGD can help diagnose local relapse. In addition, early EGD intervention may help re-staging the disease and enable early nutrition support, which helps to improve the quality of life of the patient.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Riihimaki M, Hemminki A, Sundquist K, et al. Metastatic spread in patients with gastric cancer. Oncotarget. 2016 Aug 9;7(32):52307–52316.
- Kong JH, Lee J, Yi C-A, et al. Lung metastases in metastatic gastric cancer: pattern of lung metastases and clinical outcome. Gastric Cancer. 2012;15:292–298.
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–513.
- Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215–220.
- Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32–41.
- Arnold M, Park JY, Camargo MC, et al. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut. 2020. DOI:10.1136/gutjnl-2019-320234
- Sant M, Allemani C, Santaquilani M, et al. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer. 2009;45:931–991.
- Matsuda T, Saika K. The 5-year relative survival rate of stomach cancer in the USA, Europe and Japan. Jpn J Clin Oncol. 2013;43:1157–1158.
- D’Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–816.
- Zhengyan L, Zhang L, Liu X, et al. Diagnostic utility of CT for small bowel obstruction: systematic review and meta-analysis. PLoS One. 2019;14(12):e0226740.
- Khan SL, Haris M, Munavvar M. Diagnostic accuracy of pleural fluid cytology compared to pleural biopsy histology obtained via thoracoscopy. Eur Respir J. 2014;44:P2775.
- Tringali A, Giannetti A, Adler DG. Endoscopic management of gastric outlet obstruction disease. Ann Gastroenterol. 2019 Jul-Aug;32(4):330–337.
- Glare PA, Dunwoodie D, Clark K, et al. Treatment of nausea and vomiting in terminally ill cancer patients. %SVHT. 2008;68:2575–2590.