ABSTRACT
Introduction
Obstructive sleep apnea (OSA) is an established risk factor for poor cardiovascular outcomes and coronary artery disease, but its influence on the development of peripheral artery disease (PAD) is not well established. The aim of our study was to understand the mutual prevalence of OSA and PAD and any reported statistical association between the two conditions.
Methods
PubMed, Ovid Embase, Web of Science, Cochrane library and clinicaltrials.gov databases were systematically searched up to 29 November 2018. A total of 844 articles were identified and 744 articles were screened for relevance.
Results and Conclusion
Eleven prospective cohorts qualified for inclusion with N = 63,642 (M = 28,062, F = 35,494). All studies evaluated OSA severity primarily with apnea–hypopnea index (AHI) values. The overall prevalence of PAD was 20.5% (N = 13,068). Except for two studies, all studies reported an increased prevalence of OSA in patients with PAD. OSA severity was not found to have an association with poor ankle brachial index values or increasing daytime sleepiness as measured by Epworth sleepiness scale. Further prospective clinical trials are required to further delineate this finding.
1. Introduction
Obstructive sleep apnea (OSA) is the most common type of sleep-related disordered breathing syndromes which is characterized by partial or complete obstruction of upper airway during sleep. It is accompanied by hypoxemia and forceful efforts to breathe leading to episodes of increased intrathoracic pressure [Citation1]. Patients with OSA report an increased prevalence of daytime sleepiness, systemic and pulmonary hypertension, headache and depression leading to poor concentration [Citation2] and quality of life [Citation3]. It has also been well established that OSA is associated with poor cardiovascular outcomes like coronary artery disease (CAD), atrial fibrillation, stroke [Citation4] and myocardial injury [Citation5]. Available evidence suggests that OSA is often underdiagnosed, but the general reported prevalence is over 10% [Citation6]. The high prevalence in patients with cardiovascular diseases has been consistently reported in scientific literature. Even though the presence of peripheral artery disease (PAD) is considered a poor prognostic factor for cardiovascular diseases [Citation7], it shares the similar pathophysiologic factors with CAD [Citation7], the question of prevalence of OSA in this patient population is largely unaddressed.
Multiple studies have shown that hypoxemic episodes of OSA lead to a state of systemic inflammation, increased level of circulating adhesion molecules, reactive oxygen species and endothelial injury that predispose patients to an increased risk of atherosclerosis [Citation4]. It has also been postulated that OSA leads to poor cardiovascular outcomes owing to these atherogenic vascular changes. Association of OSA with CAD and stroke is well defined; limited data exist for its association with PAD. Therefore, we conducted a comprehensive systematic review to report the association of OSA in patients with PAD.
2. Material and methods
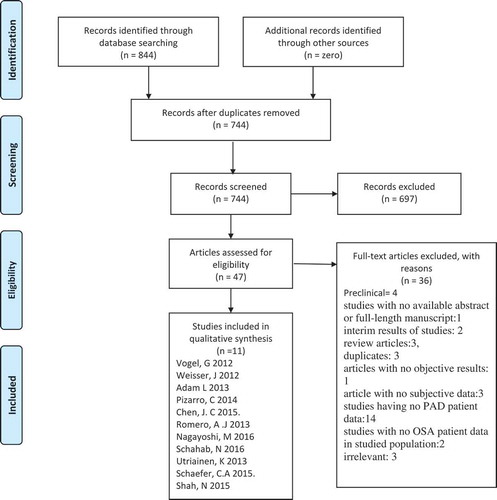
The systematic review was done according to the PRISMA statement and its summary is given in .
A systematic search of databases as PubMed, Embase, Web of Science and Cochrane library was performed using the medical search terms and their respective free words with the following search strategy: ‘Obstructive Sleep Apnea’ in combination with ‘Peripheral Arterial Disease’. Additionally, unpublished trials were identified from the clinicaltrials.gov website and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on 29 November 2018 with no filters applied for language, subjects or time period. After removing 100 duplicates, titles and abstracts of 744 articles were screened for relevance by 2 independent reviewers by reviewing the titles and abstracts of studies and conflict was settled by discussion. A total of 47 articles were deemed relevant and their abstracts and full texts were screened for eligibility.
