ABSTRACT
Transcatheter aortic valve implantation (TAVR) constitutes an established treatment in inoperable or high perioperative risk patients with severe aortic stenosis. Prosthetic valve endocarditis after ΤΑVR occurs with an incidence of 0.3–1% per patient-year. Infective endocarditis may stem from hematogenous dissemination or contact with infected adherent tissue. Few cases of infective endocarditis after TAVR have been reported. We present an interesting case of a 79-year-old male with a history of severe aortic stenosis status post TAVR greater than one year ago, and pulmonary vein isolation for atrial fibrillation six weeks ago was found to have infective endocarditis with a vegetation on the prosthetic valve leading to multiple embolic strokes as a result of Enterococcus faecalis bacteremia. The patient was not a surgical candidate with his Society of Thoracic Surgery (STS) risk score being 18%; therefore, he was managed conservatively on intravenous antibiotics. Our case had endocarditis from enterococcus bacteremia; however, the patient never had any gastrointestinal or genitourinary procedure.
1. Introduction
Transcatheter aortic valve implantation (TAVR) has become a substitute to surgical aortic valve replacement (SAVR) for severe aortic stenosis in high-risk patients or those with contraindications to surgery [Citation1–Citation4]. Following the success of the first transcatheter aortic valve replacement (TAVR) in 2002, the procedure has since been on the rise [Citation1,Citation2]. The procedure fundamentally involves a self-expandable or a balloon-expandable stent with an attached pericardial valve inserted into the native aortic annulus using a transarterial or transapical approach, which thereby compresses the cusps of the native aortic valve against the aortic root wall [Citation4]. Patients for TAVR are typically the elderly, with multiple comorbidities, and with a high surgical risk [Citation1,Citation2,Citation4].
Prosthetic valve endocarditis (PVE) in patients following a TAVR is a rare complication accounting for 1% to 3% of cases [Citation1,Citation5,Citation6]. Guidelines for PVE diagnosis and management are well curated, however, no conventional parameters are set for infective endocarditis following a TAVR [Citation1]. Until further evidence is available, diagnosis and treatment are prescribed on a case-by-case basis based on clinical judgment [Citation1].
PVE is concerning due to its low survival rates. Conservative management is often not adequate (showing a one-year mortality rate of 64% to 66%), requiring surgical valve replacement even in surgically unfit candidates [Citation3,Citation5]. The poor prognosis in these patients makes it crucial to ensure prevention to avoid detrimental events or avoidable mortalities.
We hereby report a case of Enterococcal endocarditis that occurred one year after TAVR in a 79-year-old male patient.
2. Case presentation
A 79-year-old male with a history of severe aortic stenosis had TAVR performed greater than one year ago with 26 mm bovine Edwards Sapien valve, pulmonary vein isolation with ablation for atrial fibrillation six weeks ago, coronary artery disease, chronic systolic heart failure with an ejection fraction of 30%, type 2 diabetes mellitus presented to the hospital with complaints of altered mental status (AMS), fatigue, loss of appetite, night sweats, fever, and dizzy spells. The patient had been having symptoms of fatigue, night sweats, and loss of appetite 2–4 weeks after the pulmonary vein isolation procedure, and at that time, his amiodarone had been discontinued. The following week, his colchicine was discontinued as the patient was having consistent symptoms. He presented to the emergency department due to the progression of symptoms and altered mental status. On examination, the patient was afebrile, had AMS, on cardiac exam S1/S2/S3 was audible with a systolic murmur, but no gallops or rubs were appreciated. The patient had mild elevation of jugular venous pulse, trace edema of lower extremity, and crackles at bases.
Further evaluation revealed leukocytosis with a white blood cell count of 14.1/mm3, and chest x-ray had bilateral infiltrates; therefore, the patient was admitted with a provisional diagnosis of severe sepsis with health-care-associated pneumonia. The patient underwent magnetic resonance imaging to evaluate AMS, which identified multiple embolic strokes and was also found to have Enterococcus faecalis bacteremia. A trans-esophageal echocardiogram (TEE) demonstrated a large, echogenic, mobile, irregular 14 mm x 7 mm vegetation on the non-coronary cusp (NCC) on the ventricular side of the prosthetic valve. [–] TEE did not identify any aortic regurgitation, aorta exhibited normal size, ejection fraction was 30%, mean aortic gradient was 22 mm Hg, peak aortic gradient was 35 mm Hg, mean velocity through the aortic valve was 2.2 m/s, left ventricular end-diastolic diameter was 6.6 cm.
Figure 1. A transesophageal echocardiogram showing a large echogenic mass (blue arrow) measuring 14 mm x 7 mm on ventricular aspect in outflow tract.
