ABSTRACT
Anti-synthetase syndrome (ASS) is characterized by myositis that is associated with progressive interstitial lung disease (ILD). The prognosis of the disease is affected by the type and degree of pulmonary involvement. We report a rare case of ASS with positive Anti-EJ antibody presenting with a combination of recurrent deep vein thrombosis/pulmonary embolism (DVT/PE) and progressive ILD. This case demonstrates the delayed diagnosis of ASS and the association of thromboembolic disease and ASS. Physicians should have a high index of suspicion for ASS, as early diagnosis and management alters the morbidity and prognosis of patients with ASS.
Abbreviations
ASS: Anti-synthetase syndrome; Ab: Antibody; Ag: Antigen; ANA: Anti-nuclear antibodies; CK: Creatine kinase; CRP: C-reactive protein; DVT: Deep Vein Thrombosis; ESR: Erythrocyte Sedimentation Rate; ILD: Interstitial lung disease; PE: Pulmonary Embolism; CTA: CT Angiography
1. Introduction
Anti-synthetase syndrome (ASS) is a rare systemic autoimmune condition characterized by a combination of myopathy and interstitial lung disease (ILD), a subset of idiopathic inflammatory myopathies including polymyositis/dermatomyositis [Citation1]. The main feature of ASS is the presence of antibodies directed against aminoacyl-transfer RNA synthetase [Citation2].
We present a case with a combination of recurrent deep vein thrombosis/pulmonary embolism (DVT/PE), musculoskeletal symptoms and progressive ILD.
2. Case report
2.1. Case presentation
A 59-year-old African American female with a history of recurrent DVT/PE on warfarin, was admitted for worsening dyspnea, mild hemoptysis with shoulder and back pain. She had multiple admissions of a similar presentation with the presumptive diagnosis of pneumonia/exacerbation of ILD with clinical improvement after receiving antibiotics. Her sister has a history of recurrent PE with inferior vena cava filter placed.
Physical exam demonstrated bilateral fine basal crackles, palpable cervical lymphadenopathy, bilateral shoulder tenderness and bilateral ankle joint swelling with tenderness.
2.2. Laboratory and radiological workup
Complete blood count showed chronic microcytic anemia with hemoglobin 8.9 g/dL. The chemistry was notable for K+ 3.1 mmol/L. INR was 2.17. Blood cultures, sputum culture, respiratory viral panel, urine Legionella antigen (Ag) and urine Streptococcal pneumoniae Ag were all negative. Her erythrocyte sedimentation rate (ESR) was 65 mm/hr, C-reactive protein (CRP) was 8.6 mg/dl, and creatine kinase (CK) level was 279 mg/L.
Based on elevated inflammatory markers in association with the current presentation, rheumatological workup was obtained showing positive Antinuclear Antibodies (ANA). The rheumatoid factor, Anti-dsDNA Ab, Anti-Smith Ab, Anti-Scl-70 Ab, Anti-GBM Ab, Anti-Ro Ab, and Anti-La Ab were all negative. Further testing for ASS was positive for Anti-EJ Ab. The Anti-Jo1 Ab, Anti-PL Ab, Anti-OJ Ab, Anti-KS Ab and Anti-GBM Ab were all negative.
The hypercoagulable profile including anti cardiolipin antibody, factor II and factor V Leiden mutations were negative. ACE and vitamin D levels were normal.
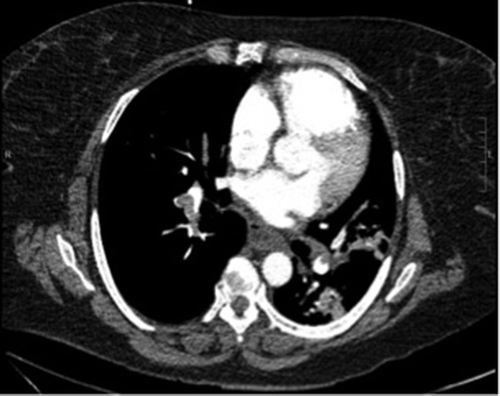
Electrocardiogram revealed sinus tachycardia with chronic T wave inversion in right chest leads. Chest radiograph showed multifocal consolidation with greater involvement on the right side. Computed tomography angiography (CTA) of the chest was done showing no new PE, but revealed new widespread broncho-vascular nodular consolidation with surrounding ground glass appearance (). CTA of the chest a month prior showed segmental and sub-segmental PE in all 5 lobes of the lung (). A bronchoscopy performed few months ago for a similar presentation showed cloudy, amber-colored fluid positive for signs of inflammation. The biopsy was negative for malignant cells and showed alveolar macrophages and multinucleated giant cells.
Figure 1. CT angiography. New widespread bronchoalveolar nodular consolidation with surrounding ground glass appearance.
