ABSTRACT
A 48-year-old male presented to the emergency room for 2 weeks of joint pain and swelling of his four extremities. His symptoms started suddenly and were quite debilitating. His hands, fingers, knees, and ankles were so swollen and painful that he was unable to get out of bed and had to use crutches to ambulate. He also complained of anorexia, nausea, and lack of energy over the past few months, but denied any other complaints. His only medical history was a traumatic left tibia fracture 1 year ago. The patient had a 30-pack year history of smoking tobacco and used marijuana daily.
The patient recently had an arthrocentesis at an outside hospital which was non-diagnostic and showed no infection. Given his symptoms, a thorough rheumatic workup was ordered. The ESR and CRP were elevated. ANA, rheumatoid factor, HLA B27, HIV, hepatitis panel, TSH, T4, Coombs antibodies, gonorrhea, chlamydia, CCP, alpha 1 antitrypsin, parvovirus, fungal antibodies, and myeloperoxidase antibodies were all within the normal range. X-rays of the hands, knees, and ankles were ordered. The images showed diffuse joint swelling with no fractures, dislocations, or hardware mispositioning. It also showed tissue swelling in the fingers that could not exclude hypertrophic pulmonary osteoarthropathy. A chest x-ray revealed a large 8.5 cm oval mass in the right upper lobe. A follow-up CT revealed a massive right upper lobe lung mass concerning for malignancy versus fungal etiology. A CT guided biopsy of the mass was performed and revealed a poorly differentiated non-small-cell lung cancer, favoring adenocarcinoma. Further CT imaging revealed limited stage disease. During the hospitalization, the patient was provided with NSAIDs for his joint pain, which provided minimal benefit. There was little to no improvement in his joint swelling. Oncology was consulted and further evaluation in the outpatient setting was recommended to determine if he would be a surgical candidate and/or to decide the best chemotherapeutic regimen. This case demonstrates an unusual presentation of non-small-cell lung cancer and highlights the importance of maintaining malignancy on the differential diagnosis for sudden arthritis.
1. Case presentation
A 48-year-old male presented to the Emergency Room with a 3-day history of worsening widespread joint pain and swelling which resulted in total debility to the point where he was bedbound. The joint swelling and pain started suddenly and was generalized to all four of his limbs. His left knee was particularly painful and swollen. He also admitted to anorexia, nausea, and a lack of energy for about 2 weeks. His review of systems was otherwise negative.
Past medical history: None
Past surgical history: ORIF left tibia, with nail placement following a traumatic tibial fracture
Social history: daily marijuana, 1 PPD smoker × 30 years, no alcohol, no drugs, unemployed
Family history: no disease
Physical exam was as follows: BP 116/65, T 98.5 degrees Fahrenheit, RR 16, SPO2 100% room air
General: Alert, cooperative, no acute distress
HEENT: Normocephalic, atraumatic, pupils equal and reactive, oropharynx normal
Lungs: Clear to auscultation bilaterally
Heart: Regular rate and rhythm, no murmurs
Abdomen: Soft, tender, nondistended
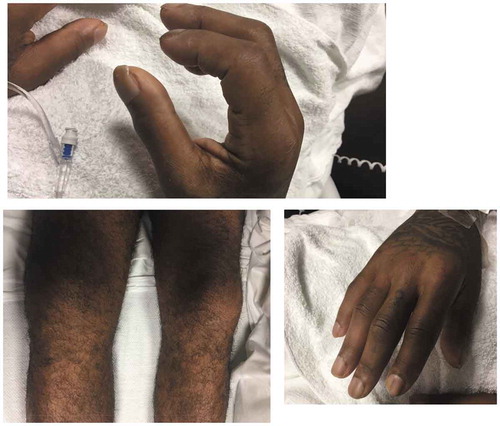
Extremities: moderate dactylitis of all fingers and toes, pain to palpation of hands and feet, L knee with pain and restricted movement, pain and swelling over left ankle, widespread finger clubbing ().
The patient was admitted to the general medical floor and initially worked up for arthritis, with leading differentials being rheumatoid arthritis, seronegative spondyloarthropathy, disseminated gonococcal infection, or septic arthritis. He also had a microcytic anemia which was new from 1 year prior.