The following eligibility criterion was used: Original articles reporting most recent results for individual studies that had evaluated the mutual prevalence or association of peripheral arterial disease and OSA in a clinical setting. The exclusion criteria were preclinical studies (n = 4); studies with no available abstract or full length manuscript (n = 1); interim results of studies with no final results (n = 2), review articles (n = 3), duplicates (n = 3), articles with no objective results (n = 1) and article with no subjective data (n = 3; these included case reports, media talks and correspondence); studies having no PAD patient data (n = 14) and studies with no OSA patient data in studied population (n = 2) and irrelevant (n = 3).
The following 11 articles with N = 63,642 qualified for this strict selection criterion: Vogel, G 2012, Weisser, J 2012, Adam, L 2013, Pizarro, C 2014, Chen, JC 2015, Romero, AJ 2013, Nagayoshi, M 2016, Schahab, N 2016, Utriainen, K 2013, Schaefer, CA 2015 and Shah, N 2015.
3. Results
3.1. Baseline characteristics
Eleven prospective cohorts qualified for inclusion with N = 63,642, including 28,062 males and 35,490 females. Adam, L 2013 did not report the gender distribution data for studied population (n = 90). Majority of patients belonged to old age group and mean (SD) age ranged from 59.6 ± 13.2 to 71.1 ± 9.8 years. The mean BMI data were reported by six studies for a total of 10,963 patients and ranged 23–39 kg/m2. Among the baseline characteristics, patients with PAD showed a significantly high prevalence of hypertension, hyperlipidemia, smoking and CAD. The data regarding the prevalence of diabetes mellitus (DM) in PAD patients were conflicting and were reported by only two studies [Citation8,Citation9]. A summary of these characteristics is given in .
Table 1. Baseline patient characteristics of included studies.
3.2. Obstructive sleep apnea
The known or suspected OSA was present in 10,628 (16.7%) patients. Polysomnography was used for diagnosis of OSA in 2121 patients (20%) while home polygraphy was used in 8507 patients (80%). All studies evaluated OSA severity primarily with apnea–hypopnea index (AHI) values. The mean AHI value was reported by six studies [Citation10–Citation15] and it ranged 11.8–39 events/h. The grades of OSA severity as per AHI values were reported by eight studies for a total of 4518 patients with OSA [Citation9–Citation12,Citation14-Citation17]. OSA was mild (AHI 5–15 events/h) in 2529 patients (55.97%), moderate severity (AHI 15–30 events/h) in 1160 patients (25.68%) and severe (AHI > 30 events/h) in 829 reported patients (18.35%). The daytime sleepiness was estimated using Epworth sleepiness scale (ESS) in six studies (N = 793) and the overall mean ESS value ranged 9.05 ± 5.1–11.02 ± 4.19. Schaefer, CA 2015 showed that ESS score significantly correlated with increasing severity of OSA (no OSA = 7.5, AHI-1 = 7.8, AHI-2 = 10.06, AHI-3 = 11.02; p = 0.022) and Shah, N 2015 reported ESS score to have no significant association with the presence of PAD (patients with PAD = 5.7 vs. no PAD = 5.9; p = 0.639). The oxygen desaturation index (ODI) measured by PSG was reported by six studies [Citation14,Citation15,Citation17] for 445 OSA patients and for 48 patients with sleep disordered breathing and it ranged 8.9 ± 14.2–30.7 ± 25 events/h. Among these six studies, only Adam, L 2013 evaluated the association of ODI with AHI values and reported a positive correlation with increasing AHI severity (R = 0.303, p = 0.024).
3.3. Peripheral artery disease
The known PAD was present in 13,068 (20.5%) patients. Ankle brachial index (ABI) was screening method in all cases, but additional screening tests including pulse wave velocity (PWV) and duplex ultrasonography were also performed to assess the influence of atherosclerotic plaque on regional blood flow.