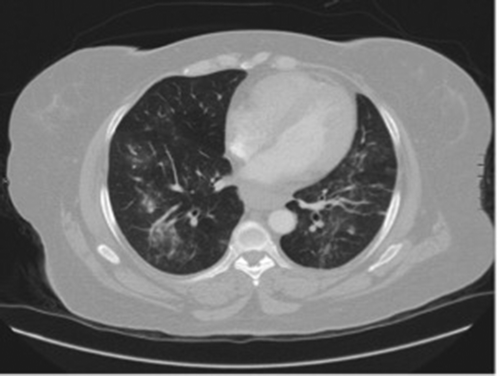
Figure 2. CT angiography. Large burden of pulmonary emboli in all segmental and sub-segmental branches of all 5 lobes with significant right heart enlargement.
The patient was initially started on antibiotics in addition to her daily warfarin dose. However, given the overall clinical presentation in conjunction with elevated ESR, CRP, CK, and positive ANA and Anti-EJ Ab with the radiologic findings on CT of the chest, this led to a diagnosis of anti-synthetase syndrome. The patient was started on prednisone with a total resolution of her symptoms. She was discharged on tapering doses of oral corticosteroids with an eventual plan for follow-up appointments with rheumatology and pulmonology.
3. Discussion
Anti-synthetase syndrome is an autoimmune condition, characterized by antibodies directed against aminoacycl-transfer RNA synthetase [Citation3]. Our case met Solomon diagnostic criteria of ASS [Citation4]: Presence of anti-aminoacyl-tRNA synthetase antibody, plus two major (1. ILD not attributable to another cause; 2. Polymyositis or dermatomyositis) or one major and two minor criteria (1. Arthritis; 2. Raynaud’s phenomenon; 3. Mechanic’s hands). ASS is treated with immunosuppressant, with first-line therapy of corticosteroids. However, most of the patients will require second-line therapy, including azathioprine or mycophenolate mofetil, and even add-on therapy of tacrolimus, rituximab or cyclophosphamide.
The clinical features would be variable based on the detected antibody. Anti Jo-1 Ab, the most common anti-aminoacyl-tRNA synthetase antibody is mostly associated with progressive myopathy. Anti-PL7 Ab and Anti-PL12 Ab were found to be more associated with severe ILD [Citation5].
The association between anti-synthetase syndrome and coagulopathy with recurrent DVT/PE has always been correlated to antiphospholipid syndrome with positive lupus anticoagulant and anti-cardiolipin in these patients [Citation6–Citation9]. Wang et el, reported a case of anti-Jo-1 myositis with right ventricular thrombus and elevated anti-cardiolipin antibody [Citation6]. This case is unique in that it’s the first case of anti-synthetase syndrome associated with recurrent DVT/PE with negative lupus anticoagulant and anti-cardiolipin antibodies. This association warrants further investigation in pathophysiology. The prognosis of patients with anti-synthetase syndrome is largely attributed to the severity of interstitial lung disease which is mostly found in patients with positive anti-PL7/PL12 autoantibodies [Citation10]. Physicians should consider referral of these patients for lung transplantation in case of disease progression and poor response to medical treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Trallero-Araguás E, Grau-Junyent JM, Labirua-Iturburu A, et al. Clinical manifestations and long-term outcome of anti Jo1 antisynthetase patients in a large cohort of Spanish patients from the GEAS-IIM group. Semin Arthritis Rheum. 2016;16(30):1–4.
- Mirrakhimov AE. Antisynthetase syndrome: a review of etiopathogenesis, diagnosis and management. Curr Med Chem. 2015;22(16):1963–1975.
- Mahler M, Miller FW, Fritzler MJ, et al. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: a comprehensive review. Autoimmun Rev. 2014;13(4–5):367–371.
- Solomon J, Swigris JJ, Brown KK. Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol. 2011;37(1):100–109.
- Witt LJ, Curran JJ, Strek ME, et al. The diagnosis and treatment of antisynthetase syndrome. Clin Pulm Med. 2016;23(5):218–226.
- Wang C-H, Wang N-C, Lin T-Y, et al. Anti-Jo-1 myositis and the antiphospholipid syndrome showing right ventricular thrombus: a novel overlap syndrome with atypical presentation. Mod Rheumatol. 2014;24(5):865–868.
- Sherer Y, Livneh A, Levy Y, et al. Dermato- myositis and polymyositis associated with the antiphospholipid syndrome—a novel overlap syndrome. Lupus. 2000;9(1):42–46.
- Ponyi A, Constantin T, Dankó K. Antiphospholipid and antisynthetase syndrome in a patient with polymyositis-rheumatoid arthritis overlap. Clin Rheumatol. 2004;23(4):371–372. MoriA, Nodera H, Nakane S, Kaji R. Transverse myelitis and polymyositis associated with antiphospholipid antibody syndrome. Clin Neurol Neurosurg. 2010;112(8):713–716.
- Souza FH, Levy-Neto M, Shinjo SK. Antiphospholipid syndrome and dermatomyositis/polymyositis: a rare association. Rev Bras Reumatol. 2012;52(4):642–644.
- Lega J-C, Fabien N, Reynaud Q, et al. The clinical phenotype associated with myositis-specific and associated autoantibodies: a meta-analysis revisiting the so-called antisynthetase syndrome. Autoimmun Rev. 2014;13(9):883–891.