A thorough laboratory workup was sent including the following ( and ):
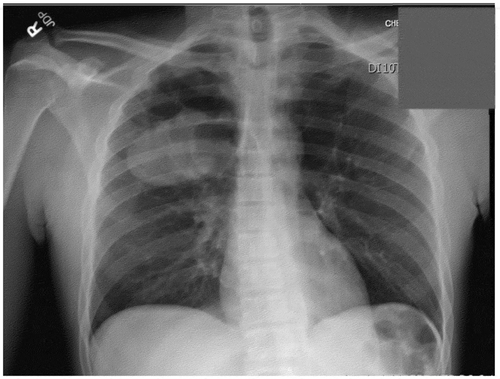
The patient was started on scheduled NSAIDs and oral glucocorticoids for symptomatic treatment of his arthritis. On hospital day 2 he reported no improvement. Therefore, the team decided to work up his clubbing with a chest X-ray, which is shown below, and was followed up with a CT scan:
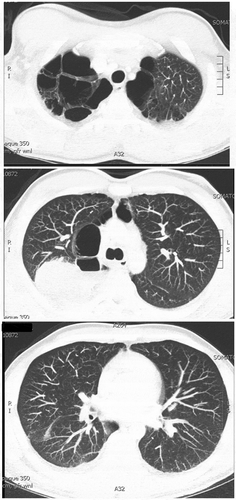
The chest x-ray revealed a large lung mass. If it had been caught earlier in the hospitalization, an expensive workup and delay in diagnosis could have been avoided. A follow-up CT scan revealed severe emphysema and a 6.5 cm x 8.7 cm x 5.8 cm mass in the right upper lobe (). Biopsy showed aggressive Non-small-cell carcinoma favoring adenocarcinoma. No metastatic disease was seen on imaging; however, staging was incomplete at the time of discharge. He was not deemed a surgical candidate given his severe underlying emphysema, and he was given instructions to follow up urgently with oncology for a PET scan, chemotherapy, and radiation.
2. Discussion
Polyarthritis is a relatively common complaint in both outpatient and inpatient settings. The patient in this case presented with polyarthritis that did not respond to NSAIDs and was diagnosed with hypertrophic osteoarthropathy secondary to Non-small-cell lung cancer. Different from most rheumatologic diseases, the evidence shows that steroids are not beneficial in this condition and the main treatment is tumor resection. Bisphosphonates are an option in non-operative candidates.
This case highlights the importance of having a high index of suspicion for malignancy in certain patient populations, but also the importance of ordering chest imaging in patients presenting with arthritic symptoms of unclear cause. This patient did not have chest imaging until day two of hospitalization since he did not have respiratory or chest symptoms.
Hypertrophic osteoarthropathy (HOA) is a rare paraneoplastic disease that can be extremely debilitating and usually resolves with treatment of the underlying lung pathology or with bisphosphonates. It develops due to underlying pulmonary pathology, most commonly a malignancy [Citation1]. Eighty percent of cases are caused by a paraneoplastic syndrome, more commonly by non-small-cell lung cancer and less often by small-cell lung cancer [Citation2]. The remaining 20% of cases can be caused by chronic respiratory disease, congenital cyanotic heart disease, chronic inflammation, or congenital pachydermoperiostosis [Citation3,Citation4]. It is sometimes the first sign of malignancy, so physicians should maintain a high index of suspicion when encountered with sudden arthritis, especially in a smoker [Citation5,Citation6].
Currently, three theories prevail regarding the pathophysiology of HOA. First, the neurogenic theory proposes direct nerve involvement. As early as 1965, several case reports were published of symptom relief following vagotomy on the side of the tumor [Citation7–Citation9]. The second theory, the mechanical hypothesis, proposes arteriovenous shunting within the pulmonary system to be the main culprit. Pulmonary capillaries are normally responsible for the degradation of inflammatory cytokines. With AV shunting secondary to chronic hypoxia, vasoactive compounds are not degraded in the pulmonary endothelium and instead are released systemically, leading to increased inflammation, tissue hypoxia, and peripheral clubbing, as well as pain [Citation10].
The third theory is currently the most widely accepted and supported by evidence. Named the biochemical hypothesis, it posits increased systemic inflammation as a direct result of biochemical compound release from the primary tumor. Multiple studies have demonstrated elevated levels of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) in patients with HOA, even when compared to other patients with chronic lung disease [Citation11,Citation12]. VEGF and PDGF are both pro-inflammatory cytokines which increase vascular permeability, cell permeability, angiogenesis, and bone formation. Tumor production of these cytokines is thought to lead to widespread inflammation, connective tissue, and bone proliferation, and the symptoms of HOA. In one study, removal of the lung tumor resulted in normalization of VEGF and PDGF levels and a remission of symptoms [Citation13].
