ABSTRACT
Pure red cell aplasia is an uncommon paraneoplastic syndrome of thymoma. Myasthenia gravis is the most common paraneoplastic syndrome associated with thymoma. We present a case of a 79-year-old Pacific Islander female who presented with profound fatigue, generalized weakness, significant unintentional weight loss, bilateral ptosis, and anemia. The bone marrow biopsy showed near absence of erythroid elements consistent with pure red cell aplasia. Ice-pack test was consistent with myasthenia gravis and computed tomography of the chest demonstrated a thymoma. The patient was started on immunosuppressive treatment with prednisone and cyclosporine. This case demonstrates a rare combination of paraneoplastic manifestations of thymoma: pure red cell aplasia and myasthenia gravis.
1. Introduction
Thymoma is often associated with paraneoplastic syndromes. Recognizing symptoms and signs of the paraneoplastic syndromes can facilitate proper work-up and treatment of the patients with thymoma. Pure red cell aplasia (PRCA) is one of rare paraneoplastic syndromes of thymoma and characterized by a low reticulocyte count, marked reduction or absence of erythroid precursors from the bone marrow. PRCA is rare in patients with a known thymoma and reports are limited. The most common paraneoplastic syndrome associated with thymoma is myasthenia gravis, which is an autoimmune disorder caused by interference with acetylcholine receptors of voluntary muscle at the neuromuscular junction [Citation1,Citation2]. We present a rare case of thymoma, which presented with concomitant PRCA and myasthenia gravis.
2. Case description
A 79-year-old Pacific Islander female presented with profound fatigue, generalized weakness and significant unintentional weight loss of 100 pounds within 3 months. The patient had multiple past medical histories including hypothyroidism status post (s/p) thyroidectomy and distal aortic aneurysm s/p abdominal aortic aneurysm repair. The patient was taking levothyroxine 50mcg daily at home, an ex-smoker of 40 pack-year. Family history was insignificant.
On physical exam, vital signs showed temperature at 37.6 degrees Celcius, pulse rate 76 per minute, respiratory rate 15 per minute, blood pressure 90/53 mmHg, and SpO2 95% on room air. Patient was chronically ill-appearing, cachectic with BMI of 18.7. She had temporal wasting, bilateral ptosis, pale conjunctiva, and dry oral mucosa. Extra-ocular muscle strength was intact. Cardiopulmonary, abdominal exam were unremarkable. Guaiac test was negative. No evidence of non-pitting edema was seen. Hypo-reflexia was present on bilateral knee reflex.
Laboratory results revealed haemoglobin 3.7 g/dL (normal 11.7–15.7 g/dL), mean corpuscular volume (MCV) 101.6 fL (normal 81–100 fL), whereas her baseline haemoglobin was 8 g/dL. Iron was at 191 ug/dL (normal 50–170 ug/dL), and ferritin was at 2,076 mg/dL (normal 4.6–274.7 mg/dL). WBC 5.2 x 103/uL (normal 4.5–11.0 x 103/uL), platelet 260 x 103/uL (normal 140–400 x 103/uL) were within normal limit. Thyroid function test showed thyroid-stimulating hormone (TSH) at 29.18 uU/ml (normal 0.35–4.94 uU/ml) and free T4 at 0.84 ng/dl (normal 0.7–1.48 ng/dL). Vitamin B12 and haptoglobin were normal. Reticulocyte absolute count of 0.0014x106/uL was markedly reduced which is consistent with reduced marrow response to the anemia.
Chest x-ray showed elevation of the right diaphragm with overlying atelectasis and no mediastinal widening noted.
The patient was resuscitated with intravenous (IV) fluid and received six packed red blood cells. She underwent Esophagogastroduodenoscopy (EGD) which did not show bleeding, only noted to be moderate chronic gastritis.
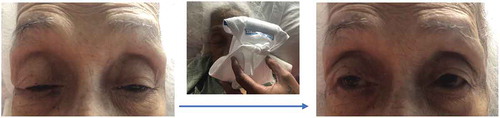
Ice pack test was performed as myasthenia gravis was suspected with symptoms of generalized weakness, and bilateral ptosis. The cold pack was placed over the eyes () and after 2 minutes, the ptosis was diminished, indicating a positive test result.
Figure 1. Positive ice pack test. A marked improvement of ptosis is noted after ice pack application for 2 minutes.