Except for one study [Citation18], all studies reported an increased prevalence of OSA in patients with PAD and vice versa. Schaefer et al. and Adam et al. ran the log regression model to find association of OSA and PAD and found that significant association persisted even when the data were adjusted for age, hyperlipidemia, diabetes, smoking and presence of systemic hypertension (OR = 1.60, p < 0.001) (adjusted OR = 1.37, p = 0.014). But in the subgroup analysis evaluating multiple testing modalities for both conditions, studies reported somehow heterogeneous data. Three studies [Citation10–Citation12] (N = 241) found a statistically significant association of worsening central PWV with increasing severity of AHI (p < 0.05, p = 0.006, p = 0.003, respectively). But data were conflicting regarding the association of AHI severity with PWV and intermittent claudication. Two studies [Citation11,Citation14] found a significant reduction in PWV with increasing apnea severity (p = 0.02, p = 0.01, respectively), while one study (Vogel, G 2012 [Citation10]) found no significant difference in PWV among AHI groups. Regarding the claudication severity, Vogel, G 2012 reported no difference among AHI groups but a significant difference (p = 0.01) was reported by Schaefer, CA 2015. Four studies [Citation10,Citation11,Citation17,Citation18] calculated any potential association of ABI values with OSA severity measured by AHI values but found no statistically significant association.
4. Discussion
Association of OSA has been well studied in numerous cardiovascular conditions including hypertension, coronary heart disease (CHD), arrhythmias, heart failure and cerebrovascular stroke. The plausible mechanisms that link OSA to the development of PAD are most likely similar to those linking it to abovementioned conditions including repetitive episodes of hypoxemia, hypercapnia promoting inflammation and oxidative stress, subsequently endothelial dysfunction. However, until the present, clinical evidence for the association between OSA and PAD is lacking. The fact that abovementioned risk conditions and PAD are highly correlated makes it difficult to interpret the causal relationship between OSA and PAD.
Current evidence suggests that OSA is associated with increased carotid artery atherosclerosis and management of OSA appears to reverse carotid artery atherosclerosis. PAD affects almost 8.5 million Americans aged 40 or older causing disabling symptoms due to severe claudication. PAD itself is a marker of underlying atherosclerosis severity and it shares large number of risk factors with OSA, i.e., obesity, smoking, hypertension and diabetes. Nagayoshi et al. while reporting a pool of 7209 patients found a significant association of OSA and PAD in African American population. This association persisted even after adjustment for confounding factors like age, diabetes, gender etc. ().
Table 2. Polysomnography results.
In their sample, both short and long sleepers were found to have higher prevalence of PAD. Likewise, Chen et al. 2015 showed that in age- and sex-matched pair analysis, moderate-to-severe OSA shows high association with PAD (OR = 1.60, p < 0.001). This effect maintained its statistical significance when baseline confounding factors were adjusted (adjusted OR = 1.37, p = 0.014) [Citation8]. However, this association was attenuated on further adjustment for relevant confounders including hypertension, hyperlipidemia and DM (adjusted OR = 1.26, p = 0.075).
Shah et al. 2015 while reporting data for 8367 patients showed that OSA is associated with severe PAD (OR = 1.67, 95% CI = 1.10–2.51, p = 0.0152) only when AHI of 15 was reached. And except for race, this association was not affected by baseline confounding factors like age, sex, BMI, waist–hip ratio, hypertension, diabetes, CHD, dyslipidemia, C reactive protein (CRP) levels, smoking, alcohol use and Hispanic/Latino background. This incoherence in outcome of different measuring variables was also shown by other studies.
Continuous positive airway pressure (CPAP) is the treatment of choice for OSA and results in significant improvement in systemic inflammation, reduction of cholesterol level, daytime sleepiness and quality of life [Citation3,Citation19]. A recent meta-analysis of case-control studies has also shown that CPAP results in significant improvement in levels of inflammatory markers as CRP, Tumor Necrosis Factor (TNF) and InterLeukin-6 (IL-6) [Citation19]. But coagulation abnormalities have been shown to persist even with prolonged therapy and treatment with CPAP has not resulted in reversal of vascular atherosclerotic plaque formation. Observational studies have documented a benefit of CPAP therapy in CAD patients with reduction in cardiovascular complications and death from cardiac cause [Citation20]. However in contrast, recent RCTs have not verified benefit of CPAP in reduction of the long-term cardiovascular outcomes, i.e., Myocardial Infarction (MI), stroke, atrial fibrillation, heart failure and unstable angina [Citation21]. Therefore, the impact of CPAP therapy and its influence on the PAD risk warrants further investigation.