HOA is important to recognize because the treatment of the primary tumor often leads to rapid improvement in symptoms. In some cases, the improvement was seen in as little as 24 h to 2 weeks [Citation14]. In the case of a debilitated patient who cannot tolerate surgery or chemotherapy, palliative treatment with bisphosphonates and octreotide is an option. However, the best treatment is cure of the underlying malignancy.
3. Comprehensive metabolic panel
Urinalysis:
Acute hepatitis panel: negative
HIV screen: negative
Sedimentation rate: 125 mm/HR (reference 0–15)
C-reactive protein: 261 mg/L (reference <10)
Iron profile:
Iron: 22 mcg/dL (reference 35–150)
TIBC: 173 mcg/dL (reference 228–428)
Iron Saturation: 13% (reference 20-55%)
Ferritin: 1330 ng/mL (reference 18–464)
ANA screen: negative
Rheumatoid factor: negative
Anti-CCP antibodies: negative
Gonorrhea and chlamydia PCR: negative
HLA B-27: negative
Parvovirus: IgG positive, IgM negative
ANCA screen: negative
Alpha 1 Antitrypsin: 231 mg/dL (reference 90–200)
Direct Coombs test: negative
Uric acid: 3 mg/dL (reference 3.5–8.5)
TSH: 2.6 uIU/mL (reference.47–4.68)
Fungal immunodiffusion for Aspergillus, Coccidioides, Histoplasma, and Blastomyces: negative
Lung biopsy report:
Right lung, upper lobe, core biopsy:
Poorly differentiated non-small-cell carcinoma with numerous giant/anaplastic tumor cells (See Comment).
4. Findings
The pulmonary vessels enhance homogeneously bilaterally without evidence for filling defects. There is no evidence for pneumothorax. Emphysematous changes are seen with a biapical predominance. There has been interval development of a 6.5 cm AP by 8.7 cm transverse by 5.8 cm craniocaudal mass within the right upper lobe. Differential considerations would include bronchogenic neoplasm versus metastatic disease versus atypical fungal/infectious etiologies. The ascending and descending thoracic aorta are of normal caliber. No mediastinal hematoma or mediastinal lymphadenopathy is seen. No acute bony abnormalities are demonstrated. The visualized upper abdomen is unremarkable.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Yao Q, Altman RD, Brahn E. Periostitis and hypertrophic pulmonary osteoarthropathy: report of 2 cases and review of the literature. Semin Arthritis Rheum. 2009;38(6):458–466.
- Qian X, Qin J. Hypertrophic pulmonary osteoarthropathy with primary lung cancer. Oncol Lett. 2014;7(6):2079–2082.
- Ito T, Goto K, Yoh K, et al. Hypertrophic pulmonary osteoarthropathy as a paraneoplastic manifestation of lung cancer. J Thorac Oncol. 2010;5(7):976–980.
- Castori M, Sinibaldi L, Mingarelli R, et al. Pachydermoperiostosis: an update. Clin Genet. 2005;68(6):477–486.
- Jajic Z, Jajic I, Nemcic T. Primary hypertrophic osteoarthropathy: clinical, radiologic, and scintigraphic characteristics. Arch Med Res. 2001;32(2):136–142.
- Pourmorteza M, Baumrucker SJ, Al-Sheyyab A, et al. Hypertrophic pulmonary osteoarthropathy: a rare but treatable condition in palliative medicine. J Pain Symptom Manage. 2015;50(2):263–267.
- Flavell G. Reversal of pulmonary hypertrophic osteoarthropathy by vagotomy. Lancet. 1956;270:260–262.
- Huckstep RL, Bodkin PE. Vagotomy in hypertrophic pulmonary osteoarthropathy associated with bronchial carcinoma. Lancet. 1958;2:343–345.
- Yacoub MH. Cervical vagotomy for pulmonary osteoarthropathy. Br J Dis Chest. 1965;59(1):28–31.
- Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res. 2000;55:15–35.
- Silveira LH, Martínez-Lavín M, Pineda C, et al. Vascular endothelial growth factor and hypertrophic osteoarthropathy. Clin Exp Rheumatol. 2000;18(1):57–62.
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–4380.
- Uchisako H, Suga K, Tanaka N, et al. Bone scintigraphy in growth hormone-secreting pulmonary cancer and hypertrophic osteoarthropathy. J Nucl Med. 1995;36(5):822–825.
- Albrecht S, Keller A. Postchemotherapeutic reversibility of hypertrophic osteoarthropathy in a patient with bronchogenic adenocarcinoma. Clin Nucl Med. 2003;28(6):463–466.