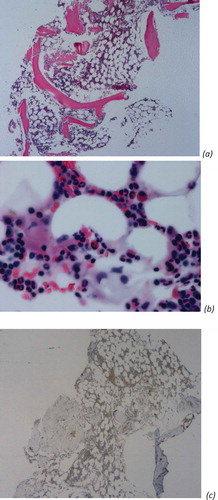
Bone marrow biopsy () demonstrated near absence of erythroid elements (erythroid 3%, myeloid 80%, monocyte 2%, lymphoid 14%, plasma 1% of differentials) and no blasts noted. CD 71 stain (transferrin receptor 1) which is essential for iron uptake and consequently for hemoglobin synthesis during erythroid differentiation, revealed negative for the patient which confirms that bone marrow biopsy findings were consistent with pure red cell aplasia [Citation3].
Figure 2. Bone marrow biopsy slides, demonstrating near absence of erythroid elements. (a) Low-power view of a HE-stained section (x20). (b) High-power view of a HE-stained section (x400). (c) Low-power view of a CD71 stain for confirmation of pure red cell aplasia.
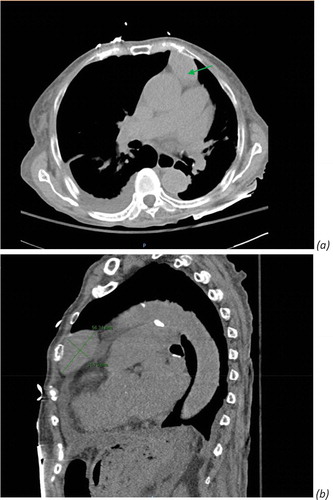
Computed tomography (CT) chest revealed a 5.6 cm ovoid anterior mediastinal mass, consistent with thymoma ().
Figure 3. Computed tomography (CT) chest without contrast revealed ovoid anterior mediastinal mass consistent with thymoma. (a) Green arrow. (b) Diameter measurement of thymoma, 5.6 × 3.1 cm ovoid anterior mediastinal mass.
Acetylcholine (Ach) receptor binding antibody and Ach receptor blocking antibody were negative. ANA was positive with titer of 1:40. Double-stranded DNA antibody and Rheumatoid factor, muscle-specific kinase/actin-related proteins 4 (MuSK/ARP4) antibody, Ryanodine receptor (RyR) autoantibody, MuSK antibody, Titin autoantibody were negative. Human immunodeficiency virus (HIV), hepatitis B, hepatitis C, and parvovirus B 19 were also negative.
The patient was given IV fluid and multiple blood transfusions and discharged home on the fourth day of hospitalization. The patient was started on prednisone and referred to cardiothoracic surgeon for removal of thymoma. Unfortunately, the patient refused surgical intervention. The patient was continued on prednisone. The patient continued to have generalized weakness with intermittent dropping of hemoglobin requiring blood transfusions.
The patient was on prednisone for 5 months without significant response. Cyclosporine was added. The patient continued to require blood transfusions and intermittently admitted to the hospital. Subsequently, the patient was started on rituximab as she did not show improvement on prednisone and cyclosporine combination.
3. Discussion
PRCA results from an autoimmune-mediated hypo-proliferation of erythrocyte precursors in the bone marrow. Anemia is resulted from an autoimmune-mediated destruction of erythroid precursors [Citation4]. PRCA can be classified with congenital and acquired forms. A congenital form of PRCA is Diamond-Blackfan anemia. Primary acquired PRCA is an autoimmune disorder associated with autoantibody that interrupts erythroid differentiation. Secondary acquired PRCA is associated with collagen vascular/autoimmune disorders; systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel disease; lymphoproliferative disorders, Parvovirus B19, thymoma and other solid tumors, drugs, or toxic agents [Citation5]. PRCA appears to be rare in known thymoma patients (<5%). However, a significant portion of PRCA (8.5–50%) has been associated with thymoma [Citation6]. It is important to note that imaging studies for thymoma are indicated when patients initially present with PRCA as clinical signs and symptoms of thymoma can be subtle and subclinical.