Our results have some limitations: The observational design of majority of included studies limits the inference of any casual association for their patient data. Moreover, our studies have not commented on the possible link of PAD with atherosclerosis in other vascular beds. The lack of homogeneity of association in results of all the clinical tests for OSA and PAD warrants further investigation. But despite these limitations, OSA has emerged as an independent risk factor for the PAD development.
5. Conclusion
To our knowledge, this is the first systematic review that has discussed the role of OSA in PAD and their mutual association. Results show that although there is a direct link between the two diseases, the results are variable regarding the clinical variables used for the evaluation of OSA and PAD, but AHI has been shown to truly depict the OSA severity and its association with peripheral arterial disease. Further prospective studies are required to further delineate the disease association and its possible pathophysiologic link with atherosclerosis in other vascular beds.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Yu X, Huang Z, Zhang Y, et al. Obstructive sleep apnea in patients with chronic thromboembolic pulmonary hypertension. J Thorac Dis. 2018;10(10):5804.
- Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating gess in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Internal Med. 2003;163(5):565–571.
- D’Ambrosio C, Bowman T, Mohsenin V. Quality of life in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure—a prospective study. Chest. 1999;115(1):123–129.
- Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6(2):131–137.
- Drager LF, McEvoy RD, Barbe F, et al. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–1850.
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014.
- Grenon SM, Vittinghoff E, Owens CD, et al. Peripheral artery disease and risk of cardiovascular events in patients with coronary artery disease: insights from the heart and soul study. Vasc Med. 2013;18(4):176–184.
- Chen J-C, Koo M, Hwang J-H. Risks of peripheral arterial occlusive disease in patients with obstructive sleep apnoea: a population-based case-control study. Clin Otolaryngol. 2015;40(5):437–442.
- Shah N, Allison M, Teng Y, et al. Sleep apnea is independently associated with peripheral arterial disease in the Hispanic community health study/study of latinos. Arterioscler Thromb Vasc Biol. 2015;35(3):710–715.
- Vogel G, Schäfer C, Adam L, et al. High prevalence of peripheral arterial occlusive disease (PAOD) in patients with obstructive sleep apnea. Pneumologie. 2012;66(S 01):V172.
- Schaefer C, Adam L, Weisser-Thomas J, et al. High prevalence of peripheral arterial disease in patients with obstructive sleep apnoea. Clin Res Cardiol. 2015;104(9):719–726.
- Adam L, Schaefer C, Weisser-Thomas J, et al. Patients with severe obstructive sleep apnea syndrome (OSAS) are at greater risk for peripheral arterial disease (PAD). Pneumologie. 2013;67(S 01):V363.
- Pizarro C, Schaefer C, Kimeu I, et al. Underdiagnosis of obstructive sleep apnoea in peripheral arterial disease. Respiration. 2015;89(3):214–220.
- Weisser-Thomas J, et al. High prevalence of early peripheral arterial occlusive disease (PAOD) in patients with obstructive sleep apnea (OSA). Vasa – J Vascular Dis. 2012;82:62.
- Schahab N, Sudan S, Schaefer C, et al. Sleep apnoea is common in severe peripheral arterial disease. PLoS ONE. 2017;12(7):e0181733. (no pagination).
- Nagayoshi M, Lutsey PL, Benkeser D, et al. Association of sleep apnea and sleep duration with peripheral artery disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2016;251:467–475.
- Utriainen KT, Airaksinen JK, Polo O, et al. Unrecognised obstructive sleep apnoea is common in severe peripheral arterial disease. Eur Respir J. 2013;41(3):616–620.
- Jimenez Romero A, Leon Acuña A, Perez Martinez P, et al. Sleep breathing disorders and peripheral arterial disease in patients with coronary artery disease. Sleep Med. 2013;1:e246–e247.
- Baessler A, Nadeem R, Harvey M, et al. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers - a meta-analysis. J Inflamm (Lond). 2013;10(1):13.
- Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-moron I, et al. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156(2):115–122.
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931.