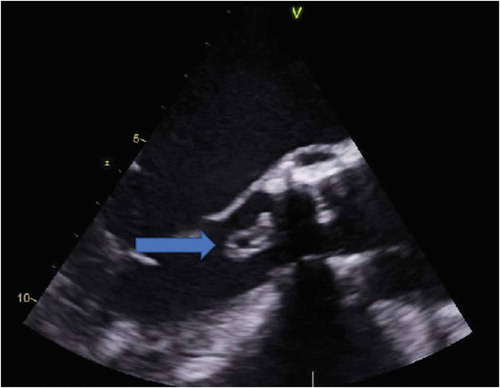
Figure 2. 3-D Echocardiogram identifying vegetation (blue arrow) on the non-coronary cusp of aortic valve.
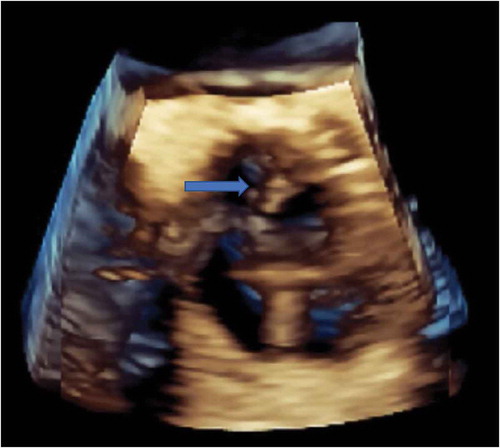
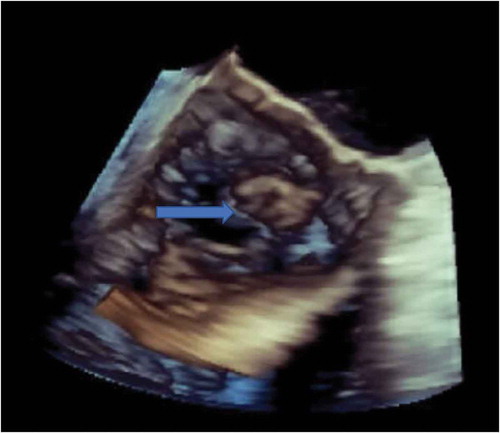
Figure 3. 3-D Echocardiogram showing vegetation (blue arrow) on the non-coronary cusp of aortic valve.
The patient was started on intravenous (IV) hydration and antibiotics. The patient was evaluated by the cardiothoracic surgeon and deemed not to be a surgical candidate, especially with a Society of Thoracic Surgery (STS) score of 18%. He was treated with IV vancomycin, ceftriaxone, and ampicillin for six weeks, followed by lifelong suppressive therapy with trimethoprim/sulfamethoxazole.
The etiology of the patient’s endocarditis is uncertain as he never had any gastrointestinal or genitourinary procedure.
3. Discussion
TAVR is a procedure used for patients with significant comorbidities and severe symptomatic stenosis [Citation1]. Owing to the novelty of the procedure, sequelae to TAVR are still being learned [Citation3,Citation6]. Pabilona et al. highlight the significant events occurring as an early sequela to TAVR, including vascular diseases, stroke, renal failure, paravalvular leak with aortic regurgitation, and atrioventricular block [Citation3]. Not much literature is available identifying the late sequelae [Citation3]. A few emerging cases of infective endocarditis after TAVR have been identified with more frequent uses. These reported incidents used antibiotics for treatment in accordance with the PVE cause [Citation6].
PVE has been recorded to have a significant mortality rate of 20% to 40%, with no possibility of improvements in the survival rate for the past three decades [Citation6]. The mortality rate in TAVR patients with PVE is congruent with the recent data at 34% [Citation6]. As per the literature, it also points out that this complication occurs more commonly in males and patients with high-risk profiles [Citation6].
While early PVE is hypothesized to arise during the implantation procedure, contamination with non-classical pathogens like enterococci in TAVR patients is suggestive of a different infective source [Citation6]. Moreover, about 15% of patients suffered from infectious complications postoperatively, serving as a possible infective source for PVE [Citation6]. Additionally, during the transcatheter valve preparation and loading, some leaflet damage can arise due to compressive handling, further favoring PVE [Citation6].
Due to discordance between the bulky-calcified native aortic valve and the implanted prosthetic valve, some amount of paravalvular leak and thence regurgitation are frequently seen after a TAVR [Citation4]. These leaks serve as breeding nests for infections, which is further exacerbated by predisposing comorbidities and older age, all favoring infective endocarditis in patients after TAVR [Citation4]. Additionally, early PVE typically occurs at the junction of the sewing ring and the annulus, hence giving rise to valve dehiscence and intensifying the paravalvular leak [Citation4].