The diagnosis of myasthenia gravis is confirmed by the combination of relevant symptoms and signs and a positive test for specific autoantibodies such as antibodies against acetylcholine receptors, MuSK, and lipoprotein receptor-related protein 4 (LRP4). Approximately 10% to 15% of myasthenia gravis cases are seronegative. In such cases, neurophysiological tests and a characteristic response to therapy can establish the diagnosis [Citation4]. As shown in above , an ice-pack test that reverses ptosis is an evidence to support the diagnosis. While severe hypothyroidism may cause similar neurologic symptoms such as ptosis and generalized weakness, her free T4 was within normal range. Positive ice pack test result would be unlikely seen in ptosis associated with severe hypothyroidism. Also, other clinical manifestations of hypothyroidism (e.g., hypothermia, protrusion of eyes, periorbital edema) were not present. If myasthenia gravis is suspected, thymus should be evaluated by mediastinal imaging studies as 10% of patients with myasthenia gravis have a thymoma, and the prevalence increases with increasing age [Citation4].
Thymectomy has been recommended as the initial treatment of thymoma-associated PRCA [Citation7]. Earlier reports suggested that thymectomy normalizes bone marrow in up to 40% of cases; however, recent observations suggest that remission rate following surgical excision alone is not greater than a third of patients [Citation8]. Adjuvant treatment for PRCA was effective with immunosuppressive therapy such as glucocorticoid, cyclosporine, or cyclophosphamide, alone or in combination. A graded approach to treatment, starting with glucocorticoids alone, and adding cyclosporine is preferred if there is no initial response [Citation6]. Agents used in patients with resistant or relapsed disease include intravenous immune globulin, azathioprine, rituximab, and bone marrow transplantation [Citation5,Citation7]. The information about the prognosis of patients with thymoma-associated PRCA is limited. It has been suggested that patients with thymoma-associated PRCA have a poor prognosis. According to a cohort study performed in Japan, the estimated overall survival time was 12 years with 66 years of the median age in patients with thymoma-associated PRCA. In comparison, life expectancies of average 65-year-old Japanese males and females are 18 and 23 years, respectively [Citation7]. The main causes of death in PRCA were infection and organ failure [Citation7].
The patients with a thymoma and myasthenia gravis, thymectomy should be performed to remove the tumor. If thymectomy cannot be performed, symptomatic and supportive therapy with acetylcholinesterase inhibitor can be used. The immunosuppressive therapy with prednisone in combination with azathioprine as first-line treatment can be added if treatment result is not satisfactory with supportive therapy alone [Citation4].
Our case demonstrates that the patients diagnosed with PRCA and/or myasthenia gravis should be evaluated for undiagnosed associated thymoma by a multidisciplinary approach including imaging studies. Combination of surgical resection of thymoma, supportive symptom relief therapy, and immunosuppressive therapy are needed to ensure an overall positive outcome.
4. Conclusion
This case shows an example that simple laboratory tests (CBC, reticulocyte count) and physical tests provide important information to guide the investigation and management of patients who present with anemia and generalized weakness. When a patient is diagnosed with PRCA and/or myasthenia gravis, these should be recognized as probable paraneoplastic syndromes of thymoma and proper work-ups including a chest-computed tomography is warranted. Thymectomy and immunosuppressive therapy can offer a potential cure of these conditions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375(26):2570–2581.
- Feinsilber D, Mears KA, Pettiford BL. Polyparaneoplastic manifestations of malignant thymoma: a unique case of myasthenia, autoimmune hepatitis, pure red cell aplasia, and keratoconjunctivitis sicca. Cureus. 2017;9(6):e1374.
- Macri S, Pavesi E, Crescitelli R, et al. Immunophenotypic profiling of erythroid progenitor-derived extracellular vesicles in Diamond-Blackfan anaemia: a new diagnostic strategy. PLoS One. 2015;10(9):e0138200. DOI:
- Lacy MQ, Kurtin PJ, Tefferi A. Pure red cell aplasia: association with large granular lymphocyte leukemia and the prognostic value of cytogenetic abnormalities. Blood. 1996;87(7):3000–3006.
- Means RT Jr. Pure red cell aplasia. Hematology Am Soc Hematol Educ Program. 2016;2016(1):51–56.
- Moriyama S, Yano M, Haneda H, et al. Pure red cell aplasia associated with thymoma: a report of a single-center experience. J Thorac Dis. 2018;10(8):5066–5072. DOI:
- Hirokawa M, Sawada K, Fujishima N, et al. Long-term response and outcome following immunosuppressive therapy in thymoma-associated pure red cell aplasia: a nationwide cohort study in Japan by the PRCA collaborative study group. Haematologica. 2008;93(1):27–33. DOI:
- Thompson CA, Steensma DP. Pure red cell aplasia associated with thymoma: clinical insights from a 50-year single-institution experience. Br J Haematol. 2006;135(3):405–407.