Studies report PVE following TAVR has a wide-ranging clinical picture, pathogen involved, and management required [Citation3]. The interim between TAVR and admission at the hospital for PVE was noted to vary between around 2 weeks to 23 months [Citation3]. Most common of the isolated organisms was Enterococcus faecalis followed by S. viridans, coagulase-negative staphylococci, Corynebacterium, Pseudomonas, Moraxella, Candida, methicillin-resistant Staphylococcus aureus, and Escherichia coli in decreasing frequency [Citation3,Citation6]
According to a study by Puls et al., a late diagnosis has led to severe morbidities, including cerebral embolization, acute renal failure, and long hospital admissions [Citation4]. Echocardiography has been used in TAVR patients to observe and monitor complications like abscesses (47%), fistulae (9%), or involvement of other valves (22%) in comparison with patients with native or surgical prosthetic valves [Citation3,Citation6]. Autopsy series and surgical explantation of infected transcatheter valves have aided in identifying predisposing structural factors such as significant inflammation and infection of the skirt and leaflets with spread and perforation of adjacent structures [Citation6]. Core for diagnosis is an echocardiogram with positive findings (from Duke’s criteria) of vegetation, abscess, new partial dehiscence of the prosthetic valve, and a new valvular regurgitation [Citation4]. Identifying small vegetations on transesophageal echocardiography can be difficult because of shadowing and reflecting the property of the prosthetic valve [Citation4].
Enterococci species are highly resistant to antibiotics, and complete elimination may require extended six weeks use of the synergistic bactericidal combination [Citation6]. In addition, these microbes can be tolerant to many drugs like aminoglycosides, beta-lactams, and vancomycin [Citation6]. This trend of high antibiotic resistance leading to treatment failure is alarming since conservative medical management is the most commonly used strategy in treating PVE following TAVR at present. Enterococcal PVE has also shown to be complicated by periprosthetic dehiscence, annular abscesses, or fistulas [Citation1]. In cases when treatment with antibiotics fails, immediate surgical intervention is required [Citation1].
Surgery is the treatment of choice for patients with PVE unless unfit [Citation7]. Patients who benefit most with surgery in terms of prognosis and overall survival are cases with additional complications stemming from PVE such as heart failure, valvular dysfunction, valvular regurgitation, or obstruction, valve dehiscence, and annular abscess [Citation7]. An absolute indication for early surgery in PVE is infection with S. aureus, even if uncomplicated, in order to prevent cerebral complications [Citation7]. PVE caused by other microbes can be managed conservatively with antibiotics if micro-organism is sensitive to antibiotics and shows no evidence of cardiac complications [Citation7]. However, cardiac surgeons should be notified early in the case, and surgery should only be postponed if adequate treatment has been achieved [Citation7]. Surgery is also recommended in hemodynamically unstable patients, those who have a recurrent infection, bacteremia, or emboli [Citation7].
To summarize, the echocardiographic criterion used to diagnose infective endocarditis is not well suited for the diagnosis of PVE in post-TAVR patients [Citation4]. Studies involving a larger population with regular follow-ups are required to understand the prevalence, the pathogen involved, and the treatment regimen effective against this complication.
4. Conclusion
It is crucial to be watchful for PVE in patients after TAVR. Currently, prophylactic antibiotic before TAVR and prior to any dental or invasive procedure in patients after TAVR is adopted on a case by case basis per the hospital protocols, naturally creating variability amongst already high-risk cases involving elderly population with multiple comorbidities. We suggest early commencement of organism-sensitive antibiotics in symptomatic TAVR patients with positive blood culture and the absence of an alternate source of infection, even with inconclusive findings on echocardiography.
Disclosure statement
Authors have no interest to disclose. The authors report no financial relationships or conflicts of interest regarding the content herein.
Additional information
Funding
References
- Sarı C, Durmaz T, Karaduman BD, et al. Prosthetic valve endocarditis 7 months after transcatheter aortic valve implantation diagnosed with 3D TEE. Hell J Cardiol. 2016;57(2):119–123.
- Loberman D, Shaefi S, Mohr R, et al. Trans-catheter aortic valve replacement program in a community hospital - Comparison with US national data. PLoS One. 2018;13(9):e0204766.
- Pabilona C, Gitler B, Lederman JA, et al. Prosthetic valve endocarditis with valvular obstruction: after transcatheter aortic valve replacement. Texas Hear Inst J. 2015;42(2):172–174.
- Puls M, Eiffert H, Hünlich M, et al. Prosthetic valve endocarditis after transcatheter aortic valve implantation: the incidence in a single-centre cohort and reflections on clinical, echocardiographic and prognostic features. EuroIntervention. 2013;8(12):1407–1418.
- Skowerski T, Grzywocz P, Bałys M, et al. Prosthetic valve endocarditis and acute heart failure in a patient after transcatheter aortic valve implantation procedure. Kardiol Pol. 2018;76(7):1116.
- Amat-Santos IJ, Ribeiro HB, Urena M, et al. Prosthetic valve endocarditis after transcatheter valve replacement: A systematic review. JACC Cardiovasc Interv. 2015;8(2):334–346.
- Attaran S, Chukwuemeka A, Punjabi PP, et al. Do all patients with prosthetic valve endocarditis need surgery? Interact Cardiovasc Thorac Surg. 2012;15(6):1057–1062.
